Chondrocalcinosis
| Chondrocalcinosis | |
 | |
|---|---|
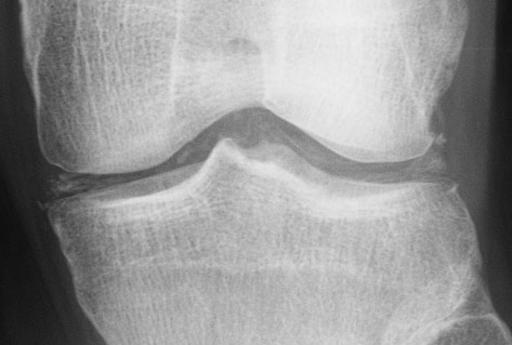
| Chondrocalcinosis of the articular and fibrocartilage of the left knee in a patient with calcium pyrophosphate dihydrate deposition disease (CPPD) | |
| ICD-10 | M11.1-M11.2 |
| ICD-9 | 712.3 |
| DiseasesDB | 10832 |
| MedlinePlus | 000421 |
| MeSH | D002805 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2] Luke Rusowicz-Orazem, B.S. Mohammed Abdelwahed M.D[3]
Synonyms and keywords:: Pyrophosphate arthropathy; chondrocalcinosis; pseudogout; CPPD; Calcium pyrophosphate dihydrate deposition disease; CPPD disease
Overview
Calcium pyrophosphate deposition disease (CPPD) is a rheumatologic disorder with varied clinical manifestations due to precipitation of calcium pyrophosphate dihydrate crystals in the connective tissues. It is more commonly known by alternative names that specify certain clinical or radiographic findings, although neither is synonymous with CPPD. Pseudogout refers to the clinically evident acute synovitis with red, tender, and swollen joints that may resemble gouty arthritis (a similar condition with joint deposition of monosodium urate crystals). Chondrocalcinosis, on the other hand, refers to the radiographic evidence of calcification in hyaline and/or fibrocartilage. Pyrophosphate arthropathy is a term that may refer to either of the above, but is uncommonly used.
Causes
Life Threatening Causes
Causes in Alphabetical Order
Epidemiology
- Calcium pyrophosphate crystal deposition (CPPD) disease has been estimated to affect 4 to 7 percent of the adult populations of Europe and the United States. [7,8]
- The average age at diagnosis of CPPD disease in one study was 72 years [3].
- The prevalence of radiographic calcium pyrophosphate deposition according to age was:
65 to 74 years – 15 percent
75 to 84 years – 36 percent
>84 years – Almost 50 percent
- There is no major gender predominance in CPPD. Attacks of acute arthritis may occur more frequently in men.
Pathogenesis
- Calcium pyrophosphate crystal formation reflects elevated levels of calcium or inorganic pyrophosphate in cartilages matrix.
- Excessive pyrophosphate levels arises from nucleoside triphosphate pyrophosphohydrolase enzyme overactivity.
- Increased concentrations of substrate (adenosine triphosphate) for this enzyme are also present in joint fluids from persons with CPPD.
- ANKH gene plays a role in formation of the crystals. There is now evidence that the ANKH gene product also promotes the release of ATP by chondrocytes. [14]
- One function of extracellular pyrophosphate appears to be to bind to and inhibit the growth of basic calcium phosphate crystals.
- In CPPD, excessive pyrophosphate levels arising from NTPPPH overactivity could provide the substrate for CPP crystal formation in the immediate environment of chondrocytes.
- Mutations in or just upstream of the chromosome 5p locus of ANKH have also been identified in some individuals with idiopathic or sporadic CPPD deposition disease [18,21].
●There are striking similarities in the pathophysiologic mechanisms and clinical appearances of acute urate gout and CPP crystal-induced arthritis [24]. Of particular note is the shared capacity of both crystal types to induce Nacht Domain-, Leucine-Rich Repeat-, and PYD-Containing Protein 3 (NALP3)-dependent inflammasome assembly and activation in synovial mononuclear phagocytes and neutrophils. Activation of the NALP3 inflammasome, in turn, activates latent caspase-1, resulting in interleukin (IL)-1 precursor processing and release of the pro-inflammatory cytokine IL-1beta [25].
●An etiologic or an amplifying role for CPP crystals in the destructive changes in osteoarthritis appears highly likely. Degenerative arthritis accompanying CPPD frequently involves such joints as the metacarpophalangeal and wrist joints, which are commonly spared in classical osteoarthritis [27], and chondrocalcinosis appears to be a primary determinant of the rate of radiographically-determined joint deterioration in osteoarthritis. Interestingly, in usual osteoarthritis, CPP crystals may not be present in early disease but appear to be secondarily associated with progression of the severity of the osteoarthritis [28]. In a study of cadaveric knees from Japanese older adults (mean age of 78), the deposition of CPP crystals correlated with the degree and depth of cartilage degeneration [29].Acute attacks of CPP crystal arthritis are typically self-limited. Although possible mechanisms for ameliorating inflammation due to CPP crystals have been suggested, a generally accepted explanation is lacking. Phagocytosis and dissolution of crystals may play a role, but observations in patients together with data from animal models indicate that inflammation can abate while crystals are still present in tissue or fluid.
Clinical presentation
- Asymptomatic CPPD disease: Most joints in which CPP crystal deposition is readily apparent on radiographs are asymptomatic, even among patients in whom acute or chronic clinical manifestations of CPPD disease in one or several other joints have occurred. However, patients with apparent asymptomatic CPPD may be found to have manifestations of an arthritic disorder upon close questioning.
- Acute CPP crystal arthritis: The knee is affected in over 50 percent of all acute attacks of acute CPP crystal arthritis.
- Other joints typically affected in acute CPP crystal arthritis include wrists, shoulders, ankles, feet, and elbows. Initial episodes of acute CPP crystal arthritis may persist longer before remitting than the one or two weeks commonly encountered in urate gout, and an upper extremity site of inflammation (wrist, elbow, shoulder) for a first attack should raise suspicion for acute CPP crystal arthritis [13].
- Predisposing factors include trauma, surgery, or severe medical illness often provoke acute attacks. Treatment with pamidronate or granulocyte-macrophage-colony-stimulating factor have also been reported to precipitate acute attacks of pseudogout.
- Chronic CPP crystal inflammatory arthritis: the chronic inflammatory arthritis of CPPD disease involves multiple joints, frequently involving peripheral joints of the upper and lower extremities, including the wrists and metacarpophalangeal (MCP) joints, as well as the knees and elbows, in a symmetric or nearly symmetric pattern. Articular inflammation may last up to several months, and inflammation in affected joints tends to wax and wane independently of one another, in distinction to RA, where synchronous flare and remission is the rule.
- Chronic CPP crystal inflammatory arthritis occurs in 5 percent or less of patients with symptomatic CPPD disease. A rare subtype of chronic CPP crystal inflammatory arthritis, occurring most often in older adult patients during an acute polyarticular attack, is characterized by prominent systemic features, such as leukocytosis, fever, and mental confusion, closely mimicking systemic sepsis [19]. In such patients, the delirium is reported to resolve with resolution of the acute polyarticular flare.
- Severe joint degeneration: A number of reports have documented CPP crystal deposition in association with severe joint degeneration which closely resembles neuropathic arthropathy [21-23]. Neuropathic arthropathy is characterized by severe joint degeneration and disruption occurring in the course of neurologic disorders leading to joint denervation; the affected joint is often called a Charcot joint. Underlying disorders associated with Charcot joints include diabetes mellitus (most common), tabes dorsalis, and syringomyelia. (See "Diabetic neuropathic arthropathy".)Spinal involvement: CPP crystal deposition in and about the spine has been associated with a number of clinical manifestations, including spine stiffness, sometimes associated with bony ankylosis, which can resemble the spinal changes of ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis (DISH). Such symptoms have been most commonly encountered in familial CPPD disease [24]. In addition, crystal deposition in the ligamentum flavum at the cervical spine level or in the posterior longitudinal ligament at lower levels of the spine may lead to spinal cord compression syndromes or to symptoms either of acute nerve compression or of chronic spinal stenosis [25-27].
Diagnosis
- Synovial fluid: Identification of CPP crystals is diagnostic. Successful identification of the crystals diminishes as the time between joint aspiration and microscopic examination increases.
Radiological findings
Three main manifestations of CPPD deposition:
- chondrocalcinosis
- crystal induced synovitis
- pyrophosphate arthropathy
Plain film radiography
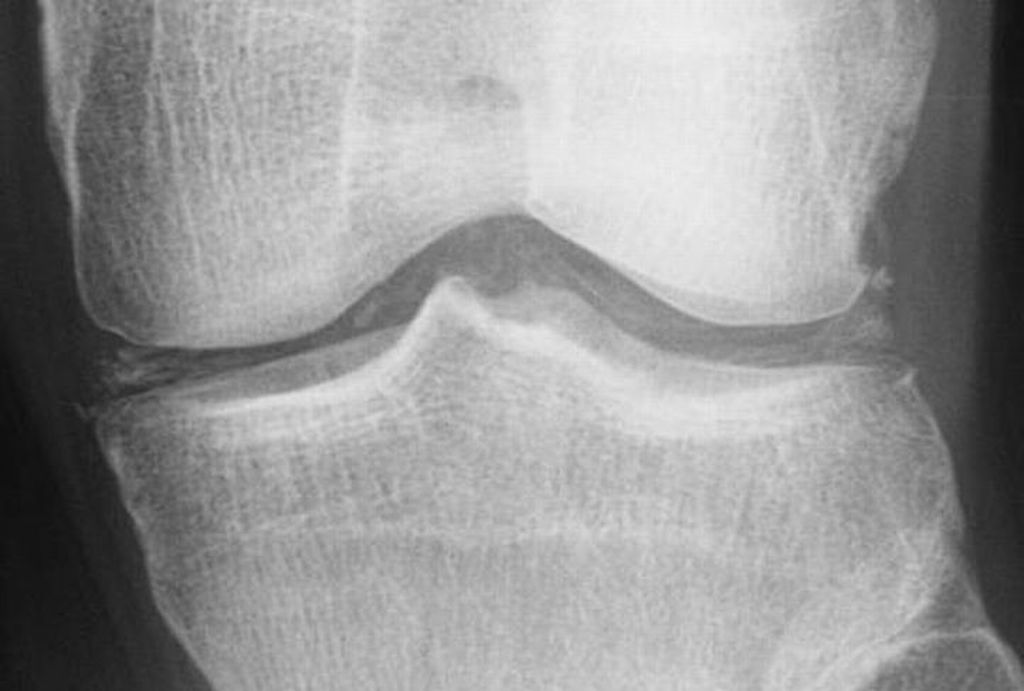
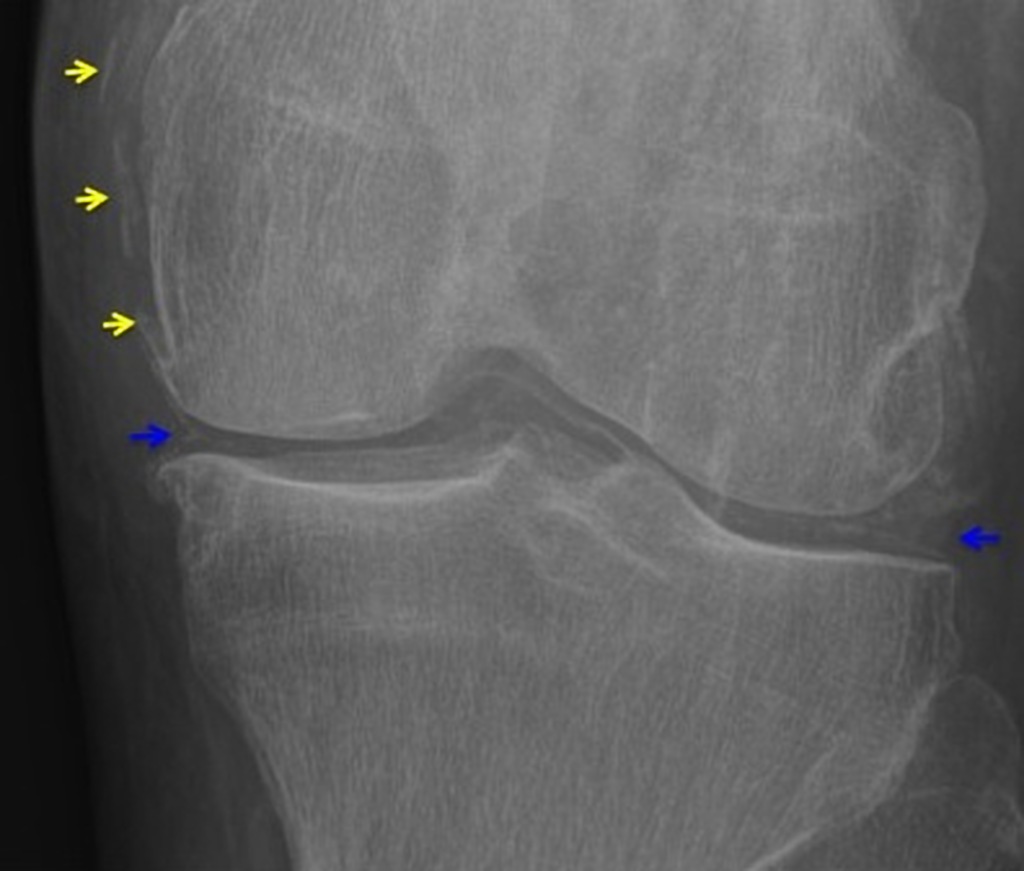
- Cartilage: CPP crystal deposits in hyaline cartilage frequently appear as a radiopaque line paralleling the surface of the underlying bone.
- Joints: Larger joints are frequently involved in CPPD disease. Synovial calcification is often fainter and more diffuse than cartilage calcification. Linear calcifications involving the Achilles tendon or plantar fascia. [32].
Degenerative changes
- CPP crystal deposition is often associated with degenerative changes in joints. It includes subchondral cysts, osteophyte formation, and bone and cartilage fragmentation.
- Radiographic features of osteoarthritis
- Stress fractures or osteonecrosis (avascular necrosis)
Ultrasonographic findings
The following findings on ultrasonography of articular and fibrocartilage may be indicative of the presence of deposits of CPP crystals [39,40]:
●A thin hyperechoic band paralleling the bone cortex and separated from it by a hypoechoic region representing cartilage. The resulting ultrasonographic appearance resembles the double contour sign (DCS) initially described in gout [41], but it often exhibits a thin, stippled appearance rather than the smooth pattern characteristic of gout.
●Small hyperechoic rounded amorphous shaped regions, often with acoustic shadowing, which are most often found in images of fibrocartilage of the wrist (image 2) and menisci of the knee, and in tendons.
●Nodular hyperechoic deposits in bursae and articular recesses.
●Hyperechoic lines of calcification running parallel to tendon fibers.
In contrast to urate crystal deposits in gout, CPP crystals often deposit within the substance of hyaline cartilage, providing a potentially attractive means to distinguish between these crystal deposition arthropathies.
Ultrasonography is a promising modality for clinical use in the diagnosis of CPPD disease and tracking the efficacy of CPPD disease therapies; however, further studies are warranted for: validation of ultrasound criteria unique to CPP crystal deposition, resolution of differences reported with regard to the sensitivity and specificity of the procedure for the diagnosis of CPPD disease [39,42-45], and comparison of imaging findings with corresponding histopathology as the gold standard.
Differential diagnosis
Acute arthritis
Pseudogout acute attacks should be differentiated from other causes of acute monoarthritis such as:
Infection
Fracture
Osteonecrosis
Meniscal tear
Plant thorn synovitis
Chronic inflammatory arthritis
Chronic CPP inflammatory arthritis should be differentiated from other forms of chronic inflammatory arthritis such as:
Rheumatoid arthritis
Spondyloarthritis
Systemic lupus erythematosus
Sarcoidosis
Osteoarthritis
Peripheral spondyloarthritis, including reactive arthritis and arthritis associated with inflammatory bowel disease, as well as psoriatic arthritis, can generally be distinguished from CPPD-related arthritis by the presence of other clinical features typical of these disorders and the absence of CPP crystals from synovial fluid and of radiographic findings of cartilage calcification.
Tumors such as:
Tenosynovial giant cell tumor
Chondrosarcoma
Osteoid osteoma
Treatment
Treatment of CPPD is mostly aimed at preventing further crystal formation and reducing symptoms from crystal deposition. CPPD crystal deposition cannot be reversed. If CPPD results from underlying metabolic abnormalities (hyperparathyroidism, hemochromatosis, hypophosphatasia, or hypomagnesemia)[1], these can be treated directly.
For symptomatic joints, treatment is similar to the treatment of gout. When a single joint is involved, joint aspiration and intra-articular corticosteroid injection is often used, in addition to NSAIDs and/or colchicine. When multiple joints are involved, joint injection is often impractical, or limited only to the most severely involved joint, and oral systemic treatment is chosen instead.
Because pyrophosphate complexes with magnesium prior to its degradation [2], and CPPD can result from pathologically low magnesium levels, magnesium supplementation may be of help, particularly in a few patients with underlying hypomagnesemia. It may be possible to end attacks by ingesting large (maximum RDA) doses of magnesium supplement accompanied by vitamin B6 to help absorption. What that does is assist the body in re-dissolving the calcium in the joint fluid, and with maintenance doses, often in the form of snacking on magnesium-rich foods such as almonds, further attacks can be prevented altogether - without medication.
Anti-inflammatory medication, usually by intraarticular injection, supported by local measures to reduce symptoms:
Joint fluid aspiration and glucocorticoid injection usually provide relief in pain and swelling within 8 to 24 hours.
application of ice or cool packs and immobilization and resting of the joint by restriction of weight bearing.
systemic anti-inflammatory therapy is warranted, employing agents typically used for the treatment of acute gout.
These include: NSAIDs, which may, however, be contraindicated in older adults, the population especially susceptible to acute attacks of pseudogout;
colchicine, which is particularly useful if a low-dose regimen is initiated within hours of attack onset;
glucocorticoids, which are efficacious in patients unable to take NSAIDs or colchicine.
contraindications to NSAIDs:
●Chronic kidney disease with creatinine clearance (CrCl) of less than 60 mL/min per 1.73 m2 (see "Nonselective NSAIDs: Overview of adverse effects", section on 'Renal effects' and "Overview of the management of chronic kidney disease in adults", section on 'Definition and classification' and "Assessment of kidney function")
●Active duodenal or gastric ulcer (see "NSAIDs (including aspirin): Primary prevention of gastroduodenal toxicity" and "NSAIDs (including aspirin): Secondary prevention of gastroduodenal toxicity")
●Cardiovascular disease, particularly heart failure or hypertension that is difficult to control (see "Nonselective NSAIDs: Adverse cardiovascular effects" and "COX-2 selective inhibitors: Adverse cardiovascular effects")
●NSAID allergy
●Ongoing treatment with anticoagulants
Colchicine
In patients in whom treatment for acute CPP crystal arthritis is initiated within about 24 hours of onset and whose clinical and concomitant medication profiles permit its consideration, we suggest oral colchicine in a low-dose regimen as initially recommended for the treatment of acute gout, rather than using oral glucocorticoids [7,9]. For regimens using low-dose colchicine, no more than 1.8 mg of colchicine is taken orally in the first 24 hours of treatment, followed by 0.6 mg colchicine taken twice daily until the attack abates [9]. This strategy is based upon a treatment regimen for acute gout and not on studies of acute CPP crystal arthritis.
Common adverse effects of colchicine may include diarrhea and abdominal cramping, but these are less likely in patients who receive no more than 1.8 mg in total on the first day compared with patients receiving higher doses, such as 0.6 mg every one to two hours until symptom relief or intolerance (as was historically employed) [11]. Gastrointestinal symptoms (diarrhea, abdominal pain, nausea, and vomiting) are the most common adverse effects. A readily reversible peripheral neuropathy, another common toxicity, does not occur frequently during the brief period of colchicine administration for acute pseudogout. More severe colchicine toxicities, which may include combinations of serious, life-threatening or fatal adverse events such as blood cytopenias, rhabdomyolysis or myopathy, peripheral neuropathy, liver failure, or severe cutaneous eruption, have only rarely been reported in patients receiving brief administration of this agent [12].
In patients in whom glucocorticoid therapy is inadequate, it is important to confirm the absence of hepatic disease that might impair the conversion of prednisone to prednisolone, in which case the glucocorticoid therapy can be switched to oral prednisolone (in similar doses to those employed for prednisone). Since pseudogout attacks are self-limited, an alternative option is managing the symptoms using supportive measures until they resolve with analgesics, rest, splinting, and icing. (See 'Initial treatment/one or two joints' above.)
●Need for parenteral (non-intraarticular) therapy – In patients who are unable to take oral agents and who are not appropriate candidates for intraarticular injection, we use parenteral glucocorticoids in doses equivalent to those suggested above for oral glucocorticoids. Although corticotropin (adrenocorticotropic hormone [ACTH]) has been reported to be beneficial for acute gout flare treatment, cost and limited availability restrict the use of parenteral ACTH treatment for gout, and ACTH therapy has been less well-studied for acute pseudogout than for gouty arthritis. (See "Treatment of gout flares", section on 'Parenteral glucocorticoids'.)
We do not administer colchicine intravenously, and we strongly advise against such use because of the risk of serious adverse effects, including death, which are associated with the intravenous administration of this drug. Although intravenous colchicine can reduce the inflammation of pseudogout, the potential dangers of this approach have led to the withdrawal of approval in the United States for the distribution of colchicine for administration by the intravenous route.
Resistant disease
The management of patients with persistent symptoms due to a confirmed acute flare of pseudogout depends upon the prior therapy and upon the patients' comorbidities:
●In some patients being treated with NSAIDs, a more prolonged than usual course of therapy may be required, especially if treatment was not started until the flare was ongoing for several days. Patients with a flare that appears resistant to an adequate course of NSAID therapy may respond to treatment with glucocorticoids. (See 'Unable to use oral NSAIDs and colchicine' above.)
●In patients who are being treated with colchicine but who do not have contraindications to NSAIDs, it may be necessary to switch to NSAID therapy if no improvement is seen within several days, especially if the attack was not treated early. In patients in whom NSAIDs are contraindicated, glucocorticoids may be required. (See 'NSAIDs' above and 'Unable to use oral NSAIDs and colchicine' above.)
●The management of recurrent (or "rebound") attacks following treatment with glucocorticoids may require slower tapering of the glucocorticoid dose with an extension of the course to 10 to 14 or even 21 days if needed. (See 'Unable to use oral NSAIDs and colchicine' above.)
●Interleukin (IL)-1 inhibitors show promise for suppressing crystal-induced inflammation, but sufficient data are not available to support treatment of acute pseudogout with these agents. We use anakinra, an IL-1 receptor antagonist protein, only in gout patients with frequent flares in whom all other available treatments have failed or in whom "rebound flares" occur even when glucocorticoid treatment is appropriately tapered. Although IL-1 antagonist agents are available in some countries for the treatment of other conditions, such as anakinra for rheumatoid arthritis and canakinumab and rilonacept for cryopyrin-associated periodic syndromes, only the first two have shown clear efficacy in treatment of acute gout (see "Treatment of gout flares", section on 'Investigational therapy'). Use of IL-1 inhibitors for either acute gout or pseudogout remains investigational in the United States.
- In patients with three or more attacks of acute CPP crystal arthritis annually, we suggest prophylaxis with colchicine (0.6 mg twice daily) rather than limiting treatment to the period of each acute attack. Several reports indicate that oral colchicine taken chronically may be effective as a prophylactic agent at this dose. In one series of 10 patients with recurrent episodes, colchicine treatment was associated with a marked reduction in the number of episodes at one year compared with the year prior to the initiation of therapy (10 versus 32 episodes) [16].
- Methotrexate (MTX) may provide benefit to patients with an inadequate response to NSAIDs, colchicine, low-dose glucocorticoids, and HCQ as the sole disease-modifying antirheumatic drug (DMARD). The benefits of MTX are uncertain in these patients, but limited observational studies involving a total of 15 patients suggest that low-dose MTX (administered once weekly in doses comparable to those recommended for rheumatoid arthritis) may be useful in instances of refractory CPP crystal-induced inflammation, presenting either as recurrent acute CPP crystal arthritis or as a more chronic inflammatory arthritis resembling rheumatoid arthritis [17-19]. By contrast, a lack of efficacy was seen in a smaller experience with three patients and in a small randomized trial with a mixed population of patients with recurrent acute attacks and chronic persistent polyarthritis [20,21]. MTX may be used in combination with HCQ. The dosing, use, monitoring, and adverse effects of MTX are described in detail separately.
References
- ↑ Wright G.D., Doherty M. Calcium pyrophosphate crystal deposition is not always 'wear and tear' or aging. Ann Rheum Dis.1997; 56: 586-588 PMID 9389218
- ↑ Wright G.D., Doherty M. Calcium pyrophosphate crystal deposition is not always 'wear and tear' or aging. Ann Rheum Dis.1997; 56: 586-588 PMID 9389218