Alectinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Alectinib is a kinase inhibitor that is FDA approved for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. Common adverse reactions include fatigue, constipation, edema and myalgia (≥20%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
ALECENSA is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Dosage
- Dosing and Administration
- The recommended dose of ALECENSA is 600 mg orally twice daily with food. Administer ALECENSA until disease progression or unacceptable toxicity.
- Do not open or dissolve the contents of the capsule.
- If a dose of ALECENSA is missed or vomiting occurs after taking a dose of ALECENSA, take the next dose at the scheduled time.
- Dose Modifications for Adverse Reactions
- The dose reduction schedule for ALECENSA is provided in TABLE 1.
- Table 1. ALECENSA Dose Reduction Schedule
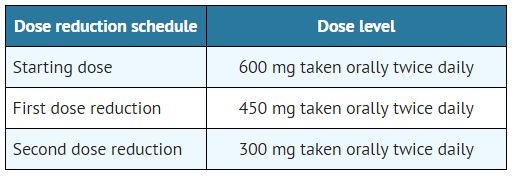
- Discontinue if patients are unable to tolerate the 300 mg twice daily dose.
- Recommendations for dose modifications of ALECENSA in case of adverse reactions are provided in TABLE 2.
- Table 2. ALECENSA Dose Modifications for Adverse Reactions
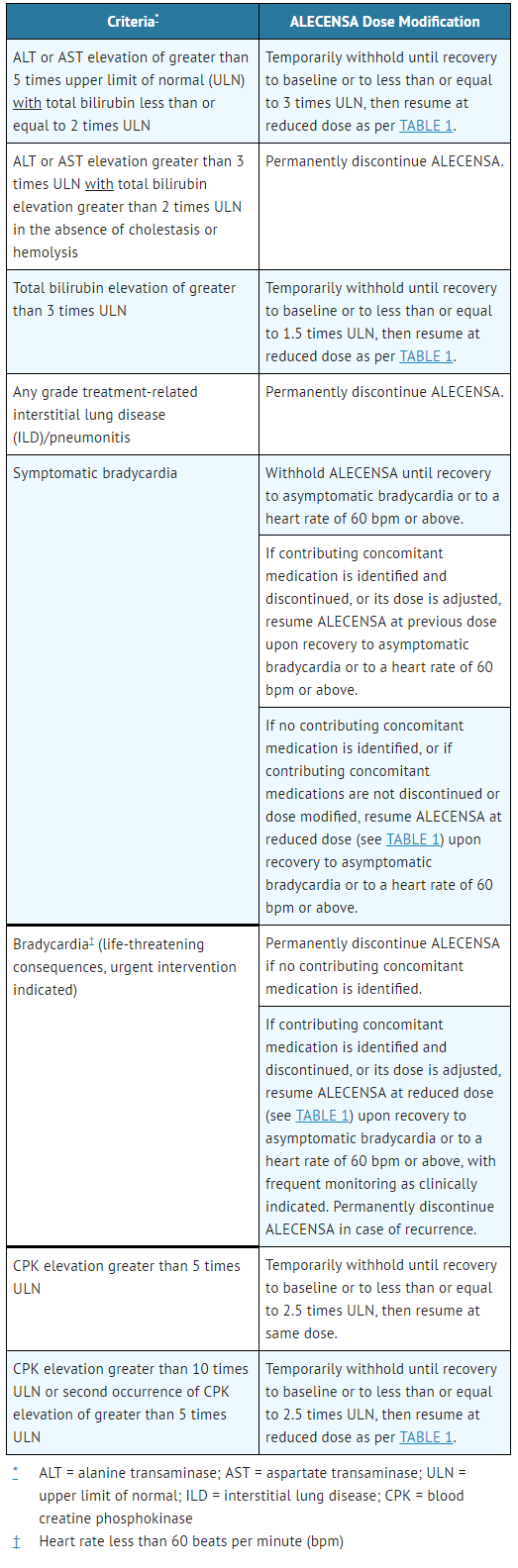
ALECENSA: Alectinib's Brand name
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alectinib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alectinib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of ALECENSA in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alectinib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alectinib in pediatric patients.
Contraindications
None
Warnings
There is limited information regarding Alectinib Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Alectinib Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Alectinib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Alectinib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Alectinib in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Alectinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Alectinib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Alectinib in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Alectinib in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Alectinib in geriatric settings.
Gender
There is no FDA guidance on the use of Alectinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Alectinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Alectinib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Alectinib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Alectinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Alectinib in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Alectinib Administration in the drug label.
Monitoring
There is limited information regarding Alectinib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Alectinib and IV administrations.
Overdosage
There is limited information regarding Alectinib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Alectinib Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Alectinib Mechanism of Action in the drug label.
Structure
There is limited information regarding Alectinib Structure in the drug label.
Pharmacodynamics
There is limited information regarding Alectinib Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Alectinib Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Alectinib Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Alectinib Clinical Studies in the drug label.
How Supplied
There is limited information regarding Alectinib How Supplied in the drug label.
Storage
There is limited information regarding Alectinib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Alectinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Alectinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Alectinib Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Alectinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Alectinib Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Alectinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.