COVID-19-associated diabetes mellitus: Difference between revisions
TayyabaAli (talk | contribs) No edit summary |
TayyabaAli (talk | contribs) No edit summary |
||
| Line 7: | Line 7: | ||
==Overview== | ==Overview== | ||
[[ACER2|ACE2 receptors]] in the [[Pancreas|endocrine pancreas]] serve the entrance for [[SARS-CoV-2|Severe acute respiratory syndrome coronavirus 2]] (SARS-CoV 2), which causes [[COVID-19|Corononavirus Disease 2019]] (COVID-19). Researchers in China observed [[COVID-19-associated diabetes mellitus|new-onset diabetes]] among [[SARS-CoV]] patients. Therefore in agreement with this, the [[SARS-CoV-2]] might enter [[pancreatic islets]] through binding to [[ACER2|ACE2]], and cause acute β-cell injury, leading to intense [[hyperglycemia]] and transient Type 2 [[Diabetes mellitus|Diabetes Mellitus]]. [[SARS-CoV-2|SARS-CoV 2]] can cause [[hyperglycemia]] by direct injuring of [[pancreatic beta cells]] and by downregulating [[ACER2|ACE2 receptors]] leading to unopposed [[Angiotensin|angiotensin II]], which may hinder [[insulin]] secretion | [[ACER2|ACE2 receptors]] in the [[Pancreas|endocrine pancreas]] serve the entrance for [[SARS-CoV-2|Severe acute respiratory syndrome coronavirus 2]] (SARS-CoV 2), which causes [[COVID-19|Corononavirus Disease 2019]] (COVID-19). Researchers in China observed [[COVID-19-associated diabetes mellitus|new-onset diabetes]] among [[SARS-CoV]] patients. Therefore in agreement with this, the [[SARS-CoV-2]] might enter [[pancreatic islets]] through binding to [[ACER2|ACE2]], and cause acute β-cell injury, leading to intense [[hyperglycemia]] and transient Type 2 [[Diabetes mellitus|Diabetes Mellitus]]. [[SARS-CoV-2|SARS-CoV 2]] can cause [[hyperglycemia]] by direct injuring of [[pancreatic beta cells]] and by downregulating [[ACER2|ACE2 receptors]] leading to unopposed [[Angiotensin|angiotensin II]], which may hinder [[insulin]] secretion | ||
To browse the complete page of COVID-19, [[COVID-19|Click here]]. | To browse the complete page of COVID-19, [[COVID-19|Click here]]. | ||
Revision as of 11:11, 16 July 2020
|
COVID-19 Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
COVID-19-associated diabetes mellitus On the Web |
|
American Roentgen Ray Society Images of COVID-19-associated diabetes mellitus |
|
Risk calculators and risk factors for COVID-19-associated diabetes mellitus |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tayyaba Ali, M.D.[2]
Synonyms and keywords: New-onset Diabetes in COVID-19 Islet cell injury by SARS-CoV 2
Overview
ACE2 receptors in the endocrine pancreas serve the entrance for Severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2), which causes Corononavirus Disease 2019 (COVID-19). Researchers in China observed new-onset diabetes among SARS-CoV patients. Therefore in agreement with this, the SARS-CoV-2 might enter pancreatic islets through binding to ACE2, and cause acute β-cell injury, leading to intense hyperglycemia and transient Type 2 Diabetes Mellitus. SARS-CoV 2 can cause hyperglycemia by direct injuring of pancreatic beta cells and by downregulating ACE2 receptors leading to unopposed angiotensin II, which may hinder insulin secretion
To browse the complete page of COVID-19, Click here.
Historical Perspective
- Diabetes mellitus is defined by insulin deficiency due to either diminished insulin release or end-organ insulin resistance.[1]
- Diabetes is an umbrella term for conditions such as type I (T1DM) and type II (T2DM) diabetes mellitus, gestational diabetes, and maturity-onset diabetes of the young (MODY).[1]
- Type 1 Diabetes Mellitus is caused by pancreatic β-cell failure or auto-immune destruction of the pancreatic β-cells. It generally presents in children and young adults.[1]
- Type 2 Diabetes Mellitus (T2DM) is defined by insulin resistance and presents in adults. Family history, hypertension, obesity, and dyslipidemia play a significant role in causing T2DM.[1]
- Around 422 million individuals worldwide have diabetes, the dominant part living in low-and middle-income nations, and 1.6 million deaths are straightforwardly credited to diabetes every year. In the course of recent decades, a consistent rise has been observed in both the incidence and the prevalence.[2]
- In China, in the year 2008, a study was done in which they compared 39 SARS-CoV patients with no previous history of diabetes, who never used steroids, with 39 matched healthy siblings.The results of the study revealed that 20 out of 39 SARS-CoV patients developed new-onset diabetes during the hospital stay. After 3 years of recovery from the SARS-CoV infection, only 5% of patients remained diabetic whereas blood sugar levels normalized in the rest of the patients with the infection recovery.[3]
- ACE2 is the primary receptor For SARS-CoV spike protein. SARS-CoV causes infection by binding to ACE2 receptors on the target cells.[4][5] The study suggested, SARS-CoV may damage islets and cause acute insulin dependent diabetes mellitus.[3]
To browse the historical perspectives of COVID-19, Click here.
Classification
- There is no established system for the classification of COVID-19-associated Diabetes.
- Future research is needed to provide a better understanding of the type of Diabetes, SARS-CoV-2 can cause. Whether SARS-CoV causes T1DM or T2DM or a new type of Diabetes.
To browse the classification of COVID-19, Click here.
Pathophysiology
- Angiotensin-converting enzyme 2 (ACE2) receptors expressed in the tissues that are highly involved in body metabolism. These tissues comprise of pancreatic beta cells, adipose tissue, small intestine, and the kidneys. ACE2 receptors in the endocrine pancreas serve the entrance for Severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2), which causes Corononavirus Disease 2019 (COVID-19). [6]
- Expression of ACE2 receptors and effector protease TMPRSS2 in pancreas are associated with SARS-CoV 2 infection.[7]
- The pancreas consists of nine different cell types such as acinar cells, ductal cells, beta cells, alpha cells, mesenchymal cells, and endothelial cells. These pancreatic cells express both ACE2 and TMPRSS2. The expression of ACE2 in pancreatic alpha and beta cells is further proved by immunohistochemistry. Both beta cells that secrete insulin and alpha cells that secrete glucagon, stained positive for SARS-CoV 2 Spike protein and thus, it is postulated that SARS-CoV-2 can infect pancreatic islet cells.[7]
- A recent experiement was conducted to study SARS-CoV-2 tropism that is the cellular response to an external stimulus in human cells and organoids. Researchers infect human pluripotent stem cells (hPSC)-derived pancreatic endocrine cells with SARS-CoV-2.[8]
- Researchers found when SARS-CoV-2 infect pancreatic cells, it downregulates the pathways including calcium signaling pathways, glucagon signaling pathways of alpha cells, and metabolic pathways that assist in insulin secretion from pancreatic beta cells.[8]
- Researchers further stained SARS-CoV-2 infected hPSC-derived pancreatic endocrine cells with a cell apoptotic marker (CASP3). As a result of this staining, they found a large number of CASP3 cells in infected hPSC-derived pancreatic cells. This indicates that change in metabolic pathways of the pancreas is mainly due to cell apoptosis, trigger by SARS-CoV-2. This experiment suggest that when SARS-CoV-2 binds to ACE2 in pancreas, this will upregulate the genes responsible for apoptosis and downregulate the genes responsible for the cell survival. [8]
- ACE2 is the target receptor for both SARS-CoV and SARS-CoV-2. Researchers in China observed new-onset diabetes among SARS-CoV patients. Therefore in agreement with this, the SARS-CoV-2 might enter pancreatic islets through binding to ACE2, and cause acute β-cell injury, leading to intense hyperglycemia and transient Type 2 Diabetes Mellitus.[3]
- ACE2 serves as the negative regulator of the Renin-Angiotensin System (RAS) mainly by converting Ang (angiotensin) I and Ang II into Ang 1-9 and Ang 1-7, respectively.[9][10] When SARS-CoV and SARS-CoV-2 bind to ACE2 receptors, this will lead to the subsequent downregulation of surface ACE2 expression.[5][11][12] SARS-CoV-2 differs from SARS-CoV by 380 amino acid substitutions and thus has a stronger binding affinity than SARS-CoV, which explains the global impact of SARS-CoV-2 than the previous SARS-CoV outbreak.[13][14]
- ACE2 is the negative regulator of the Renin-Angiotensin system (RAS) and has protective benefits against many diseases and complications. SARS-CoV-2 binds to ACE2 receptors, this blocks all the protective benefits of the ACE2 pathway and shifts the cascade back to ACE/Ang II/AT1R-pathway, increasing Ang II, decreasing ACE2 and Ang-( 1-7)[15][16] as shown in the figure.
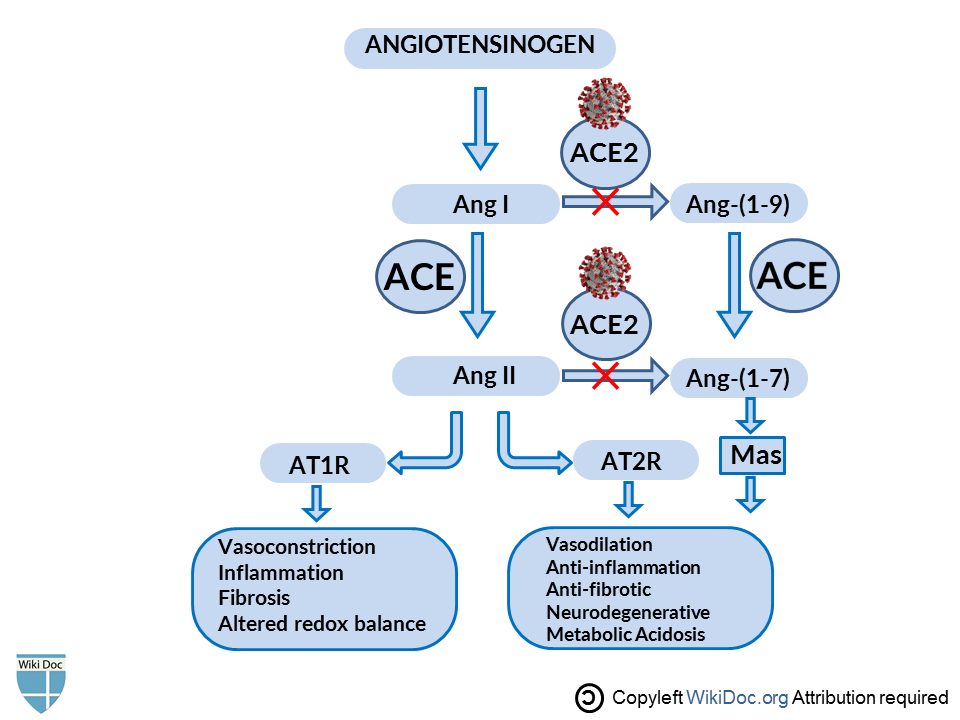
- Inhibition of RAS(Angiotensin→Ang1→Ang2→AT1R) protects pancreatic β-cells from oxidative stress-related tissue damage, therefore improves insulin synthesis and secretion.[17]Hyperactivity of RAS works in contrast. In adipose tissue, Ras decreases insulin sensitivity, decreases glucose uptake. In pancreatic tissue, it decreases insulin secretion, increases islet oxidative stress and fibrosis, decrease perfusion.[18]
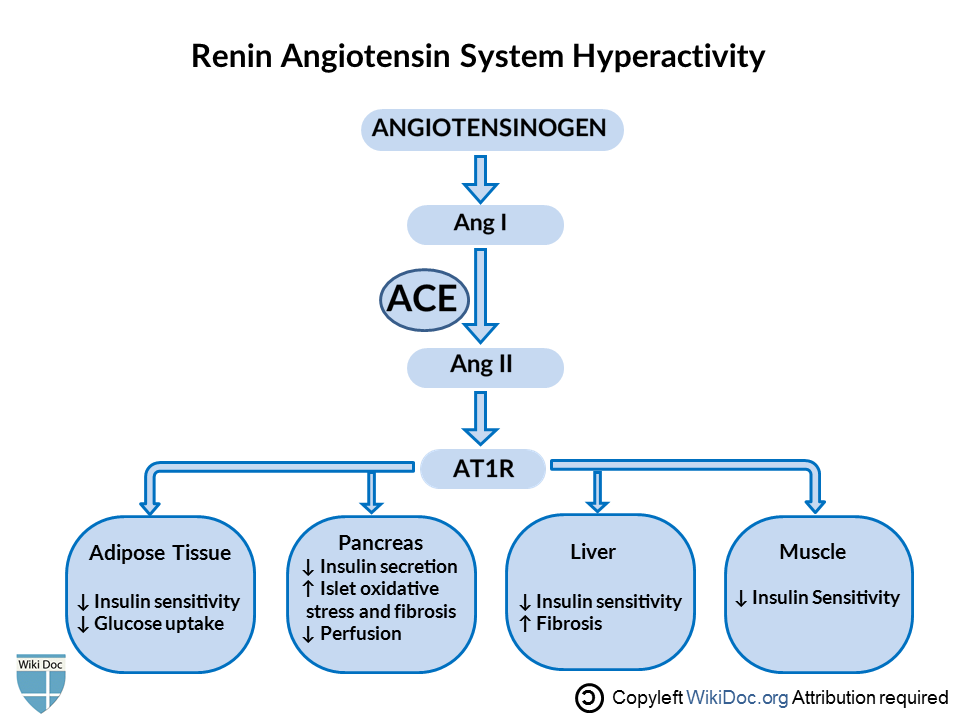
- In an experimental trial, pressor doses of Ang-II were given to healthy human subjects. As a result, researchers observed suppression of basal, pulsatile, and glucose-stimulated insulin release.[19]This loss of insulin release is supposed to be the contributing factor in the development of T2DM.[20]
- SARS-CoV 2 can cause hyperglycemia by direct injuring of pancreatic beta cells[3] and by downregulating ACE2 receptors leading to unopposed angiotensin II, which may hinder insulin secretion.[21]
To browse the pathophysiology of COVID-19, Click here.
Causes
1- Direst damage of pancreatic beta-cells by SARS-CoV 2 [3]
2- Downregulation of ACE2 by SARS-CoV 2 shift the cascade to the ACE/AngII/AT1R pathway which further leads to decrease insulin release and islet cell oxidative damage.[21]
Epidemiology and Demographics
- There is not enough data available on incidence and prevalence of COVID-19-associated Diabetes Mellitus.
- To browse the epidemiology and Demographics of Diabetes Mellitus. Click here.
Risk Factors
There are no established risk factors for COVID-19-associated Diabetes.
To browse the risk factors for different types of Diabetes Mellitus, Click here.
Screening
There is insufficient evidence to recommend routine screening for COVID-19-associated Diabetes.
To browse the screening performed for Diabetes Mellitus, Click here.
Natural History, Complications, and Prognosis
To browse the history and complications of Diabetes Mellitus, Click here.
Diagnosis
History and Symptoms
According to a recent case report of Diabetic ketoacidoses precipitated by COVID-19 in a patient with newly diagnosed diabetes mellitus. He was a previously healthy man presented with 1-week history of:
- Fever (38.5 °C)
- Vomiting,
- Polydipsia (intense thirst)
- Polyuria (production of abnormally large volumes of dilute urine)[22]
Physical Examination
- Mildly tachycardic
- Kusmmaul's breathing was not observed.[22]
Laboratory Findings
- Hyperglycemia
- High anion gap metabolic acidosis
- Ketonemia [22]
- COVID-19 infection can cause ketosis and ketoacidosis.[23]When the body doesn’t make enough insulin to break down sugar, it uses ketones as an alternative source of fuel.[24]
To browse the diagnosis of Diabetes mellitus, Click here.
Treatment
To browse the treatment of Diabetes Mellitus, Click here.
References
- ↑ 1.0 1.1 1.2 1.3 King, H.; Aubert, R. E.; Herman, W. H. (1998). "Global Burden of Diabetes, 1995-2025: Prevalence, numerical estimates, and projections". Diabetes Care. 21 (9): 1414–1431. doi:10.2337/diacare.21.9.1414. ISSN 0149-5992.
- ↑ "Diabetes".
- ↑ 3.0 3.1 3.2 3.3 3.4 Yang, Jin-Kui; Lin, Shan-Shan; Ji, Xiu-Juan; Guo, Li-Min (2009). "Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes". Acta Diabetologica. 47 (3): 193–199. doi:10.1007/s00592-009-0109-4. ISSN 0940-5429.
- ↑ Turner, Anthony J; Hiscox, Julian A; Hooper, Nigel M (2004). "ACE2: from vasopeptidase to SARS virus receptor". Trends in Pharmacological Sciences. 25 (6): 291–294. doi:10.1016/j.tips.2004.04.001. ISSN 0165-6147.
- ↑ 5.0 5.1 Li, Wenhui; Moore, Michael J.; Vasilieva, Natalya; Sui, Jianhua; Wong, Swee Kee; Berne, Michael A.; Somasundaran, Mohan; Sullivan, John L.; Luzuriaga, Katherine; Greenough, Thomas C.; Choe, Hyeryun; Farzan, Michael (2003). "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus". Nature. 426 (6965): 450–454. doi:10.1038/nature02145. ISSN 0028-0836.
- ↑ Bornstein, Stefan R.; Dalan, Rinkoo; Hopkins, David; Mingrone, Geltrude; Boehm, Bernhard O. (2020). "Endocrine and metabolic link to coronavirus infection". Nature Reviews Endocrinology. 16 (6): 297–298. doi:10.1038/s41574-020-0353-9. ISSN 1759-5029.
- ↑ 7.0 7.1 Hoffmann, Markus; Kleine-Weber, Hannah; Schroeder, Simon; Krüger, Nadine; Herrler, Tanja; Erichsen, Sandra; Schiergens, Tobias S.; Herrler, Georg; Wu, Nai-Huei; Nitsche, Andreas; Müller, Marcel A.; Drosten, Christian; Pöhlmann, Stefan (2020). "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor". Cell. 181 (2): 271–280.e8. doi:10.1016/j.cell.2020.02.052. ISSN 0092-8674.
- ↑ 8.0 8.1 8.2 Yang, Liuliu; Han, Yuling; Nilsson-Payant, Benjamin E.; Gupta, Vikas; Wang, Pengfei; Duan, Xiaohua; Tang, Xuming; Zhu, Jiajun; Zhao, Zeping; Jaffré, Fabrice; Zhang, Tuo; Kim, Tae Wan; Harschnitz, Oliver; Redmond, David; Houghton, Sean; Liu, Chengyang; Naji, Ali; Ciceri, Gabriele; Guttikonda, Sudha; Bram, Yaron; Nguyen, Duc-Huy T.; Cioffi, Michele; Chandar, Vasuretha; Hoagland, Daisy A.; Huang, Yaoxing; Xiang, Jenny; Wang, Hui; Lyden, David; Borczuk, Alain; Chen, Huanhuan Joyce; Studer, Lorenz; Pan, Fong Cheng; Ho, David D.; tenOever, Benjamin R.; Evans, Todd; Schwartz, Robert E.; Chen, Shuibing (2020). "A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids". Cell Stem Cell. doi:10.1016/j.stem.2020.06.015. ISSN 1934-5909.
- ↑ Patel, Vaibhav B.; Zhong, Jiu-Chang; Grant, Maria B.; Oudit, Gavin Y. (2016). "Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure". Circulation Research. 118 (8): 1313–1326. doi:10.1161/CIRCRESAHA.116.307708. ISSN 0009-7330.
- ↑ Wang, Kaiming; Gheblawi, Mahmoud; Oudit, Gavin Y. (2020). "Angiotensin Converting Enzyme 2: A Double-Edged Sword". Circulation. doi:10.1161/CIRCULATIONAHA.120.047049. ISSN 0009-7322.
- ↑ Walls, Alexandra C.; Park, Young-Jun; Tortorici, M. Alejandra; Wall, Abigail; McGuire, Andrew T.; Veesler, David (2020). "Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein". Cell. 181 (2): 281–292.e6. doi:10.1016/j.cell.2020.02.058. ISSN 0092-8674.
- ↑ Zhou, Peng; Yang, Xing-Lou; Wang, Xian-Guang; Hu, Ben; Zhang, Lei; Zhang, Wei; Si, Hao-Rui; Zhu, Yan; Li, Bei; Huang, Chao-Lin; Chen, Hui-Dong; Chen, Jing; Luo, Yun; Guo, Hua; Jiang, Ren-Di; Liu, Mei-Qin; Chen, Ying; Shen, Xu-Rui; Wang, Xi; Zheng, Xiao-Shuang; Zhao, Kai; Chen, Quan-Jiao; Deng, Fei; Liu, Lin-Lin; Yan, Bing; Zhan, Fa-Xian; Wang, Yan-Yi; Xiao, Geng-Fu; Shi, Zheng-Li (2020). "A pneumonia outbreak associated with a new coronavirus of probable bat origin". Nature. 579 (7798): 270–273. doi:10.1038/s41586-020-2012-7. ISSN 0028-0836.
- ↑ Yan, Renhong; Zhang, Yuanyuan; Li, Yaning; Xia, Lu; Guo, Yingying; Zhou, Qiang (2020). "Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2". Science. 367 (6485): 1444–1448. doi:10.1126/science.abb2762. ISSN 0036-8075.
- ↑ Shang, Jian; Ye, Gang; Shi, Ke; Wan, Yushun; Luo, Chuming; Aihara, Hideki; Geng, Qibin; Auerbach, Ashley; Li, Fang (2020). "Structural basis of receptor recognition by SARS-CoV-2". Nature. 581 (7807): 221–224. doi:10.1038/s41586-020-2179-y. ISSN 0028-0836.
- ↑ Gheblawi, Mahmoud; Wang, Kaiming; Viveiros, Anissa; Nguyen, Quynh; Zhong, Jiu-Chang; Turner, Anthony J.; Raizada, Mohan K.; Grant, Maria B.; Oudit, Gavin Y. (2020). "Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System". Circulation Research. 126 (10): 1456–1474. doi:10.1161/CIRCRESAHA.120.317015. ISSN 0009-7330.
- ↑ D’Ardes, Damiano; Boccatonda, Andrea; Rossi, Ilaria; Guagnano, Maria Teresa; Santilli, Francesca; Cipollone, Francesco; Bucci, Marco (2020). "COVID-19 and RAS: Unravelling an Unclear Relationship". International Journal of Molecular Sciences. 21 (8): 3003. doi:10.3390/ijms21083003. ISSN 1422-0067.
- ↑ Grankvist, K; Marklund, S L; Täljedal, I B (1981). "CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse". Biochemical Journal. 199 (2): 393–398. doi:10.1042/bj1990393. ISSN 0264-6021.
- ↑ Bindom, Sharell M.; Lazartigues, Eric (2009). "The sweeter side of ACE2: Physiological evidence for a role in diabetes". Molecular and Cellular Endocrinology. 302 (2): 193–202. doi:10.1016/j.mce.2008.09.020. ISSN 0303-7207.
- ↑ Fliser, Danilo; Schaefer, Franz; Schmid, Daniela; Veldhuis, Johannes D.; Ritz, Eberhard (1997). "Angiotensin II Affects Basal, Pulsatile, and Glucose-Stimulated Insulin Secretion in Humans". Hypertension. 30 (5): 1156–1161. doi:10.1161/01.HYP.30.5.1156. ISSN 0194-911X.
- ↑ Gerich, J. E. (2002). "Is Reduced First-Phase Insulin Release the Earliest Detectable Abnormality in Individuals Destined to Develop Type 2 Diabetes?". Diabetes. 51 (Supplement 1): S117–S121. doi:10.2337/diabetes.51.2007.S117. ISSN 0012-1797.
- ↑ 21.0 21.1 Carlsson, P.-O.; Berne, C.; Jansson, L. (1998). "Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats". Diabetologia. 41 (2): 127–133. doi:10.1007/s001250050880. ISSN 0012-186X.
- ↑ 22.0 22.1 22.2 Chee, Ying Jie; Ng, Shereen Jia Huey; Yeoh, Ester (2020). "Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus". Diabetes Research and Clinical Practice. 164: 108166. doi:10.1016/j.diabres.2020.108166. ISSN 0168-8227.
- ↑ Li, Juyi; Wang, Xiufang; Chen, Jian; Zuo, Xiuran; Zhang, Hongmei; Deng, Aiping (2020). "COVID
‐19 infection may cause ketosis and ketoacidosis". Diabetes, Obesity and Metabolism. doi:10.1111/dom.14057. ISSN 1462-8902. line feed character in
|title=at position 6 (help) - ↑ "Mounting clues suggest the coronavirus might trigger diabetes".