Measles laboratory findings: Difference between revisions
| Line 9: | Line 9: | ||
Laboratory confirmation is essential for all sporadic measles cases and all outbreaks. Detection of measles-specific [[IgM]] antibody and measles RNA by real-time polymerase chain reaction (RT-PCR) are the most common methods for confirming measles infection. Healthcare providers should obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients suspected to have measles at first contact with them. Urine samples may also contain virus, and when feasible to do so, collecting both respiratory and urine samples can increase the likelihood of detecting measles virus. <ref name="CDC"> {{ cite web| title= CDC Measles Laboratory Findings | url=http://www.cdc.gov/measles/lab-tools/index.html}} </ref> | Laboratory confirmation is essential for all sporadic measles cases and all outbreaks. Detection of measles-specific [[IgM]] antibody and measles RNA by real-time polymerase chain reaction (RT-PCR) are the most common methods for confirming measles infection. Healthcare providers should obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients suspected to have measles at first contact with them. Urine samples may also contain virus, and when feasible to do so, collecting both respiratory and urine samples can increase the likelihood of detecting measles virus. <ref name="CDC"> {{ cite web| title= CDC Measles Laboratory Findings | url=http://www.cdc.gov/measles/lab-tools/index.html}} </ref> | ||
===Serology=== | |||
Detection of specific [[IgM]] antibodies in a [[serum]] sample collected within the first few days of rash onset can provide presumptive evidence of a current or recent [[measles virus]] infection. However, because no assay is 100% specific, [[serologic]] testing of non-measles cases using any assay will occasionally produce [[false positive]] [[IgM ]]results. | |||
In countries such as the United States where endemic circulation of measles has been eliminated, most suspected cases are not measles, and [[rash]] and [[fever]] illnesses are more likely due to a number of other rash–causing illnesses such as [[parvovirus B19]], [[enterviruses]], or human [[herpesvirus–6]]([[roseola]]). In addition, testing for measles is frequently requested for people with ear infections or sore throats who were given antibiotics which resulted in a rash. The presence of [[rheumatoid factor]] can also result in a [[false positive]] [[IgM]]. However, ongoing measles activity in many other countries will result in sporadic cases of measles in the United States. | |||
In cases of [[measles]] infection following secondary [[vaccine]] failure [[IgM]] antibody may not be present. In these cases serological confirmation may be made by showing [[IgG]] antibody rises by [[Enzyme immunoassay]] or [[complement fixation]]. [[IgM]] antibodies are tested in a blood sample taken at least 4-5 days after appearance of rash and persist for 30-60 days thereafter. False positives are sometimes seen. More than 4 fold rise in [[IgG]] antibodies between actue and convalescent phase sera, is also diagnostic. | |||
===Specimens for Detection of Measles RNA by RT–PCR or Virus Isolation=== | |||
Detection of measles [[RNA]] in a clinical sample can provide laboratory confirmation of infection. Real–time [[RT–PCR]] (rRT–PCR) and conventional, endpoint [[RT–PCR]] to detect measles [[RNA]] are performed at CDC. The rRT–PCR is more sensitive than endpoint [[RT–PCR]] assay for detection of measles [[RNA]] in clinical sample, while the endpoint assay is routinely used to amplify the region of the measles genome required to determine the genotype. | |||
Throat (oropharyngeal), nasal or nasopharyngeal swabs are the preferred samples for virus isolation or detection of measles [[RNA]] by [[RT–PCR]]. Synthetic swabs are recommended. Urine samples may also contain [[virus]] and when feasible to do so, collection of both samples can increase the likelihood of detecting the [[virus]]. Collect samples as soon after [[rash]] as possible. The sample should be collected at the first contact with a suspected case of measles when the serum sample for diagnosis is drawn. | |||
Detection of measles [[RNA]] and measles virus isolation are most successful when samples are collected on the first day of rash through the 3 days following onset of rash. However, virus may still be recovered by cell culture through day 10 following rash onset. Detection of measles [[RNA]] by [[RT–PCR]] may be successful as late as 10–14 days post rash onset. | |||
===Histological findings=== | |||
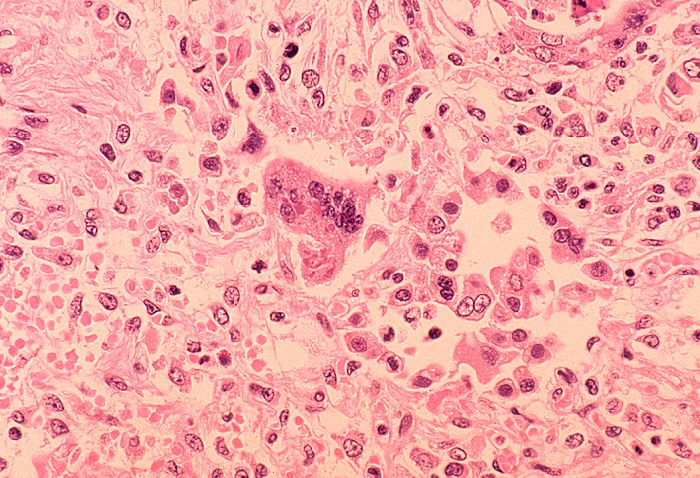
Some patients will develop [[pneumonia]] as a sequela to the measles. Histologically, a unique cell can be found in the paracortical region of hyperplastic [[lymph nodes]] in patients affected with this condition. This cell, known as the Warthin-Finkeldey cell, is a multinucleated giant with [[eosinophilic]] cytoplasmic and nuclear inclusions. Shown below is an image depicting histological section of [[lung]] specimen in a patient with measles associated [[pneumonia]]: | Some patients will develop [[pneumonia]] as a sequela to the measles. Histologically, a unique cell can be found in the paracortical region of hyperplastic [[lymph nodes]] in patients affected with this condition. This cell, known as the Warthin-Finkeldey cell, is a multinucleated giant with [[eosinophilic]] cytoplasmic and nuclear inclusions. Shown below is an image depicting histological section of [[lung]] specimen in a patient with measles associated [[pneumonia]]: | ||
Revision as of 16:02, 24 June 2014
|
Measles Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Measles laboratory findings On the Web |
|
American Roentgen Ray Society Images of Measles laboratory findings |
|
Risk calculators and risk factors for Measles laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Measles is a disease best diagnosed clinically, however laboratory tests have a limited role to play especially in those conditions where the diagnosis is uncertain and certain cases of atypical pneumonia. Detecting a positive rise in measles IgM antibodies is one of the methods used for laboratory diagnosis of measles.
Laboratory Findings
Laboratory confirmation is essential for all sporadic measles cases and all outbreaks. Detection of measles-specific IgM antibody and measles RNA by real-time polymerase chain reaction (RT-PCR) are the most common methods for confirming measles infection. Healthcare providers should obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients suspected to have measles at first contact with them. Urine samples may also contain virus, and when feasible to do so, collecting both respiratory and urine samples can increase the likelihood of detecting measles virus. [1]
Serology
Detection of specific IgM antibodies in a serum sample collected within the first few days of rash onset can provide presumptive evidence of a current or recent measles virus infection. However, because no assay is 100% specific, serologic testing of non-measles cases using any assay will occasionally produce false positive IgM results.
In countries such as the United States where endemic circulation of measles has been eliminated, most suspected cases are not measles, and rash and fever illnesses are more likely due to a number of other rash–causing illnesses such as parvovirus B19, enterviruses, or human herpesvirus–6(roseola). In addition, testing for measles is frequently requested for people with ear infections or sore throats who were given antibiotics which resulted in a rash. The presence of rheumatoid factor can also result in a false positive IgM. However, ongoing measles activity in many other countries will result in sporadic cases of measles in the United States.
In cases of measles infection following secondary vaccine failure IgM antibody may not be present. In these cases serological confirmation may be made by showing IgG antibody rises by Enzyme immunoassay or complement fixation. IgM antibodies are tested in a blood sample taken at least 4-5 days after appearance of rash and persist for 30-60 days thereafter. False positives are sometimes seen. More than 4 fold rise in IgG antibodies between actue and convalescent phase sera, is also diagnostic.
Specimens for Detection of Measles RNA by RT–PCR or Virus Isolation
Detection of measles RNA in a clinical sample can provide laboratory confirmation of infection. Real–time RT–PCR (rRT–PCR) and conventional, endpoint RT–PCR to detect measles RNA are performed at CDC. The rRT–PCR is more sensitive than endpoint RT–PCR assay for detection of measles RNA in clinical sample, while the endpoint assay is routinely used to amplify the region of the measles genome required to determine the genotype.
Throat (oropharyngeal), nasal or nasopharyngeal swabs are the preferred samples for virus isolation or detection of measles RNA by RT–PCR. Synthetic swabs are recommended. Urine samples may also contain virus and when feasible to do so, collection of both samples can increase the likelihood of detecting the virus. Collect samples as soon after rash as possible. The sample should be collected at the first contact with a suspected case of measles when the serum sample for diagnosis is drawn.
Detection of measles RNA and measles virus isolation are most successful when samples are collected on the first day of rash through the 3 days following onset of rash. However, virus may still be recovered by cell culture through day 10 following rash onset. Detection of measles RNA by RT–PCR may be successful as late as 10–14 days post rash onset.
Histological findings
Some patients will develop pneumonia as a sequela to the measles. Histologically, a unique cell can be found in the paracortical region of hyperplastic lymph nodes in patients affected with this condition. This cell, known as the Warthin-Finkeldey cell, is a multinucleated giant with eosinophilic cytoplasmic and nuclear inclusions. Shown below is an image depicting histological section of lung specimen in a patient with measles associated pneumonia: