Thrombotic thrombocytopenic purpura
| Thrombotic thrombocytopenic purpura | |
 | |
|---|---|
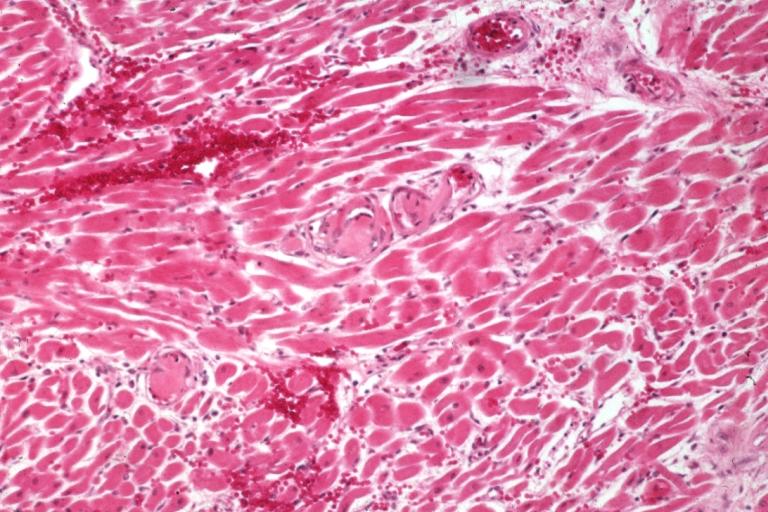
| Thrombotic Thrombocytopenic Purpura: Micro H&E low mag; myocardial cells: an excellent example of this condition. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | M31.1 |
| ICD-9 | 446.6 |
| DiseasesDB | 13052 |
| MedlinePlus | 000552 |
| MeSH | D011697 |
Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Thrombotic thrombocytopenic purpura (TTP or Moschcowitz disease) is a rare disorder of the blood-coagulation system, causing multiple blood clots to form in blood vessels around the body.[1] Most cases of TTP arise from deficiency or inhibition of the enzyme ADAMTS13, which is responsible for cleaving large multimers of von Willebrand factor.[2] This leads to hemolysis and end-organ damage, and may require plasmapheresis therapy.
Signs and symptoms
Classically, the following five symptoms are indicative of this elusive disease. The full 'pentad' is not seen in all cases and clinical suspicion and acumen are the foremost necessity.
- Fluctuating neurological symptoms, such as bizarre behavior, altered mental status, stroke or headaches (65%);
- Kidney failure (46%);
- Fever (33%);
- Thrombocytopenia (low platelet count; most < 50,000), leading to bruising or purpura;
- Microangiopathic hemolytic anemia (anemia, jaundice, elevated LDH and a characteristic blood film showing schistocytes or fragmented erythrocytes).
A patient may notice dark urine from the hemolytic anemia. Because of the many small areas of ischemia produced by clots in the microvasculature, symptoms may be diffuse and fluctuating, including the classical bruising, confusion, or headache, but also nausea and vomiting (from ischemia in the GI tract or from central nervous system involvement), chest pain from cardiac ischemia, seizures, muscle and joint pain, etc.
Causes
ADAMTS13 is a zinc-requiring and calcium-requiring 190,000 Dalton glycosylated protein that is encoded on chromosome 9q34. It is a disintegrin and a metalloprotease with 8 thrombospondin 1-like domains composed of an aminoterminal metalloprotease followed by a disintegrin domain; a thrombospondin 1-like domain; a cysteine-rich domain and an adjacent spacer portion; seven additional thrombospondin 1-like domains and 2 other different types of domains that resemble each other at the carboxyl-terminal end of the molecule. It cleaves a tyrosine 1605-1606 methionine peptide bond of VWF. This protease is #13 in a family of 19 distinct ADAMTS-type metalloprotease enzymes. It is produced predominantly in endothelial cells for slow, constitutive release into the circulation. Endothelial cells can be stimulated to secrete long VWF strings by inflammatory cytokines (TNF, IL8 & IL6, shiga toxins or estrogen). ADAMTS13 is inhibited by EDTA and therefore functional assays of the enzyme are usually performed using plasma anticoagulated with citrate (a weaker divalent cation binder than EDTA).
TTP, as with other microangiopathic hemolytic anemias (MAHAs), is caused by a spontaneous aggregation of platelets and activation of coagulation in the small blood vessels. When stimulated, endothelial cells secrete the ultra-large VWF multimers in long strips that remain anchored to the cell membrane. The long VWF multimeric strings are EXTREMELY "sticky" to the glycoprotein Iba components of platelet GPIb-IX-V surface receptors. The initial adherence of platelets via the GPIb receptors to the long VWF strings and the subsequent coherence of additional platelets to each other (aggregation) via activated GPIIb/IIIa receptors produces potentially occlusive platelet thrombi. Platelets are consumed in the coagulation process, and bind fibrin, the end product of the coagulation pathway. These platelet-fibrin complexes form microthrombi which circulate in the vasculature and cause shearing of red blood cells, resulting in hemolysis.
Roughly, there are two forms of TTP: idiopathic and secondary TTP. A special case is the inherited deficiency of ADAMTS13, known as the Upshaw-Schulman syndrome.
The differential diagnosis of TTP includes hemolytic-uremic syndrome (HUS; which has neurosymptoms, renal failure, hypertension and fever). Note that ADAMTS13 activity is normal in HUS.
Idiopathic TTP
The idiopathic form of TTP was recently linked to the inhibition of the enzyme ADAMTS13 by antibodies, rendering TTP as an autoimmune disease. von Willebrand factor (vWF) is a protein that links platelets, blood clots, and the blood vessel wall in the process of blood coagulation. ADAMTS13 is a proteinase responsible for the breakdown of VWF; very large VWF molecules are prone to coagulation. Without proper cleavage of VWF by ADAMTS13, these unusually large VWF cause coagulation at a higher rate, especially in the part of the circulatory system where VWF is most active due to high shear stress - in the microvascualture, thereby causing thrombi.
In idiopathic TTP, severely decreased (<5% of normal) ADAMTS13 activity can be detected in most (80%) patients, and inhibitors are often found in this subgroup (44-56%). The relationship of reduced ADAMTS13 to the pathogenesis of TTP is known as the Furlan-Tsai hypothesis, after the two independent researchers who published their research in the same issue of the New England Journal of Medicine in 1998.[3][4][5] This theory is seen as insufficient to explain the etiology of TTP, since many patients with a hereditary lack of ADAMTS13 activity do not manifest clinical symptoms of TTP.
Congenital or acquired ADAMTS13 deficiency causes TTP; acute TTP in adults is usually due to an acquired atuoantibody to ADAMTS13. However, as stated before, cases of plasma exchange-responsive acute TTP have been reported in patients who have no evidence of an autoantibody to ADAMTS13 and patients with congenital ADAMTS13 deficiency may not manifest TTP until adulthood. Autoantibodies against ADAMTS13 present in a majority of patients with idiopathic TTP and, additionally, ticlopidine and clopidogrel associated TTP. Severe deficiency of ADAMTS13 activity (<5%) is a specific feature of TTP. Normal levels of ADAMTS13 do NOT rule out the diagnosis of TTP. Normally there is only a slight increase in D-dimers, FDP and thrombin-antithrombin complexes in acute TTP. Secondary DIC may arise due to prolonged tissue ischemia and is an ominous prognostic sign.
Secondary TTP
Secondary TTP is diagnosed when the patient's history mentions one of the known features associated with TTP. It comprises about 40% of all cases of TTP. Predisposing factors are:
- Cancer
- Bone marrow transplantation; (TBI is a risk factor).
- Pregnancy; rare.
- Medication use:
- Platelet aggregation inhibitors (ticlopidine and clopidogrel)
- Immunosuppressants (cyclosporine, mitomycin, tacrolimus/FK506, interferon-α)
- HIV-1 infection
The mechanism of secondary TTP is poorly understood, as ADAMTS13 activity is generally not as depressed as in idiopathic TTP, and inhibitors cannot be detected. The probable etiology may involve, at least in some cases, endothelial damage. A small fraction of patients treated for arterial thrombosis with the platelet P2Y12 adenosine diphosphate receptor inhibiting thienopyridine drugs ticopidine (Ticlid) or clopidogrel (Plavix) develop TTP within a few weeks after the initiation of treatment. Autoantibodies that inhibit plasma ADAMTS13 have been demonstrated in a few patients with Ticlid-associated or Plavix-associated TTP, indicating a possible immune dysregulation induced by these similar thienpyridine compounds. Ticlodipine-associated TTP may respond to drug withdrawal and plasma exchange whereas TTP-like syndromes occuring after transplantation (often in associated with cyclosporine or FK506) are less likely to be responsive to plasma exchange treatment.
Upshaw-Schulman syndrome
A hereditary form of TTP is called the Upshaw-Schulman syndrome; this is generally due to inherited deficiency of ADAMTS13 (frameshift and point mutations). Patients with this inherited ADAMTS13 deficiency have a surprisingly mild phenotype, but develop TTP in clinical situations with increased von Willebrand factor levels, e.g. infection. Reportedly, 5-10% of all TTP cases are due to Upshaw-Schulman syndrome.
Treatment
Since the early 1990s, plasmapheresis has become the treatment of choice for TTP.[6] This is an exchange transfusion involving removal of the patient's blood plasma through apheresis and replacement with donor plasma (fresh frozen plasma or cryosupernatant); the procedure has to be repeated daily to eliminate the inhibitor and ablate the symptoms. Plasma infusion may be necessary and preferred if plasma exchange is not readily available. Exchange tranfusion has a complete response rate of 76%; plasma exchange allows ~80% of acquired ADAMTS13 autoantibody mediated TTP patients to survive an episode(compared to ~80% mortality without it), usually with minimal organ damage. FFP is the replacement fluid of choice in TTP and an exchange of a single plasma volume is the standard initial treatment. All patients should receive folate. Lactate dehydrogenase levels are generally used to monitor disease activity. The serum level increases as erythrocytes are destroyed. Plasmapheresis may need to be continued for 1-8 weeks before patients with idiopathic TTP cease to consume platelets and begin to normalize their hemoglobin. No single laboratory test (platelet count, LDH, ADAMTS13 level, or inhibitory factor) is indicative of recovery; research protocols have used improvement or normalization of LDH as a measure for ending plasmapheresis. Although patients may be critically ill with failure of multiple organ systems during the acute illness, including renal failure, myocardial ischemia, and neurologic symptoms, recovery over several months may be complete in the absence of a frank myocardial infarct, stroke, or CNS hemorrhage. Platelet transfusions are contraindicated unless there is life-threatening hemorrhage.
Many TTP patients need additional immunosuppressive therapy, with glucocorticoid steroids (e.g. prednisolone or prednisone), vincristine, cyclophosphamide, splenectomy or a combination of the above. Rituximab, a monoclonal antibody targeting CD20 on B cells, has been successfully used to treat patients with refractory disease.
Children with Upshaw-Schulman syndrome receive plasma every three weeks prophylactically; this maintains adequate levels of functioning ADAMTS13.
Epidemiology
The incidence of TTP is about 4-6 per million people per year. As with most other autoimmune disorders, idiopathic TTP occurs more often in women and blacks, while the secondary forms do not show this distribution.
Prognosis
The mortality rate is approximately 95% for untreated cases, but the prognosis is reasonably favorable (80-90%) for patients with idiopathic TTP diagnosed and treated early with plasmapheresis.
Approximately one-third of patients experiencing a TTP episode have a relapse within 10 years following their first attack.
Secondary TTP still has a dismal prognosis, with mortality rates despite treatment being reported as 59% to 100%.
History
TTP was initially described in a 16-year old girl by Dr Eli Moschcowitz of New York City in 1924.[7] Moschcowitz ascribed the disease (incorrectly) to a toxic cause. Moschcowitz noted that his 16 year-old patient had anemia; petechiae; microscopic hematuria; and at autopsy, disseminated microvascular thrombi. Since that time, the pathophysiology, etiology, and medical management of TTP has expanded.
See also
References
- ↑ "MedlinePlus: Thrombotic thrombocytopenic purpura", MedlinePlus Medical Encyclopedia, 2007, webpage: NLM-552.
- ↑ Moake JL (2004). "von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura". Semin. Hematol. 41 (1): 4–14. PMID 14727254.
- ↑ Moake JL (1998). "Moschcowitz, multimers, and metalloprotease". N. Engl. J. Med. 339 (22): 1629–31. PMID 9828253.
- ↑ Furlan M, Robles R, Galbusera M; et al. (1998). "von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome". N. Engl. J. Med. 339 (22): 1578–84. PMID 9828245.
- ↑ Tsai HM, Lian EC (1998). "Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura". N. Engl. J. Med. 339 (22): 1585–94. PMID 9828246.
- ↑ Zheng XL, Kaufman RM, Goodnough LT, Sadler JE (2004). "Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura". Blood. 103 (11): 4043–9. doi:10.1182/blood-2003-11-4035. PMID 14982878.
- ↑ Moschcowitz E (1924). "An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: an undescribed disease". Proc NY Pathol Soc. 24: 21–4. Reprinted in Mt Sinai J Med 2003;70(5):322-5, PMID 14631522.
Template:Hematology Template:SIB
bn:তঞ্চনসংক্রান্ত অণুচক্রিকাস্বল্পতাজনিত পারপ্যুরা de:Thrombotisch-thrombozytopenische Purpura it:Porpora trombotica trombocitopenica nl:Thrombotische thrombocytopenische purpura