COVID-19-associated coagulopathy: Difference between revisions
(Created page with "__NOTOC__ {{SI}} {{CMG}}; {{AE}} {{SK}} ==Overview== ==Historical Perspective== [Disease name] was first discovered by [name of scientist], a [nationality + occupation],...") |
No edit summary |
||
| (135 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{Main|COVID-19}} | |||
'''For COVID-19 frequently asked inpatient questions, click [[COVID-19 frequently asked inpatient questions|here]]''' | |||
{{SK}} | '''For COVID-19 frequently asked outpatient questions, click [[COVID-19 frequently asked outpatient questions|here]]''' | ||
{{CMG}}; {{AE}} {{IF}} | |||
{{SK}} Hematological findings and COVID-19, hypercoagulability in COVID-19, clotting disorder in COVID-19 | |||
==Overview== | ==Overview== | ||
[[Hypercoagulability]] is a major [[complication]] seen in as many as 31% of patients with [[COVID-19]]. It leads to many life-threatening outcomes, [[pulmonary embolism]] being the most common [[thrombotic]] complication. [[Hypercoagulability]] is characterized by elevated [[Fibrinogen]] and [[D-dimer]] levels. [[Coagulopathy]] in [[COVID-19]] must be differentiated from other diseases that cause disseminated intravascular coagulation ([[Disseminated intravascular coagulation|DIC]]). [[Prophylactic]] [[anticoagulation]] with [[low molecular weight heparin]] is given to all inpatients in the absence of active [[bleeding]]. Full dose [[anticoagulation]] is administered in patients with documented and confirmed [[venous thromboembolism]] ([[Venous thromboembolism|VTE]]) . | |||
==Historical Perspective== | ==Historical Perspective== | ||
* The etiological agent is [[SARS-CoV-2]], named for the similarity of its symptoms to those induced by the [[severe acute respiratory syndrome]], causing [[coronavirus]] disease 2019 ([[COVID-19]]), is a [[virus]] identified as the cause of an outbreak of [[respiratory illness]] first detected in Wuhan, China.<ref name="LuCui2020">{{cite journal|last1=Lu|first1=Jian|last2=Cui|first2=Jie|last3=Qian|first3=Zhaohui|last4=Wang|first4=Yirong|last5=Zhang|first5=Hong|last6=Duan|first6=Yuange|last7=Wu|first7=Xinkai|last8=Yao|first8=Xinmin|last9=Song|first9=Yuhe|last10=Li|first10=Xiang|last11=Wu|first11=Changcheng|last12=Tang|first12=Xiaolu|title=On the origin and continuing evolution of SARS-CoV-2|journal=National Science Review|volume=7|issue=6|year=2020|pages=1012–1023|issn=2095-5138|doi=10.1093/nsr/nwaa036}}</ref> | |||
*The rapidly increasing number of infected [[patients]] suggest that human-to-human transmission is actively occurring. | |||
*The [[outbreak]] was declared a Public Health Emergency of International Concern on 30 January 2020. | |||
*On March 12, 2020, the [[World Health Organization]] declared the [[COVID-19]] outbreak a [[pandemic]]. | |||
==Classification== | ==Classification== | ||
[ | * There is no established system for the classification of the [[hypercoagulability]] seen in [[COVID-19]]. | ||
* The [[coagulopathy]] may be classified according to the type of vessels and organs involved into:<ref name="pmid324155792">{{cite journal| author=Becker RC| title=COVID-19 update: Covid-19-associated coagulopathy. | journal=J Thromb Thrombolysis | year= 2020 | volume= 50 | issue= 1 | pages= 54-67 | pmid=32415579 | doi=10.1007/s11239-020-02134-3 | pmc=7225095 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32415579 }}</ref> | |||
**[[Venous thrombosis]] | |||
**[[Arterial thrombosis]] | |||
**[[Microvascular disease|Microvascular]] [[thrombosis]] | |||
* To view the classification of COVID-19, [[COVID-19 classification|click here]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
* [[COVID-19]] induces a [[Hypercoagulable state|hypercoagulable]] state in the body. An increased risk of [[mortality]] has been noted in patient’s with [[coagulopathy]] in COVID-19. It is thought that the [[coagulopathy]] in COVID-19 is the result of:<ref name="pmid32415579" /><ref name="pmid320732132">{{cite journal| author=Tang N, Li D, Wang X, Sun Z| title=Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 4 | pages= 844-847 | pmid=32073213 | doi=10.1111/jth.14768 | pmc=7166509 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32073213 }}</ref> | |||
**[[Virchow's triad|Virchow]]’s triad | |||
It is thought that [ | ** Vascular [[Endothelium|endothelial]] damage | ||
**Endothelitis- direct invasion of endothelial cells by SARS-CoV-2 which exposes the [[vWF]] associated with a massive release of [[vWF]], eventually activating the [[coagulation cascade]].<ref name="pmid32305740">{{cite journal| author=Escher R, Breakey N, Lämmle B| title=Severe COVID-19 infection associated with endothelial activation. | journal=Thromb Res | year= 2020 | volume= 190 | issue= | pages= 62 | pmid=32305740 | doi=10.1016/j.thromres.2020.04.014 | pmc=7156948 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32305740 }}</ref> | |||
**[[Complement system|Complement]] mediated damage to pericytes | |||
**Pro-inflammatory [[Cytokine|cytokines]]- [[IL-1]], [[Interleukin 6|IL-6]], and [[TNF-α|TNF- α]], that activate the [[Coagulation cascade|coagulation]] pathway and the [[fibrinolytic]] system.<ref name="pmid32418715">{{cite journal| author=Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G| title=COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. | journal=Cytokine Growth Factor Rev | year= 2020 | volume= 53 | issue= | pages= 66-70 | pmid=32418715 | doi=10.1016/j.cytogfr.2020.05.002 | pmc=7204669 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32418715 }}</ref><ref name="pmid7204669">{{cite journal| author=Luiten PG| title=Two visual pathways to the telencephalon in the nurse shark (Ginglymostoma cirratum). I. Retinal projections. | journal=J Comp Neurol | year= 1981 | volume= 196 | issue= 4 | pages= 531-8 | pmid=7204669 | doi=10.1002/cne.901960402 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7204669 }}</ref><ref name="pmid32513566">{{cite journal| author=Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L| title=SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. | journal=Cytokine Growth Factor Rev | year= 2020 | volume= | issue= | pages= | pmid=32513566 | doi=10.1016/j.cytogfr.2020.06.001 | pmc=7265853 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32513566 }}</ref> | |||
[ | *[[Stasis (medicine)|Stasis]]- Prolonged hospital admissions causing immobilization of the patient. | ||
*[[Hypercoagulable state]]- Evidenced by elevated [[fibrinogen]], prothrombotic factors and [[Hyperviscosity syndrome|hyperviscosity]].<ref name="pmid32464112">{{cite journal| author=Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A| title=COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? | journal=Lancet | year= 2020 | volume= 395 | issue= 10239 | pages= 1758-1759 | pmid=32464112 | doi=10.1016/S0140-6736(20)31209-5 | pmc=7247793 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32464112 }}</ref> | |||
*Some patients have been found to have [[Lupus anticoagulant]] ([[Anti-cardiolipin antibodies|anti-cardiolipin]]) and anti-β2GP1 [[antibodies]] that may be contributory.<ref name="pmid32369280">{{cite journal| author=Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP | display-authors=etal| title=Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. | journal=N Engl J Med | year= 2020 | volume= 383 | issue= 3 | pages= 288-290 | pmid=32369280 | doi=10.1056/NEJMc2013656 | pmc=7217555 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32369280 }}</ref> | |||
[ | |||
==Causes== | ==Causes== | ||
* [[COVID-19|Coronavirus disease 2019 (COVID-19)]] is caused by a novel [[coronavirus]] called [[SARS-CoV-2]] and is the cause of [[thrombocytopenia]] in [[COVID-19]] infection. | |||
* To view causes of COVID-19, [[COVID-19 causes|click here]]. | |||
==Differentiating COVID-19 associated coagulopathy from other Diseases== | |||
The | * The main feature of COVID-19 coagulopathy is [[thrombosis]] while the acute phase of [[DIC]] presents with [[bleeding]].<ref name="pmid32407672">{{cite journal| author=Levi M, Thachil J, Iba T, Levy JH| title=Coagulation abnormalities and thrombosis in patients with COVID-19. | journal=Lancet Haematol | year= 2020 | volume= 7 | issue= 6 | pages= e438-e440 | pmid=32407672 | doi=10.1016/S2352-3026(20)30145-9 | pmc=7213964 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32407672 }}</ref> | ||
*Similar laboratory findings are: marked increase in [[D-dimer]] and normal/slightly low [[platelets]] and prolonged [[Prothrombin time|PT.]] | |||
[ | *Findings distinct in patients with COVID 19 are: high [[fibrinogen]] and high [[factor VIII]] activity | ||
*The scoring system of the [https://www.isth.org/ International Society on Thrombosis and Hemostasis] should be used to detect DIC ([[platelet]] count, PT, [[fibrinogen]], D‐dimer, [[antithrombin]] and [[protein C]] activity monitoring), but the diagnosis and subsequent treatment should be done clinically.<ref name="pmid19222477">{{cite journal| author=Levi M, Toh CH, Thachil J, Watson HG| title=Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. | journal=Br J Haematol | year= 2009 | volume= 145 | issue= 1 | pages= 24-33 | pmid=19222477 | doi=10.1111/j.1365-2141.2009.07600.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19222477 }}</ref> | |||
* Coagulopathy in COVID-19 must also be differentiated from other diseases that cause [[Disseminated intravascular coagulation|DIC]] resulting in DVT and pulmonary embolism such as: | |||
**[[Antithrombin III deficiency]] | |||
** [[Factor V Leiden mutation]] | |||
** [[Protein C deficiency]] | |||
** [[Protein S deficiency]] | |||
** [[Prothrombin gene mutation G20210A|Prothrombin gene mutation]] | |||
** [[Antiphospholipid antibody syndrome]] | |||
For further information about the differential diagnosis, click [[COVID-19 associated coagulopathy differential diagnosis|here]]. | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
===Incidence=== | |||
* The [[Incidence (epidemiology)|incidence]] of venous [[thromboembolism]] in [[Intensive care unit|ICU]] patients with COVID-19 was analyzed in a study by Klok et al.<ref name="pmid322910942">{{cite journal| author=Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM | display-authors=etal| title=Incidence of thrombotic complications in critically ill ICU patients with COVID-19. | journal=Thromb Res | year= 2020 | volume= 191 | issue= | pages= 145-147 | pmid=32291094 | doi=10.1016/j.thromres.2020.04.013 | pmc=7146714 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32291094 }}</ref> | |||
* It concluded that the cumulative [[incidence]] of acute [[pulmonary embolism]] ([[Pulmonary embolism|PE]]), [[deep vein thrombosis]] ([[Deep vein thrombosis|DVT]]), [[ischemic stroke]], [[ST elevation myocardial infarction|MI]], or systemic [[Arterial embolism|arterial embolism was 31%.]] | |||
* The [[Incidence (epidemiology)|incidence]] of most common [[Thrombosis|thrombotic]] complication was [[pulmonary embolism]] seen in 81% of patients. All these patients were on at least standard doses of thromboprophylaxis. <ref name="pmid32291094">{{cite journal| author=Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM | display-authors=etal| title=Incidence of thrombotic complications in critically ill ICU patients with COVID-19. | journal=Thromb Res | year= 2020 | volume= 191 | issue= | pages= 145-147 | pmid=32291094 | doi=10.1016/j.thromres.2020.04.013 | pmc=7146714 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32291094 }} </ref><ref name="pmid7146714">{{cite journal| author=Woringer V, Renevey F| title=[A case of gonococcal arthritis at a young age]. | journal=Rev Med Suisse Romande | year= 1982 | volume= 102 | issue= 9 | pages= 863-5 | pmid=7146714 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7146714 }}</ref> | |||
* The cumulative [[Incidence (epidemiology)|incidence]] of [[Venous thromboembolism|venous thromboembolism (VTE)]] were 16% (95% CI, 10-22) at 7 days, 33% (95% CI, 23-43) at 14 days and 42% (95% CI 30-54) at 21 days. | |||
* Comparatively, the cumulative [[incidence]] of [[venous thromboembolism]] ([[Venous thromboembolism|VTE]]) was higher in the '''ICU''' patients - 26% (95% CI, 17-37) at 7 days, 47% (95% CI, 34-58) at 14 days and 59% (95% CI, 42-72) at 21 days) than on the floor.<ref name="pmid32369666">{{cite journal| author=Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA | display-authors=etal| title=Incidence of venous thromboembolism in hospitalized patients with COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=32369666 | doi=10.1111/jth.14888 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32369666 }}</ref> | |||
To view the epidemiology and demographics for COVID-19, [[COVID-19 epidemiology and demographics|click here]].<br /> | |||
=== Age === | |||
* There is insufficient information regarding age-specific [[prevalence]] or incidence of [[COVID-19]]-associated [[coagulopathy]]. | |||
=== Gender === | |||
* There is insufficient information regarding gender-specific [[prevalence]] or incidence of [[COVID-19]]-associated [[coagulopathy]]. | |||
=== Race === | |||
* There is insufficient information regarding race-specific [[prevalence]] or incidence of [[COVID-19]]-associated [[coagulopathy]]. | |||
[ | ==Risk Factors== | ||
Common hypothesized [[Risk factor|risk factors]] for [[coagulopathy]] in [[COVID-19]] [[pneumonia]] based on studies include:<ref name="pmid32291094" /><ref name="WuChen2020">{{cite journal|last1=Wu|first1=Chaomin|last2=Chen|first2=Xiaoyan|last3=Cai|first3=Yanping|last4=Xia|first4=Jia’an|last5=Zhou|first5=Xing|last6=Xu|first6=Sha|last7=Huang|first7=Hanping|last8=Zhang|first8=Li|last9=Zhou|first9=Xia|last10=Du|first10=Chunling|last11=Zhang|first11=Yuye|last12=Song|first12=Juan|last13=Wang|first13=Sijiao|last14=Chao|first14=Yencheng|last15=Yang|first15=Zeyong|last16=Xu|first16=Jie|last17=Zhou|first17=Xin|last18=Chen|first18=Dechang|last19=Xiong|first19=Weining|last20=Xu|first20=Lei|last21=Zhou|first21=Feng|last22=Jiang|first22=Jinjun|last23=Bai|first23=Chunxue|last24=Zheng|first24=Junhua|last25=Song|first25=Yuanlin|title=Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China|journal=JAMA Internal Medicine|volume=180|issue=7|year=2020|pages=934|issn=2168-6106|doi=10.1001/jamainternmed.2020.0994}}</ref><ref name="urlManagement of Patients with Confirmed 2019-nCoV | CDC">{{cite web |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html |title=Management of Patients with Confirmed 2019-nCoV | CDC |format= |work= |accessdate=}}</ref> | |||
*[[Intensive care unit|ICU]] admission | |||
* Age (> 40 years) | |||
*[[Hypoxia]] | |||
[ | Other general [[Risk factor|risk factors]] for [[venous thromboembolism]] ([[Venous thromboembolism|VTE]]) are: | ||
* Male | |||
* Active [[cancer]] | |||
* Recent major [[surgery]] | |||
* High [[Body mass index|BMI]] ([[obesity]]) | |||
* Prior [[Venous thromboembolism|VTE]] | |||
* Immobilization | |||
*[[Trauma]] | |||
To view the risk factors of COVID-19, [[COVID-19 risk factors|click here]]. | |||
==Screening== | |||
* Every patient with [[COVID-19]] infection admitted to the hospital should have a baseline of basic blood investigations such as:<ref name="LevyConnors2020">{{cite journal|last1=Levy|first1=Jerrold H.|last2=Connors|first2=Jean M.|title=COVID-19 and its implications for thrombosis and anticoagulation|journal=Blood|volume=135|issue=23|year=2020|pages=2033–2040|issn=0006-4971|doi=10.1182/blood.2020006000}}</ref> | |||
**[[Complete blood count]] ([[Complete blood count|CBC]]) | |||
**[[Platelet count]] | |||
**[[Prothrombin time]] ([[Prothrombin time|PT]]), [[Activated partial thromboplastin time]] ([[Partial thromboplastin time|aPTT]]) | |||
**[[Fibrinogen]] | |||
**[[D-dimer]] | |||
**[[C-reactive protein]] | |||
* Routine [[Screening (medicine)|screening]] with [[imaging]] is not done as there is no evidence to indicate an improvement in clinical outcomes. | |||
* Depending on the clinical state of the patient and suspicion for the development of [[Venous thromboembolism|VTE]] or [[Artery|arterial]] thrombi, repeat testing and further imaging investigations are done. | |||
To view screening for COVID-19, [[COVID-19 screening|click here]].<br /> | |||
==Natural History, Complications, and Prognosis== | |||
===Natural History=== | |||
[ | * If left untreated, patients with [[coagulopathy]] may progress to develop [[Venous thromboembolism|VTE]], [[arterial thrombosis]], or microvascular [[thrombosis]] and ultimately succumb to death. | ||
===Complications=== | |||
*[[Thrombosis|Thrombotic]] complications : <ref name="pmid32415579">{{cite journal| author=Becker RC| title=COVID-19 update: Covid-19-associated coagulopathy. | journal=J Thromb Thrombolysis | year= 2020 | volume= | issue= | pages= | pmid=32415579 | doi=10.1007/s11239-020-02134-3 | pmc=7225095 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32415579 }} </ref><ref name="pmid32302462">{{cite journal| author=Barrett CD, Moore HB, Yaffe MB, Moore EE| title=ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=32302462 | doi=10.1111/jth.14860 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302462 }}</ref> | |||
**[[Deep vein thrombosis|Deep Vein Thrombosis]] | |||
**[[Pulmonary embolism|Pulmonary Embolism]] | |||
**[[Ischemic stroke]] | |||
**[[Myocardial infarction]] | |||
**[[Ischemia|Ischemic]] limbs | |||
**Systemic arterial events | |||
**Clotting of [[central venous catheter]]<nowiki/>s, [[dialysis]] catheter<nowiki/>s, and dialysis filters | |||
=== Prognosis === | |||
[[Prognosis|Progn]][[Prognosis|osis]] depends on numerous factors:<ref name="pmid32306492">{{cite journal| author=Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z | display-authors=etal| title=D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 6 | pages= 1324-1329 | pmid=32306492 | doi=10.1111/jth.14859 | pmc=7264730 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32306492 }}</ref> | |||
* Increased [[D-dimer]] levels- poor [[prognosis]] | |||
* Increased [[fibrin degradation product]] (FDP) levels <ref name="pmid32073213">{{cite journal| author=Tang N, Li D, Wang X, Sun Z| title=Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 4 | pages= 844-847 | pmid=32073213 | doi=10.1111/jth.14768 | pmc=7166509 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32073213 }} </ref> | |||
*[[Intensive care unit|ICU]] admission | |||
Independent predictors of thrombotic complications include: | |||
*[[Age]] | |||
*[[Coagulopathy]] (defined as spo<nowiki/>ntaneou<nowiki/>s prolongatio<nowiki/>n of th<nowiki/>e [[prothrombin time]] > 3 s or [[activated partial thromboplastin time]] > 5 s) | |||
*Active [[cancer]] | |||
To view natural history, complications, and prognosis of [[COVID-19]], [[COVID-19 natural history, complications, and prognosis|click here]]. | |||
==Diagnosis== | ==Diagnosis== | ||
===Diagnostic Study of Choice=== | ===Diagnostic Study of Choice=== | ||
*The diagnosis of [[coagulopathy]] in [[COVID-19]] is based mainly on the laboratory findings showing a pro-coagulant profile. | |||
*The pre-test probability of [[DVT]] and [[PE]] can be calculated using the [[Wells Score|Wells' criteria]] | |||
*[[Computed tomography]] with [[pulmonary angiography]] ([[CT pulmonary angiogram|CTPA]]) is the diagnostic test of choice. [[Ventilation/perfusion scan|Ventilation/Perfusion]] scan may also be done, but may not be of much yield in patients with [[COVID-19]]. | |||
*To view the study of choice for diagnosis of [[COVID-19]], [[COVID-19 diagnostic study of choice|click here]].<br /> | |||
The | ===History and Symptoms=== | ||
The [[symptoms]] depend on the vessels and the organ systems involved. | |||
'''Pulmonary Embolism:''' Many symptoms of [[Pulmonary embolism|PE]] overlap with the [[respiratory]] symptoms seen in [[COVID-19]]. | |||
* Maybe [[asymptomatic]] | |||
* [[Dyspnea]] | |||
* [[Chest pain]] | |||
* [[Cough]] | |||
* Some other rare presentations include- [[hemoptysis]], [[shock]], [[hypotension]], death. | |||
A positive history of the following is suggestive of and contributory: | |||
* Immobilization or prolonged [[hospitalization]] | |||
* Recent [[surgery]] | |||
*[[Trauma]] | |||
*[[Obesity]] | |||
* History of previous venous [[thromboembolism]] ([[Venous thromboembolism|VTE]]) | |||
*[[Malignancy]] | |||
* Stroke with [[hemiplegia]] or [[immobility]] | |||
* Age >65 years | |||
'''Deep Vein Thrombosis''' | |||
* [[Swelling]] | |||
* [[Edema]] | |||
* [[Pain]] | |||
* Warmth | |||
[[Arterial thrombosis]] involving various systems show the following symptoms: | |||
* '''Ischemic Stroke:''' Various focal [[neurological]] deficits depending on the large artery involved | |||
* '''Myocardial infarction:''' [[Chest pain]] radiating to left arm and neck, sweating, [[dyspnea]] | |||
* '''Acute ischemic limb:''' Pain, pallor, [[poikilothermia]], [[pulselessness]], [[paresthesia]], [[paralysis]] | |||
To view the history and symptoms of COVID-19, [[COVID-19 history and symptoms|click here]]. | |||
===Physical Examination=== | ===Physical Examination=== | ||
'''Pulmonary Embolism''' | |||
Physical examination of patients with [[Pulmonary embolism|Pulmonary Embolism]] is usually remarkable for: | |||
*[[Tachycardia]] | |||
*[[Tachypnea]] | |||
*[[Diaphoresis]] | |||
'''Deep Vein Thrombosis''' | |||
Physical examination of patients with [[Deep Vein Thrombosis]] includes: | |||
* Unilateral swelling/[[edema]] with a difference in diameters | |||
* [[Warmth]] | |||
* [[Tenderness]] | |||
* Dilated [[veins]] | |||
* [[Homan's sign]] may also be seen but is unreliable. | |||
[[Arterial]] [[thrombosis]]: | |||
* '''Ischemic Stroke:''' Focal neurological deficits depending on the vessel involved | |||
* '''Myocardial Infarction:''' Uncomfortable appearing patient with [[diaphoresis]] | |||
* '''Ischemic Limb:''' Pallor, [[poikilothermia]], [[pulselessness]], [[paresthesia]], [[paralysis]] | |||
To view the complete physical examination in [[COVID-19]], [[COVID-19 physical examination|click here]]. | |||
===Laboratory Findings=== | ===Laboratory Findings=== | ||
An elevated | * An elevated concentration of serum/blood pro-coagulant factors is diagnostic of [[coagulopathy]] associated with [[COVID-19]]. | ||
* Laboratory findings consistent with the diagnosis of [[COVID-19]] associated [[coagulopathy]] include:<ref name="pmid2302448">{{cite journal| author=Wasserbauer R, Beranová M, Vancurová D, Dolezel B| title=Biodegradation of polyethylene foils by bacterial and liver homogenates. | journal=Biomaterials | year= 1990 | volume= 11 | issue= 1 | pages= 36-40 | pmid=2302448 | doi=10.1016/0142-9612(90)90049-v | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2302448 }}</ref><ref name="pmid32302448">{{cite journal| author=Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M | display-authors=etal| title=The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 7 | pages= 1747-1751 | pmid=32302448 | doi=10.1111/jth.14854 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302448 }}</ref> | |||
**[[Coagulation]] testing- pro-coagulant profile which includes: | |||
***[[Platelet]] Counts- Normal or increased | |||
Laboratory findings consistent with the diagnosis of [ | ***[[Prothrombin time]] (PT) and [[activated partial thromboplastin time]] ([[aPTT]])- normal or slightly prolonged | ||
***[[Fibrinogen]]- increased | |||
***[[D-dimer]]- increased | |||
***[[Factor VIII]] activity- increased | |||
[ | ***[[Von Willebrand factor|VWF]] antigen- increased | ||
***[[Protein C]], [[Protein S]], [[Antithrombin III]] - slightly decreased | |||
* [[TEGT|TEG]] findings:<ref name="pmid32302438">{{cite journal| author=Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V | display-authors=etal| title=Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 7 | pages= 1738-1742 | pmid=32302438 | doi=10.1111/jth.14850 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302438 }}</ref><ref name="pmid323024482">{{cite journal| author=Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M | display-authors=etal| title=The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 7 | pages= 1747-1751 | pmid=32302448 | doi=10.1111/jth.14854 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302448 }}</ref> | |||
** Reaction time (R) - decreased | |||
**[[Clot]] formation time (K)- decreased | |||
** Maximum amplitude (MA)- increased | |||
** Clot lysis at 30 minutes (LY30)- decreased | |||
To view the laboratory findings on COVID-19, [[COVID-19 laboratory findings|click here]]. | |||
===Electrocardiogram=== | ===Electrocardiogram=== | ||
An [[The electrocardiogram|ECG]] may be helpful in the diagnosis of [[pulmonary embolism]] or [[myocardial infarction]] caused due to hypercoagulability in [[COVID-19]]. | |||
*Findings on an [[The electrocardiogram|ECG]] suggestive of/diagnostic of [[pulmonary embolism]] include tachycardia and S1Q3T3 pattern. | |||
*Findings on an [[The electrocardiogram|ECG]] suggestive of/diagnostic of [[myocardial infarction]] include STE elevation in various leads. | |||
*To view the electrocardiogram findings on COVID-19, [[COVID-19 electrocardiogram|click here]].<br /> | |||
===X-ray=== | ===X-ray=== | ||
There are no x-ray findings associated with [ | * There are no specific x-ray findings associated with [[PE]]. | ||
* However, an x-ray may be helpful in ruling out other causes with similar symptoms like [[pneumonia]], [[cardiogenic]] causes of [[dyspnea]], and [[pneumothorax]]. | |||
*To view the x-ray finidings on COVID-19, [[COVID-19 x ray|click here]].<br /> | |||
===Echocardiography or Ultrasound=== | ===Echocardiography or Ultrasound=== | ||
*[[Echocardiography]] may be helpful in the diagnosis of [[pulmonary embolism]]. | |||
*[[Ultrasound|Compressive Ultrasound]] may be helpful in the diagnosis of [[deep vein thrombosis]] | |||
*To view the echocardiographic findings on COVID-19, [[COVID-19 echocardiography and ultrasound|click here]].<br /> | |||
===CT scan=== | ===CT scan=== | ||
===== CTPA and Ventilation Perfusion(V/Q) Scan ===== | |||
*Prompt diagnosis of PE in COVID-19 patient is difficult in this regard that various symptoms of [[COVID-19]] overlap with that of [[pulmonary embolism]]. American Society of Hematology provides the following guidelines regarding the diagnosis of pulmonary embolism:<ref name="pmid32383092">{{cite journal| author=Lu Y, Macapinlac HA| title=Perfusion SPECT/CT to diagnose pulmonary embolism during COVID-19 pandemic. | journal=Eur J Nucl Med Mol Imaging | year= 2020 | volume= 47 | issue= 9 | pages= 2064-2065 | pmid=32383092 | doi=10.1007/s00259-020-04851-6 | pmc=7205478 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32383092 }}</ref> | |||
** Normal [[d-dimers]] level in a patient with low to moderate [[Pretest probability of DVT|pretest probability]] is sufficient to rule out the diagnosis of PE. [[D-dimers|D-dimers level]] is usually elevated in COVID-19 patients. This is not applicable to a patient with a high pretest probability. | |||
** Inpatient with suspected PE with symptoms like [[hypotension]], [[tachycardia]], and sudden drop in [[oxygen saturation]] with a high pretest probability of PE, computed [[tomography]] with [[pulmonary angiography]] is used for the diagnosis. Contraindication to the use of [[CT pulmonary angiogram|CTPA]] warrants investigation with [[Ventilation/perfusion scan|ventilation/perfusion scan.]] | |||
[ | |||
[[File:Covid-19-pneumonia-and-pulmonary-emboli.jpg|thumb|800x800px| '''Right-sided segmental and subsegmental pulmonary arterial filling defects (yellow arrows) in keeping with acute distal pulmonary emboli.''' Case courtesy of Dr Gianluca Martinelli, <a href="https://radiopaedia.org/">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/76817">rID: 76817</a> |center]] | |||
To view the [[Computed tomography|CT]] scan findings on [[COVID-19]], [[COVID-19 CT scan|click here]]. | |||
===MRI=== | ===MRI=== | ||
There are no MRI findings associated with [ | *There are no [[Magnetic resonance imaging|MRI]] findings associated with [[coagulopathy]] of [[COVID-19]] unless it is used to diagnose and evaluate an [[ischemic stroke]] caused by it. | ||
*To view the [[Magnetic resonance imaging|MRI]] findings on [[COVID-19|COVID-19,]] [[COVID-19 MRI|click here]].<br /> | |||
[ | |||
===Other Imaging Findings=== | ===Other Imaging Findings=== | ||
There are no other imaging findings associated with [ | There are no other imaging findings associated with [[coagulopathy]] of [[COVID-19]]. | ||
[ | |||
===Other Diagnostic Studies=== | ===Other Diagnostic Studies=== | ||
* To view other diagnostic studies for [[COVID-19]], [[COVID-19 other diagnostic studies|click here]].<br /> | |||
[ | |||
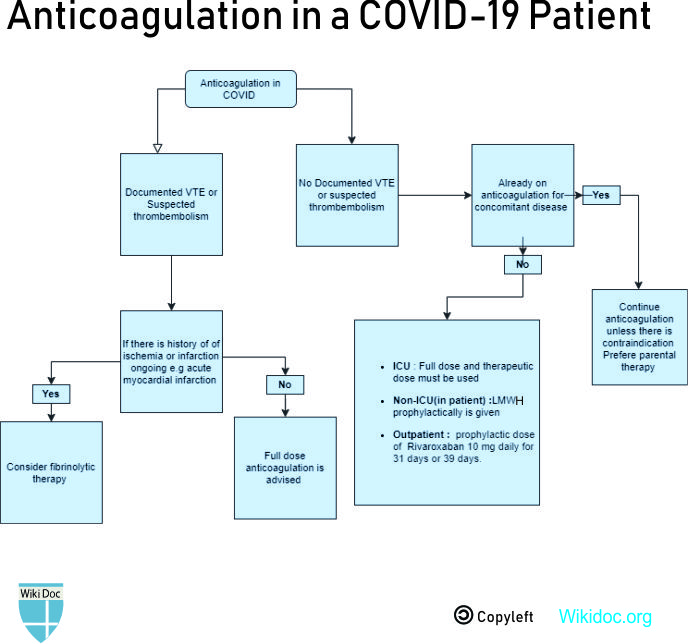
==Treatment== | ==Treatment== | ||
===Medical Therapy=== | ===Medical Therapy=== | ||
'''Prophylactic dose of anticoagulation''' | |||
* Drug- [[Low-molecular-weight heparin]] ([[Low molecular weight heparin|LMWH]]) is preferred over [[unfractionated heparin]] to reduce contact with the patient. | |||
*[[Unfractionated heparin]] may be used in case of unavailability or severe [[Renal insufficiency|renal impairment]]. | |||
[ | Indications: | ||
* All inpatients in the absence of active [[bleeding]] | |||
* To be held only if [[Platelet|platelet counts]] fall below 25 x 109/L, or [[fibrinogen]] less than 0.5 g/L | |||
* In case of a history of [[heparin-induced thrombocytopenia]](HIT), [[fondaparinux]] is used. | |||
'''Intermediate or therapeutic dose anticoagulation''' | |||
* Preferred regimen: [[Enoxaparin]] 40 to 60 mg once daily<ref name="pmid322201122">{{cite journal| author=Tang N, Bai H, Chen X, Gong J, Li D, Sun Z| title=Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 5 | pages= 1094-1099 | pmid=32220112 | doi=10.1111/jth.14817 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32220112 }}</ref> | |||
Indications: | |||
*Critically ill patients or [[Intensive care unit|ICU]] patients<ref name="pmid323024422">{{cite journal| author=Akima S, McLintock C, Hunt BJ| title=RE: ISTH interim guidance to recognition and management of coagulopathy in COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=32302442 | doi=10.1111/jth.14853 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302442 }}</ref> | |||
* According to a study, a better [[prognosis]] was seen in patients who met the SIC ([[Sepsis]]-induced [[coagulopathy]]) criteria or had marked elevated [[D-dimer]] levels and were put on anticoagulant therapy(mainly with [[low molecular weight heparin]]) <ref name="pmid32220112">{{cite journal| author=Tang N, Bai H, Chen X, Gong J, Li D, Sun Z| title=Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 5 | pages= 1094-1099 | pmid=32220112 | doi=10.1111/jth.14817 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32220112 }} </ref> | |||
'''Therapeutic/ full-dose anticoagulation''' | |||
* Preferred regimen: [[Enoxaparin]] 1 mg/kg every 12 hours | |||
Indications: | |||
* Suspected [[Venous thromboembolism|VTE]]/[[Pulmonary embolism|PE]] | |||
* Confirmed [[Venous thromboembolism|VTE]]/[[Pulmonary embolism|PE]] | |||
* Clotting in [[Catheter|catheters]], or [[extracorporeal]] circuits like [[Extracorporeal membrane oxygenation|ECMO]] and CRRT. <ref name="pmid32302442">{{cite journal| author=Akima S, McLintock C, Hunt BJ| title=RE: ISTH interim guidance to recognition and management of coagulopathy in COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=32302442 | doi=10.1111/jth.14853 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302442 }} </ref> | |||
'''Post-discharge thromboprophylaxis''' | |||
* Drug and dose- Regulatory-approved regimen<ref name="pmid27232649">{{cite journal| author=Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A | display-authors=etal| title=Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. | journal=N Engl J Med | year= 2016 | volume= 375 | issue= 6 | pages= 534-44 | pmid=27232649 | doi=10.1056/NEJMoa1601747 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27232649 }}</ref> | |||
**Preferred regimen (1): [[Betrixaban]] 160 mg on day 1, followed by 80 mg once daily for 35-42 days | |||
**Preferred regimen (2): [[Rivaroxaban]] 10 mg daily for 31-39 days | |||
Indications: | |||
* Patients with documented venous thromboembolism [[Venous thromboembolism|(VTE]]) require thromboprophylaxis for up to 90 days after discharge. | |||
* Some patients who do not have [[Venous thromboembolism|VTE]] but require extended thromboprophylaxis include: | |||
**Acute medical illness, older age, immobilization, recent surgery, or trauma. | |||
*Most of these criteria are met by patients with COVID-19, and they require thromboprophylaxis for up to 90 days after discharge.<ref name="pmid32311448">{{cite journal| author=Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E | display-authors=etal| title=COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. | journal=J Am Coll Cardiol | year= 2020 | volume= 75 | issue= 23 | pages= 2950-2973 | pmid=32311448 | doi=10.1016/j.jacc.2020.04.031 | pmc=7164881 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32311448 }}</ref> | |||
'''Bleeding in COVID-19''' | |||
*[[Transfusion]] and replacement; discontinuation or reversal of [[Anticoagulant|anticoagulation]] are the mainstay of treatment | |||
* Transfuse [[Platelet|platelets]] if the platelet count is less than 50 x 109/L | |||
* Administer [[Blood plasma|plasma]] if the [[INR]] is above 1.8 | |||
* Order [[fibrinogen]] concentrate or [[cryoprecipitate]] if the [[fibrinogen]] level is less than 1.5 g/L | |||
*To view medical treatment for COVID-19, click here. | |||
===Surgery=== | ===Surgery=== | ||
Surgical intervention is not recommended for the management of | Surgical intervention is not recommended for the management of COVID-19 associated coagulopathy. | ||
===Primary Prevention=== | ===Primary Prevention=== | ||
* Since there is no vaccine for COVID-19 there are plenty of primary prevention suggested from CDC such as:<ref>{{Cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/index.html|title=|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> | |||
** Hand washing every 10 minutes. | |||
** Using alcoholic hand sanitizer. | |||
** Self [[quarantine]] for two weeks if [[symptomatic]]. | |||
* To view the primary prevention measures of COVID-19, click [[COVID-19 primary prevention|here]]. | |||
===Secondary Prevention=== | ===Secondary Prevention=== | ||
*[[World Health Organization|WHO]] recommends home care for patients with suspected [[COVID-19]] who present with mild symptoms:<ref>{{cite web |url=https://www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts |title=Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts |format= |work= |accessdate=}}</ref> | |||
**Family members of an infected patient are better to wear masks. | |||
**Using separate bathroom and bedroom by the infected person. | |||
**Using [[antipyretics]] and analgesics for [[fever]], [[myalgias]], and [[headaches]] | |||
* To view the secondary prevention measures of COVID-19, click [[COVID-19 secondary prevention|here]]. | |||
[[File:Patho covid anticoagulation.jpg|600px|center]] | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{Covid-19}} | |||
{{ | [[Category:Up-To-Date]] | ||
Latest revision as of 18:30, 25 August 2020
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ifrah Fatima, M.B.B.S[2]
Synonyms and keywords: Hematological findings and COVID-19, hypercoagulability in COVID-19, clotting disorder in COVID-19
Overview
Hypercoagulability is a major complication seen in as many as 31% of patients with COVID-19. It leads to many life-threatening outcomes, pulmonary embolism being the most common thrombotic complication. Hypercoagulability is characterized by elevated Fibrinogen and D-dimer levels. Coagulopathy in COVID-19 must be differentiated from other diseases that cause disseminated intravascular coagulation (DIC). Prophylactic anticoagulation with low molecular weight heparin is given to all inpatients in the absence of active bleeding. Full dose anticoagulation is administered in patients with documented and confirmed venous thromboembolism (VTE) .
Historical Perspective
- The etiological agent is SARS-CoV-2, named for the similarity of its symptoms to those induced by the severe acute respiratory syndrome, causing coronavirus disease 2019 (COVID-19), is a virus identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China.[1]
- The rapidly increasing number of infected patients suggest that human-to-human transmission is actively occurring.
- The outbreak was declared a Public Health Emergency of International Concern on 30 January 2020.
- On March 12, 2020, the World Health Organization declared the COVID-19 outbreak a pandemic.
Classification
- There is no established system for the classification of the hypercoagulability seen in COVID-19.
- The coagulopathy may be classified according to the type of vessels and organs involved into:[2]
- To view the classification of COVID-19, click here.
Pathophysiology
- COVID-19 induces a hypercoagulable state in the body. An increased risk of mortality has been noted in patient’s with coagulopathy in COVID-19. It is thought that the coagulopathy in COVID-19 is the result of:[3][4]
- Virchow’s triad
- Vascular endothelial damage
- Endothelitis- direct invasion of endothelial cells by SARS-CoV-2 which exposes the vWF associated with a massive release of vWF, eventually activating the coagulation cascade.[5]
- Complement mediated damage to pericytes
- Pro-inflammatory cytokines- IL-1, IL-6, and TNF- α, that activate the coagulation pathway and the fibrinolytic system.[6][7][8]
- Stasis- Prolonged hospital admissions causing immobilization of the patient.
- Hypercoagulable state- Evidenced by elevated fibrinogen, prothrombotic factors and hyperviscosity.[9]
- Some patients have been found to have Lupus anticoagulant (anti-cardiolipin) and anti-β2GP1 antibodies that may be contributory.[10]
Causes
- Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus called SARS-CoV-2 and is the cause of thrombocytopenia in COVID-19 infection.
- To view causes of COVID-19, click here.
Differentiating COVID-19 associated coagulopathy from other Diseases
- The main feature of COVID-19 coagulopathy is thrombosis while the acute phase of DIC presents with bleeding.[11]
- Similar laboratory findings are: marked increase in D-dimer and normal/slightly low platelets and prolonged PT.
- Findings distinct in patients with COVID 19 are: high fibrinogen and high factor VIII activity
- The scoring system of the International Society on Thrombosis and Hemostasis should be used to detect DIC (platelet count, PT, fibrinogen, D‐dimer, antithrombin and protein C activity monitoring), but the diagnosis and subsequent treatment should be done clinically.[12]
- Coagulopathy in COVID-19 must also be differentiated from other diseases that cause DIC resulting in DVT and pulmonary embolism such as:
For further information about the differential diagnosis, click here.
Epidemiology and Demographics
Incidence
- The incidence of venous thromboembolism in ICU patients with COVID-19 was analyzed in a study by Klok et al.[13]
- It concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31%.
- The incidence of most common thrombotic complication was pulmonary embolism seen in 81% of patients. All these patients were on at least standard doses of thromboprophylaxis. [14][15]
- The cumulative incidence of venous thromboembolism (VTE) were 16% (95% CI, 10-22) at 7 days, 33% (95% CI, 23-43) at 14 days and 42% (95% CI 30-54) at 21 days.
- Comparatively, the cumulative incidence of venous thromboembolism (VTE) was higher in the ICU patients - 26% (95% CI, 17-37) at 7 days, 47% (95% CI, 34-58) at 14 days and 59% (95% CI, 42-72) at 21 days) than on the floor.[16]
To view the epidemiology and demographics for COVID-19, click here.
Age
- There is insufficient information regarding age-specific prevalence or incidence of COVID-19-associated coagulopathy.
Gender
- There is insufficient information regarding gender-specific prevalence or incidence of COVID-19-associated coagulopathy.
Race
- There is insufficient information regarding race-specific prevalence or incidence of COVID-19-associated coagulopathy.
Risk Factors
Common hypothesized risk factors for coagulopathy in COVID-19 pneumonia based on studies include:[14][17][18]
Other general risk factors for venous thromboembolism (VTE) are:
To view the risk factors of COVID-19, click here.
Screening
- Every patient with COVID-19 infection admitted to the hospital should have a baseline of basic blood investigations such as:[19]
- Routine screening with imaging is not done as there is no evidence to indicate an improvement in clinical outcomes.
- Depending on the clinical state of the patient and suspicion for the development of VTE or arterial thrombi, repeat testing and further imaging investigations are done.
To view screening for COVID-19, click here.
Natural History, Complications, and Prognosis
Natural History
- If left untreated, patients with coagulopathy may progress to develop VTE, arterial thrombosis, or microvascular thrombosis and ultimately succumb to death.
Complications
- Thrombotic complications : [3][20]
- Deep Vein Thrombosis
- Pulmonary Embolism
- Ischemic stroke
- Myocardial infarction
- Ischemic limbs
- Systemic arterial events
- Clotting of central venous catheters, dialysis catheters, and dialysis filters
Prognosis
Prognosis depends on numerous factors:[21]
- Increased D-dimer levels- poor prognosis
- Increased fibrin degradation product (FDP) levels [22]
- ICU admission
Independent predictors of thrombotic complications include:
- Age
- Coagulopathy (defined as spontaneous prolongation of the prothrombin time > 3 s or activated partial thromboplastin time > 5 s)
- Active cancer
To view natural history, complications, and prognosis of COVID-19, click here.
Diagnosis
Diagnostic Study of Choice
- The diagnosis of coagulopathy in COVID-19 is based mainly on the laboratory findings showing a pro-coagulant profile.
- The pre-test probability of DVT and PE can be calculated using the Wells' criteria
- Computed tomography with pulmonary angiography (CTPA) is the diagnostic test of choice. Ventilation/Perfusion scan may also be done, but may not be of much yield in patients with COVID-19.
- To view the study of choice for diagnosis of COVID-19, click here.
History and Symptoms
The symptoms depend on the vessels and the organ systems involved.
Pulmonary Embolism: Many symptoms of PE overlap with the respiratory symptoms seen in COVID-19.
- Maybe asymptomatic
- Dyspnea
- Chest pain
- Cough
- Some other rare presentations include- hemoptysis, shock, hypotension, death.
A positive history of the following is suggestive of and contributory:
- Immobilization or prolonged hospitalization
- Recent surgery
- Trauma
- Obesity
- History of previous venous thromboembolism (VTE)
- Malignancy
- Stroke with hemiplegia or immobility
- Age >65 years
Deep Vein Thrombosis
Arterial thrombosis involving various systems show the following symptoms:
- Ischemic Stroke: Various focal neurological deficits depending on the large artery involved
- Myocardial infarction: Chest pain radiating to left arm and neck, sweating, dyspnea
- Acute ischemic limb: Pain, pallor, poikilothermia, pulselessness, paresthesia, paralysis
To view the history and symptoms of COVID-19, click here.
Physical Examination
Pulmonary Embolism
Physical examination of patients with Pulmonary Embolism is usually remarkable for:
Deep Vein Thrombosis
Physical examination of patients with Deep Vein Thrombosis includes:
- Unilateral swelling/edema with a difference in diameters
- Warmth
- Tenderness
- Dilated veins
- Homan's sign may also be seen but is unreliable.
- Ischemic Stroke: Focal neurological deficits depending on the vessel involved
- Myocardial Infarction: Uncomfortable appearing patient with diaphoresis
- Ischemic Limb: Pallor, poikilothermia, pulselessness, paresthesia, paralysis
To view the complete physical examination in COVID-19, click here.
Laboratory Findings
- An elevated concentration of serum/blood pro-coagulant factors is diagnostic of coagulopathy associated with COVID-19.
- Laboratory findings consistent with the diagnosis of COVID-19 associated coagulopathy include:[23][24]
- Coagulation testing- pro-coagulant profile which includes:
- Platelet Counts- Normal or increased
- Prothrombin time (PT) and activated partial thromboplastin time (aPTT)- normal or slightly prolonged
- Fibrinogen- increased
- D-dimer- increased
- Factor VIII activity- increased
- VWF antigen- increased
- Protein C, Protein S, Antithrombin III - slightly decreased
- Coagulation testing- pro-coagulant profile which includes:
- TEG findings:[25][26]
- Reaction time (R) - decreased
- Clot formation time (K)- decreased
- Maximum amplitude (MA)- increased
- Clot lysis at 30 minutes (LY30)- decreased
To view the laboratory findings on COVID-19, click here.
Electrocardiogram
An ECG may be helpful in the diagnosis of pulmonary embolism or myocardial infarction caused due to hypercoagulability in COVID-19.
- Findings on an ECG suggestive of/diagnostic of pulmonary embolism include tachycardia and S1Q3T3 pattern.
- Findings on an ECG suggestive of/diagnostic of myocardial infarction include STE elevation in various leads.
- To view the electrocardiogram findings on COVID-19, click here.
X-ray
- There are no specific x-ray findings associated with PE.
- However, an x-ray may be helpful in ruling out other causes with similar symptoms like pneumonia, cardiogenic causes of dyspnea, and pneumothorax.
- To view the x-ray finidings on COVID-19, click here.
Echocardiography or Ultrasound
- Echocardiography may be helpful in the diagnosis of pulmonary embolism.
- Compressive Ultrasound may be helpful in the diagnosis of deep vein thrombosis
- To view the echocardiographic findings on COVID-19, click here.
CT scan
CTPA and Ventilation Perfusion(V/Q) Scan
- Prompt diagnosis of PE in COVID-19 patient is difficult in this regard that various symptoms of COVID-19 overlap with that of pulmonary embolism. American Society of Hematology provides the following guidelines regarding the diagnosis of pulmonary embolism:[27]
- Normal d-dimers level in a patient with low to moderate pretest probability is sufficient to rule out the diagnosis of PE. D-dimers level is usually elevated in COVID-19 patients. This is not applicable to a patient with a high pretest probability.
- Inpatient with suspected PE with symptoms like hypotension, tachycardia, and sudden drop in oxygen saturation with a high pretest probability of PE, computed tomography with pulmonary angiography is used for the diagnosis. Contraindication to the use of CTPA warrants investigation with ventilation/perfusion scan.
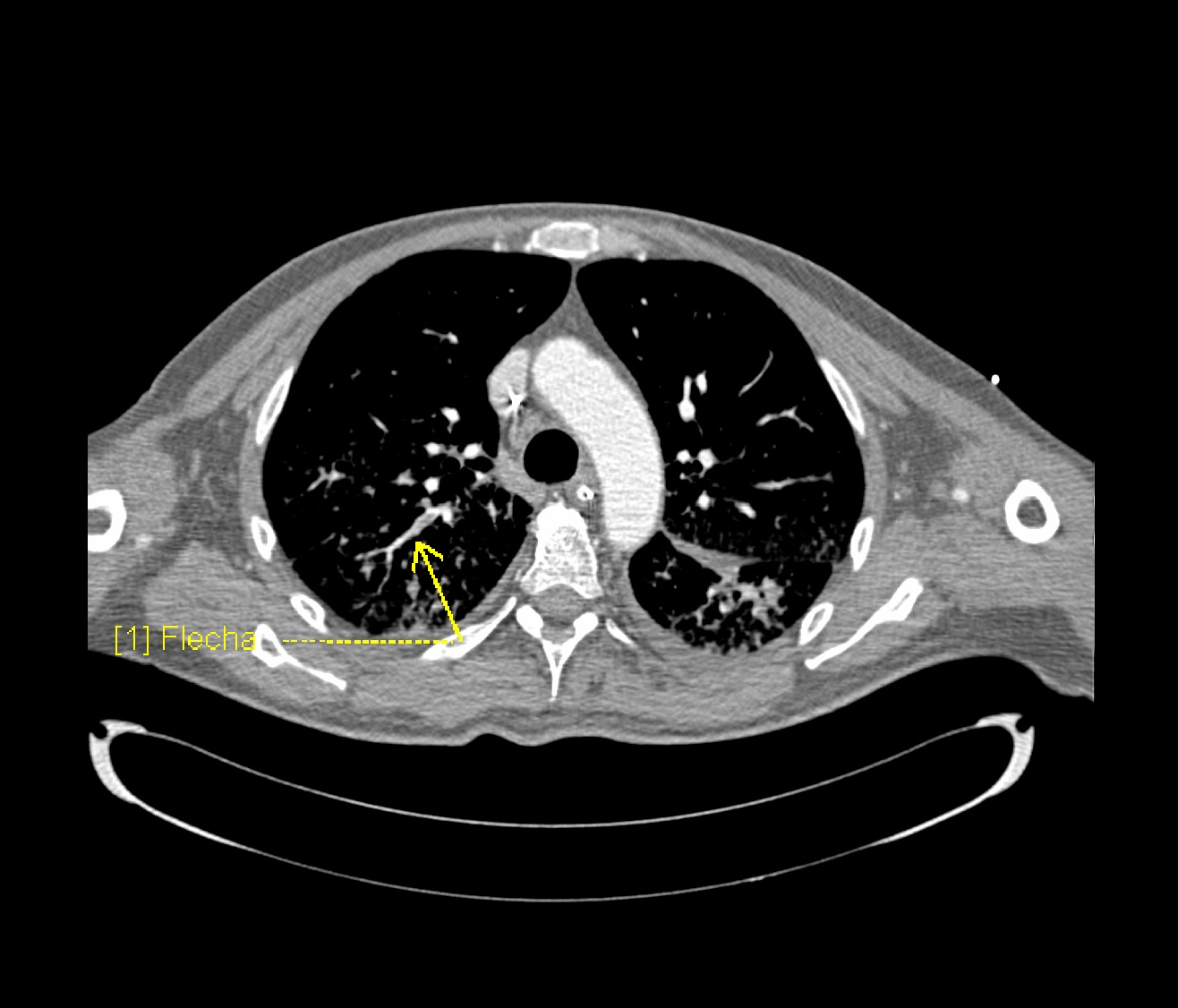
To view the CT scan findings on COVID-19, click here.
MRI
- There are no MRI findings associated with coagulopathy of COVID-19 unless it is used to diagnose and evaluate an ischemic stroke caused by it.
- To view the MRI findings on COVID-19, click here.
Other Imaging Findings
There are no other imaging findings associated with coagulopathy of COVID-19.
Other Diagnostic Studies
- To view other diagnostic studies for COVID-19, click here.
Treatment
Medical Therapy
Prophylactic dose of anticoagulation
- Drug- Low-molecular-weight heparin (LMWH) is preferred over unfractionated heparin to reduce contact with the patient.
- Unfractionated heparin may be used in case of unavailability or severe renal impairment.
Indications:
- All inpatients in the absence of active bleeding
- To be held only if platelet counts fall below 25 x 109/L, or fibrinogen less than 0.5 g/L
- In case of a history of heparin-induced thrombocytopenia(HIT), fondaparinux is used.
Intermediate or therapeutic dose anticoagulation
- Preferred regimen: Enoxaparin 40 to 60 mg once daily[28]
Indications:
- Critically ill patients or ICU patients[29]
- According to a study, a better prognosis was seen in patients who met the SIC (Sepsis-induced coagulopathy) criteria or had marked elevated D-dimer levels and were put on anticoagulant therapy(mainly with low molecular weight heparin) [30]
Therapeutic/ full-dose anticoagulation
- Preferred regimen: Enoxaparin 1 mg/kg every 12 hours
Indications:
- Suspected VTE/PE
- Confirmed VTE/PE
- Clotting in catheters, or extracorporeal circuits like ECMO and CRRT. [31]
Post-discharge thromboprophylaxis
- Drug and dose- Regulatory-approved regimen[32]
- Preferred regimen (1): Betrixaban 160 mg on day 1, followed by 80 mg once daily for 35-42 days
- Preferred regimen (2): Rivaroxaban 10 mg daily for 31-39 days
Indications:
- Patients with documented venous thromboembolism (VTE) require thromboprophylaxis for up to 90 days after discharge.
- Some patients who do not have VTE but require extended thromboprophylaxis include:
- Acute medical illness, older age, immobilization, recent surgery, or trauma.
- Most of these criteria are met by patients with COVID-19, and they require thromboprophylaxis for up to 90 days after discharge.[33]
Bleeding in COVID-19
- Transfusion and replacement; discontinuation or reversal of anticoagulation are the mainstay of treatment
- Transfuse platelets if the platelet count is less than 50 x 109/L
- Administer plasma if the INR is above 1.8
- Order fibrinogen concentrate or cryoprecipitate if the fibrinogen level is less than 1.5 g/L
- To view medical treatment for COVID-19, click here.
Surgery
Surgical intervention is not recommended for the management of COVID-19 associated coagulopathy.
Primary Prevention
- Since there is no vaccine for COVID-19 there are plenty of primary prevention suggested from CDC such as:[34]
- Hand washing every 10 minutes.
- Using alcoholic hand sanitizer.
- Self quarantine for two weeks if symptomatic.
- To view the primary prevention measures of COVID-19, click here.
Secondary Prevention
- WHO recommends home care for patients with suspected COVID-19 who present with mild symptoms:[35]
- Family members of an infected patient are better to wear masks.
- Using separate bathroom and bedroom by the infected person.
- Using antipyretics and analgesics for fever, myalgias, and headaches
- To view the secondary prevention measures of COVID-19, click here.
References
- ↑ Lu, Jian; Cui, Jie; Qian, Zhaohui; Wang, Yirong; Zhang, Hong; Duan, Yuange; Wu, Xinkai; Yao, Xinmin; Song, Yuhe; Li, Xiang; Wu, Changcheng; Tang, Xiaolu (2020). "On the origin and continuing evolution of SARS-CoV-2". National Science Review. 7 (6): 1012–1023. doi:10.1093/nsr/nwaa036. ISSN 2095-5138.
- ↑ Becker RC (2020). "COVID-19 update: Covid-19-associated coagulopathy". J Thromb Thrombolysis. 50 (1): 54–67. doi:10.1007/s11239-020-02134-3. PMC 7225095 Check
|pmc=value (help). PMID 32415579 Check|pmid=value (help). - ↑ 3.0 3.1 Becker RC (2020). "COVID-19 update: Covid-19-associated coagulopathy". J Thromb Thrombolysis. doi:10.1007/s11239-020-02134-3. PMC 7225095 Check
|pmc=value (help). PMID 32415579 Check|pmid=value (help). - ↑ Tang N, Li D, Wang X, Sun Z (2020). "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia". J Thromb Haemost. 18 (4): 844–847. doi:10.1111/jth.14768. PMC 7166509 Check
|pmc=value (help). PMID 32073213 Check|pmid=value (help). - ↑ Escher R, Breakey N, Lämmle B (2020). "Severe COVID-19 infection associated with endothelial activation". Thromb Res. 190: 62. doi:10.1016/j.thromres.2020.04.014. PMC 7156948 Check
|pmc=value (help). PMID 32305740 Check|pmid=value (help). - ↑ Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G (2020). "COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons". Cytokine Growth Factor Rev. 53: 66–70. doi:10.1016/j.cytogfr.2020.05.002. PMC 7204669 Check
|pmc=value (help). PMID 32418715 Check|pmid=value (help). - ↑ Luiten PG (1981). "Two visual pathways to the telencephalon in the nurse shark (Ginglymostoma cirratum). I. Retinal projections". J Comp Neurol. 196 (4): 531–8. doi:10.1002/cne.901960402. PMID 7204669.
- ↑ Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020). "SARS-CoV-2 infection: The role of cytokines in COVID-19 disease". Cytokine Growth Factor Rev. doi:10.1016/j.cytogfr.2020.06.001. PMC 7265853 Check
|pmc=value (help). PMID 32513566 Check|pmid=value (help). - ↑ Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A (2020). "COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia?". Lancet. 395 (10239): 1758–1759. doi:10.1016/S0140-6736(20)31209-5. PMC 7247793 Check
|pmc=value (help). PMID 32464112 Check|pmid=value (help). - ↑ Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP; et al. (2020). "Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19". N Engl J Med. 383 (3): 288–290. doi:10.1056/NEJMc2013656. PMC 7217555 Check
|pmc=value (help). PMID 32369280 Check|pmid=value (help). - ↑ Levi M, Thachil J, Iba T, Levy JH (2020). "Coagulation abnormalities and thrombosis in patients with COVID-19". Lancet Haematol. 7 (6): e438–e440. doi:10.1016/S2352-3026(20)30145-9. PMC 7213964 Check
|pmc=value (help). PMID 32407672 Check|pmid=value (help). - ↑ Levi M, Toh CH, Thachil J, Watson HG (2009). "Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology". Br J Haematol. 145 (1): 24–33. doi:10.1111/j.1365-2141.2009.07600.x. PMID 19222477.
- ↑ Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM; et al. (2020). "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thromb Res. 191: 145–147. doi:10.1016/j.thromres.2020.04.013. PMC 7146714 Check
|pmc=value (help). PMID 32291094 Check|pmid=value (help). - ↑ 14.0 14.1 Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM; et al. (2020). "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thromb Res. 191: 145–147. doi:10.1016/j.thromres.2020.04.013. PMC 7146714 Check
|pmc=value (help). PMID 32291094 Check|pmid=value (help). - ↑ Woringer V, Renevey F (1982). "[A case of gonococcal arthritis at a young age]". Rev Med Suisse Romande. 102 (9): 863–5. PMID 7146714.
- ↑ Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA; et al. (2020). "Incidence of venous thromboembolism in hospitalized patients with COVID-19". J Thromb Haemost. doi:10.1111/jth.14888. PMID 32369666 Check
|pmid=value (help). - ↑ Wu, Chaomin; Chen, Xiaoyan; Cai, Yanping; Xia, Jia’an; Zhou, Xing; Xu, Sha; Huang, Hanping; Zhang, Li; Zhou, Xia; Du, Chunling; Zhang, Yuye; Song, Juan; Wang, Sijiao; Chao, Yencheng; Yang, Zeyong; Xu, Jie; Zhou, Xin; Chen, Dechang; Xiong, Weining; Xu, Lei; Zhou, Feng; Jiang, Jinjun; Bai, Chunxue; Zheng, Junhua; Song, Yuanlin (2020). "Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China". JAMA Internal Medicine. 180 (7): 934. doi:10.1001/jamainternmed.2020.0994. ISSN 2168-6106.
- ↑ "Management of Patients with Confirmed 2019-nCoV | CDC".
- ↑ Levy, Jerrold H.; Connors, Jean M. (2020). "COVID-19 and its implications for thrombosis and anticoagulation". Blood. 135 (23): 2033–2040. doi:10.1182/blood.2020006000. ISSN 0006-4971.
- ↑ Barrett CD, Moore HB, Yaffe MB, Moore EE (2020). "ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment". J Thromb Haemost. doi:10.1111/jth.14860. PMID 32302462 Check
|pmid=value (help). - ↑ Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z; et al. (2020). "D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19". J Thromb Haemost. 18 (6): 1324–1329. doi:10.1111/jth.14859. PMC 7264730 Check
|pmc=value (help). PMID 32306492 Check|pmid=value (help). - ↑ Tang N, Li D, Wang X, Sun Z (2020). "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia". J Thromb Haemost. 18 (4): 844–847. doi:10.1111/jth.14768. PMC 7166509 Check
|pmc=value (help). PMID 32073213 Check|pmid=value (help). - ↑ Wasserbauer R, Beranová M, Vancurová D, Dolezel B (1990). "Biodegradation of polyethylene foils by bacterial and liver homogenates". Biomaterials. 11 (1): 36–40. doi:10.1016/0142-9612(90)90049-v. PMID 2302448.
- ↑ Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M; et al. (2020). "The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome". J Thromb Haemost. 18 (7): 1747–1751. doi:10.1111/jth.14854. PMID 32302448 Check
|pmid=value (help). - ↑ Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V; et al. (2020). "Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis". J Thromb Haemost. 18 (7): 1738–1742. doi:10.1111/jth.14850. PMID 32302438 Check
|pmid=value (help). - ↑ Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M; et al. (2020). "The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome". J Thromb Haemost. 18 (7): 1747–1751. doi:10.1111/jth.14854. PMID 32302448 Check
|pmid=value (help). - ↑ Lu Y, Macapinlac HA (2020). "Perfusion SPECT/CT to diagnose pulmonary embolism during COVID-19 pandemic". Eur J Nucl Med Mol Imaging. 47 (9): 2064–2065. doi:10.1007/s00259-020-04851-6. PMC 7205478 Check
|pmc=value (help). PMID 32383092 Check|pmid=value (help). - ↑ Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020). "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy". J Thromb Haemost. 18 (5): 1094–1099. doi:10.1111/jth.14817. PMID 32220112 Check
|pmid=value (help). - ↑ Akima S, McLintock C, Hunt BJ (2020). "RE: ISTH interim guidance to recognition and management of coagulopathy in COVID-19". J Thromb Haemost. doi:10.1111/jth.14853. PMID 32302442 Check
|pmid=value (help). - ↑ Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020). "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy". J Thromb Haemost. 18 (5): 1094–1099. doi:10.1111/jth.14817. PMID 32220112 Check
|pmid=value (help). - ↑ Akima S, McLintock C, Hunt BJ (2020). "RE: ISTH interim guidance to recognition and management of coagulopathy in COVID-19". J Thromb Haemost. doi:10.1111/jth.14853. PMID 32302442 Check
|pmid=value (help). - ↑ Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A; et al. (2016). "Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients". N Engl J Med. 375 (6): 534–44. doi:10.1056/NEJMoa1601747. PMID 27232649.
- ↑ Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E; et al. (2020). "COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review". J Am Coll Cardiol. 75 (23): 2950–2973. doi:10.1016/j.jacc.2020.04.031. PMC 7164881 Check
|pmc=value (help). PMID 32311448 Check|pmid=value (help). - ↑ https://www.cdc.gov/coronavirus/2019-ncov/index.html. Missing or empty
|title=(help) - ↑ "Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts".