Idiopathic interstitial pneumonia pathophysiology: Difference between revisions
Ahmed Zaghw (talk | contribs) No edit summary |
Ahmed Zaghw (talk | contribs) |
||
| (126 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{Idiopathic interstitial pneumonia}} | ||
{{CMG}}; {{AE}} {{chetan}} | {{CMG}}; {{AE}} {{chetan}} | ||
==Overview== | ==Overview== | ||
Idiopathic interstitial pneumonia (IIP) is a disease entity that can be histologically classified into different categories. [[Idiopathic pulmonary fibrosis]] has the same features as that of usual interstitial pneumonia (UIP) whereas no specific pattern or common feature is noted among the other types of IIP. The pathophysiology of IIP can be summarized in the following three stages: recruitment of inflammatory cells, abnormal [[collagen]] deposition and fibroblastic proliferation and lastly progression to [[fibrosis]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
===[[Idiopathic pulmonary fibrosis]]=== | ===Idiopathic Pulmonary Fibrosis=== | ||
Idiopathic pulmonary fibrosis (IPF) has often been considered an [[autoimmunity|autoimmune disease]]. However, it is perhaps better characterized as an abnormal and excessive deposition of fibrotic tissue in the pulmonary interstitium with minimal associated [[inflammation]].<ref name="Selman">{{cite journal |last=Selman |first=Moisés |coauthors=Talmadge E. King, Jr.; and Annie Pardo |title=Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy |journal=Annals of Internal Medicine |year=2001 |volume=134 |number=2 |pages=136-51|url=http://www.annals.org/cgi/content/abstract/134/2/136}}</ref> [[Autoantibodies]], a hallmark of autoimmune diseases, are found in a minority of patients with true idiopathic pulmonary fibrosis. Moreover, many autoimmune diseases that are associated with pulmonary fibrosis such as [[scleroderma]], are more frequently associated with a related but more inflammatory disease, nonspecific interstitial pneumonitis.<ref>{{cite journal |last=King, Jr. |first=Talmadge E. |title=Centennial review: clinical advances in the diagnosis and therapy of the interstitial lung diseases|url=http://ajrccm.atsjournals.org/cgi/content/full/172/3/268 |journal=American Journal of Respiratory and Critical Care Medicine |year=2005 |volume=172 |number=3 |pages=268-79}}</ref> IPF is associated with [[smoking]]<ref>{{cite journal |last=Nagai |first=Sonoko |coauthors=Yuma Hoshino, Michio Hayashi, Isao Ito |title=Smoking-related interstitial lung diseases |url=http://www.co-pulmonarymedicine.com/pt/re/copulmonary/abstract.00063198-200009000-00005.htm |journal=Current Opinion in Pulmonary Medicine |volume=6 |issue=5 |pages=415-9 |year=2000 |pmid=10958232}}</ref> and exhibits some dependency on the amount of smoking.<ref>{{cite journal |last=Baumgartner |first=KB |coauthors=Samet JM, Stidley CA, Colby TV, Waldron JA |title=Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis |url= http://ajrccm.atsjournals.org/cgi/content/short/155/1/242 |journal=American Journal of Respiratory and Critical Care Medicine |volume=155 |number=1 |pages=242-248 |year=1997 |pmid=9001319}}</ref> | |||
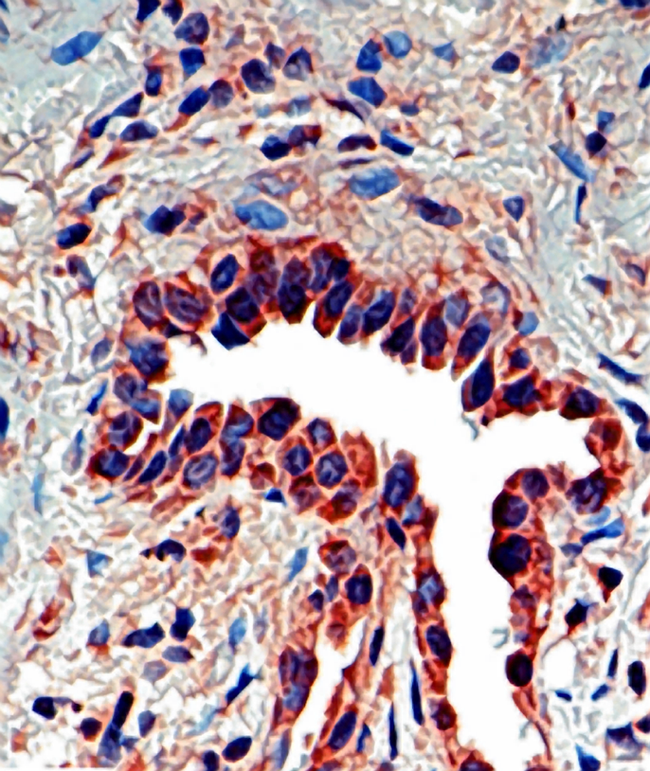
Shown below is an image depicting IPF: | |||
[[File:Journal.pmed.0020314.g001 Osteopontin .png|300px|Idiopathic Pulmonary Fibrosis]] | |||
===Idiopathic Non-specific Interstitial Pneumonia === | |||
As the name implies, idiopathic non-specific interstitial pneumonia (NSIP) has very inconsistent and non-specific findings.<ref name="Flaherty-2001">{{Cite journal | last1 = Flaherty | first1 = KR. | last2 = Travis | first2 = WD. | last3 = Colby | first3 = TV. | last4 = Toews | first4 = GB. | last5 = Kazerooni | first5 = EA. | last6 = Gross | first6 = BH. | last7 = Jain | first7 = A. | last8 = Strawderman | first8 = RL. | last9 = Flint | first9 = A. | title = Histopathologic variability in usual and nonspecific interstitial pneumonias. | journal = Am J Respir Crit Care Med | volume = 164 | issue = 9 | pages = 1722-7 | month = Nov | year = 2001 | doi = 10.1164/ajrccm.164.9.2103074 | PMID = 11719316 }}</ref><ref name="Cottin-1998">{{Cite journal | last1 = Cottin | first1 = V. | last2 = Donsbeck | first2 = AV. | last3 = Revel | first3 = D. | last4 = Loire | first4 = R. | last5 = Cordier | first5 = JF. | title = Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients. | journal = Am J Respir Crit Care Med | volume = 158 | issue = 4 | pages = 1286-93 | month = Oct | year = 1998 | doi = 10.1164/ajrccm.158.4.9802119 | PMID = 9769293 }}</ref><ref name="Katzenstein-2000">{{Cite journal | last1 = Katzenstein | first1 = AL. | last2 = Myers | first2 = JL. | title = Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: classification and diagnostic criteria. | journal = Am J Surg Pathol | volume = 24 | issue = 1 | pages = 1-3 | month = Jan | year = 2000 | doi = | PMID = 10632482 }}</ref> | |||
Changes similar to other cases of interstitial pneumonia are seen which are migration of inflammatory cells in the alveolar septa and its widening with or without fibrosis. | |||
NSIP can be divided into three groups based on histopathological changes.<ref name="Katzenstein-1994">{{Cite journal | last1 = Katzenstein | first1 = AL. | last2 = Fiorelli | first2 = RF. | title = Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. | journal = Am J Surg Pathol | volume = 18 | issue = 2 | pages = 136-47 | month = Feb | year = 1994 | doi = | PMID = 8291652 }}</ref> Shown below is a table summarizing the pathological findings in the three groups of NSIP: | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0" | '''Stage''' | |||
| align="center" style="background:#f0f0f0 " | '''Pathological Feature ''' | |||
|- | |||
| Group I || Inflammatory cells predominant stage | |||
|- | |||
| Group II|| Accompanying fibrosis | |||
|- | |||
| Group III|| Fibrosis prevalent | |||
|- | |||
|} | |||
=== | Bronchoalveloar lavage (BAL) reveals the presence of [[lymphocytes]] in the alveolar septum which is an evidence of the involvement of the immune system.<ref name="Cottin-1998">{{Cite journal | last1 = Cottin | first1 = V. | last2 = Donsbeck | first2 = AV. | last3 = Revel | first3 = D. |last4 = Loire | first4 = R. | last5 = Cordier | first5 = JF. | title = Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients. | journal = Am J Respir Crit Care Med | volume = 158 | issue = 4 | pages = 1286-93 | month = Oct | year = 1998 | doi = 10.1164/ajrccm.158.4.9802119 | PMID = 9769293 }}</ref><ref name="Fujita-1999">{{Cite journal | last1 = Fujita |first1 = J. | last2 = Yamadori | first2 = I. | last3 = Suemitsu | first3 = I. | last4 = Yoshinouchi | first4 = T. | last5 = Ohtsuki | first5 = Y. | last6 = Yamaji | first6 = Y. | last7 = Kamei | first7 = T. |last8 = Kobayashi | first8 = M. | last9 = Nakamura | first9 = Y. | title = Clinical features of non-specific interstitial pneumonia. | journal = Respir Med | volume = 93 | issue = 2 | pages = 113-8 | month = Feb | year = 1999 | doi = | PMID = 10464862 }}</ref><ref name="Nagai-1998">{{Cite journal | last1 = Nagai | first1 = S. | last2 = Kitaichi | first2 = M. | last3 = Itoh | first3 = H. | last4 = Nishimura |first4 = K. | last5 = Izumi | first5 = T. | last6 = Colby | first6 = TV. | title = Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. | journal = Eur Respir J | volume = 12 | issue = 5 | pages = 1010-9 | month = Nov | year = 1998 | doi = | PMID = 9863989 }}</ref><ref name="Park-1996">{{Cite journal | last1 = Park | first1 = CS. | last2 = Jeon | first2 = JW. | last3 = Park | first3 = SW. | last4 = Lim | first4 = GI. | last5 = Jeong | first5 = SH. | last6 = Uh | first6 = ST. | last7 = Park | first7 = JS. | last8 = Choi | first8 = DL. | last9 = Jin | first9 = SY. | title = Nonspecific interstitial pneumonia/fibrosis: clinical manifestations, histologic and radiologic features. | journal = Korean J Intern Med | volume = 11 | issue = 2 | pages = 122-32 | month = Jun| year = 1996 | doi = | PMID = 8854648 }}</ref> | ||
A greater number of [[dendritic cells]] (DC), which help in antigen presentation, are visualized in close association with [[CD4]] and [[CD8]] lymphocytes in the biopsy of NSIS patients than in UIP.<ref name="Shimizu-2002">{{Cite journal | last1 = Shimizu | first1 = S. | last2 = Yoshinouchi | first2 = T. | last3 = Ohtsuki | first3 = Y. | last4 = Fujita | first4 = J. |last5 = Sugiura | first5 = Y. | last6 = Banno | first6 = S. | last7 = Yamadori | first7 = I. | last8 = Eimoto | first8 = T. | last9 = Ueda | first9 = R. | title = The appearance of S-100 protein-positive dendritic cells and the distribution of lymphocyte subsets in idiopathic nonspecific interstitial pneumonia. | journal = Respir Med | volume = 96 | issue = 10 | pages = 770-6 | month = Oct | year = 2002 | doi = | PMID = 12412975 }}</ref> | |||
[[Fibroblasts]] are the key cells involved in fibrotic lung diseases.<ref name="Selman-2001">{{Cite journal | last1 = Selman | first1 = M. | last2 = King | first2 = TE. |last3 = Pardo | first3 = A. | title = Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. | journal = Ann Intern Med | volume = 134 | issue = 2 | pages = 136-51 | month = Jan | year = 2001 | doi = | PMID = 11177318 }}</ref> | |||
The pathological mechanism of NSIP involves: | |||
:* Epithelial injury and dysregulated repair (major role)<ref name="Ishii-">{{Cite journal | last1 = Ishii | first1 = H. | last2 = Mukae | first2 = H. | last3 = Kadota | first3 = J. | last4 = Fujii |first4 = T. | last5 = Abe | first5 = K. | last6 = Ashitani | first6 = J. | last7 = Kohno | first7 = S. | title = Increased levels of interleukin-18 in bronchoalveolar lavage fluid of patients with idiopathic nonspecific interstitial pneumonia. | journal = Respiration | volume = 72 | issue = 1 | pages = 39-45 | month = | year = | doi = 10.1159/000083399 | PMID = 15753633 }}</ref> | |||
:*[[Cytokines]] | |||
:*Proteins like epimorphin (a cell surface associated protein)<ref name="Terasaki-2000">{{Cite journal | last1 = Terasaki | first1 = Y. |last2 = Fukuda | first2 = Y. | last3 = Ishizaki | first3 = M. | last4 = Yamanaka | first4 = N. | title = Increased expression of epimorphin in bleomycin-induced pulmonary fibrosis in mice. | journal = Am J Respir Cell Mol Biol | volume = 23 | issue = 2 | pages = 168-74 | month = Aug | year = 2000 | doi = 10.1165/ajrcmb.23.2.3973 | PMID = 10919982 }}</ref> [[matrix metalloproteinases]],<ref name="Suga-2000">{{Cite journal | last1 = Suga |first1 = M. | last2 = Iyonaga | first2 = K. | last3 = Okamoto | first3 = T. | last4 = Gushima | first4 = Y. | last5 = Miyakawa | first5 = H. | last6 = Akaike | first6 = T. | last7 = Ando | first7 = M. | title = Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. | journal = Am J Respir Crit Care Med | volume = 162 | issue = 5 | pages = 1949-56 | month = Nov | year = 2000 | doi = 10.1164/ajrccm.162.5.9906096 | PMID = 11069839 }}</ref> heat shock protein,<ref name="Kakugawa-2005">{{Cite journal | last1 = Kakugawa | first1 = T. | last2 = Mukae | first2 = H. | last3 = Hayashi | first3 = T. |last4 = Ishii | first4 = H. | last5 = Nakayama | first5 = S. | last6 = Sakamoto | first6 = N. | last7 = Yoshioka | first7 = S. | last8 = Sugiyama | first8 = K. | last9 = Mine | first9 = M. | title = Expression of HSP47 in usual interstitial pneumonia and nonspecific interstitial pneumonia. | journal = Respir Res | volume = 6 | issue = | pages = 57 | month = | year = 2005 | doi = 10.1186/1465-9921-6-57 | PMID = 15955241 }}</ref> surfactant protein C<ref name="Thomas-2002">{{Cite journal | last1 = Thomas | first1 = AQ. | last2 = Lane | first2 = K. | last3 = Phillips | first3 = J. | last4 = Prince | first4 = M. | last5 = Markin | first5 = C. | last6 = Speer | first6 = M. | last7 = Schwartz | first7 = DA. | last8 = Gaddipati | first8 = R. | last9 = Marney | first9 = A. | title = Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. | journal = Am J Respir Crit Care Med | volume = 165 | issue = 9 | pages = 1322-8 | month = May | year = 2002 | doi = 10.1164/rccm.200112-123OC | PMID = 11991887 }}</ref><ref name="Brasch-2004">{{Cite journal | last1 = Brasch | first1 = F. | last2 = Griese | first2 = M. | last3 = Tredano | first3 = M. | last4 = Johnen | first4 = G. | last5 = Ochs | first5 = M. | last6 = Rieger | first6 = C. | last7 = Mulugeta | first7 = S. | last8 = Müller | first8 = KM. | last9 = Bahuau | first9 = M. | title = Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. | journal = Eur Respir J | volume = 24 | issue = 1 | pages = 30-9 | month = Jul | year = 2004 | doi = | PMID = 15293602 }}</ref><ref name="Nogee-2001">{{Cite journal | last1 = Nogee | first1 = LM. | last2 = Dunbar | first2 = AE. | last3 = Wert | first3 = SE. | last4 = Askin | first4 = F. | last5 = Hamvas | first5 = A. | last6 = Whitsett | first6 = JA.| title = A mutation in the surfactant protein C gene associated with familial interstitial lung disease. | journal = N Engl J Med | volume = 344 | issue = 8 | pages = 573-9 | month = Feb | year = 2001 | doi = 10.1056/NEJM200102223440805 | PMID = 11207353 }}</ref><ref name="Stevens-2005">{{Cite journal | last1 = Stevens | first1 = PA. | last2 = Pettenazzo | first2 = A. | last3 = Brasch | first3 = F. | last4 = Mulugeta | first4 = S. | last5 = Baritussio | first5 = A. | last6 = Ochs | first6 = M. | last7 = Morrison | first7 = L. | last8 = Russo | first8 = SJ. | last9 = Beers | first9 = MF. | title = Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene. | journal = Pediatr Res | volume = 57 | issue = 1 |pages = 89-98 | month = Jan | year = 2005 | doi = 10.1203/01.PDR.0000147567.02473.5A | PMID = 15557112 }}</ref><ref name="Chibbar-2004">{{Cite journal | last1 = Chibbar | first1 = R. | last2 = Shih | first2 = F. | last3 = Baga | first3 = M. | last4 = Torlakovic | first4 = E. | last5 = Ramlall | first5 = K. | last6 = Skomro | first6 = R. | last7 = Cockcroft | first7 = DW. | last8 = Lemire | first8 = EG. | title = Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis. | journal = Mod Pathol | volume = 17 | issue = 8 | pages = 973-80 |month = Aug | year = 2004 | doi = 10.1038/modpathol.3800149 | PMID = 15133475 }}</ref> | |||
:*The coagulation system<ref name="Eitzman-1996">{{Cite journal | last1 = Eitzman | first1 = DT. | last2 = McCoy | first2 = RD. | last3 = Zheng |first3 = X. | last4 = Fay | first4 = WP. | last5 = Shen | first5 = T. | last6 = Ginsburg | first6 = D. | last7 = Simon | first7 = RH. | title = Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. | journal = J Clin Invest | volume = 97 | issue = 1 | pages = 232-7 | month = Jan | year = 1996 | doi = 10.1172/JCI118396 | PMID = 8550840 }}</ref><ref name="Kim-">{{Cite journal | last1 = Kim | first1 = KK. | last2 = Flaherty | first2 = KR. | last3 = Long | first3 = Q. | last4 = Hattori | first4 = N. | last5 = Sisson | first5 = TH. |last6 = Colby | first6 = TV. | last7 = Travis | first7 = WD. | last8 = Martinez | first8 = FJ. | last9 = Murray | first9 = S. | title = A plasminogen activator inhibitor-1 promoter polymorphism and idiopathic interstitial pneumonia. | journal = Mol Med | volume = 9 | issue = 1-2 | pages = 52-6 | month = | year = | doi = | PMID = 12765340 }}</ref> | |||
:*Intercellular adhesion molecules-1,<ref name="Takehara-2001">{{Cite journal | last1 = Takehara | first1 = H. | last2 = Tada | first2 = S. | last3 = Kataoka | first3 = M. | last4 = Matsuo | first4 = K. | last5 = Ueno | first5 = Y. | last6 = Ozaki | first6 = S. | last7 = Miyake | first7 = T. | last8 = Fujimori |first8 = Y. | last9 = Yamadori | first9 = I. | title = Intercellular adhesion molecule-1 in patients with idiopathic interstitial pneumonia. | journal = Acta Med Okayama | volume = 55 | issue = 4 | pages = 205-11 | month = Aug | year = 2001 | doi = | PMID = 11512562 }}</ref> IL-4, IL-13, IL-18<ref name="Jakubzick-2004">{{Cite journal | last1 = Jakubzick | first1 = C. | last2 = Choi | first2 = ES. | last3 = Kunkel | first3 = SL. |last4 = Evanoff | first4 = H. | last5 = Martinez | first5 = FJ. | last6 = Puri | first6 = RK. | last7 = Flaherty | first7 = KR. | last8 = Toews | first8 = GB. | last9 = Colby | first9 = TV. | title = Augmented pulmonary IL-4 and IL-13 receptor subunit expression in idiopathic interstitial pneumonia. | journal = J Clin Pathol | volume = 57 | issue = 5 | pages = 477-86 | month = May | year = 2004 | doi = | PMID = 15113854 }}</ref> | |||
:*Interferon-gamma | |||
:*Pro fibrotic chemokines<ref name="Choi-2004">{{Cite journal | last1 = Choi | first1 = ES.| last2 = Jakubzick | first2 = C. | last3 = Carpenter | first3 = KJ. | last4 = Kunkel | first4 = SL. | last5 = Evanoff | first5 = H. | last6 = Martinez | first6 = FJ. | last7 = Flaherty | first7 = KR. | last8 = Toews | first8 = GB. | last9 = Colby | first9 = TV. | title = Enhanced monocyte chemoattractant protein-3/CC chemokine ligand-7 in usual interstitial pneumonia. | journal = Am J Respir Crit Care Med | volume = 170 | issue = 5 | pages = 508-15 | month = Sep | year = 2004 | doi = 10.1164/rccm.200401-002OC | PMID = 15191918 }}</ref> | |||
=== | :*CCL7, and CCL5 | ||
:*Lymphocytes<ref name="Keogh-2005">{{Cite journal | last1 = Keogh | first1 = KA. | last2 = Limper | first2 = AH. | title = Characterization of lymphocyte populations in nonspecific interstitial pneumonia.| journal = Respir Res | volume = 6 | issue = | pages = 137 | month = | year = 2005 | doi = 10.1186/1465-9921-6-137 | PMID = 16287509 }}</ref> | |||
:*Dendritic cells | |||
:*Fibroblasts | |||
Some common associations between Idiopathic Non-specific Interstitial Pneumonia (NSIP) and Usual Interstitial Pneumonia (UIP) have been noted. Histologically patients can manifest lesions of both UIP and NSIP simultaneously. The reason for this presentation is still unknown but environmental exposures and genetic mutations could be some of the causes.<ref name="Monaghan-2004">{{Cite journal | last1 = Monaghan | first1 = H. | last2 = Wells | first2 = AU. | last3 = Colby | first3 = TV. | last4 = du Bois | first4 = RM. | last5 = Hansell | first5 = DM. | last6 = Nicholson | first6 = AG. |title = Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. | journal = Chest | volume = 125 | issue = 2 | pages = 522-6 |month = Feb | year = 2004 | doi = | PMID = 14769733 }}</ref><ref name="Flaherty-2001">{{Cite journal | last1 = Flaherty | first1 = KR. | last2 = Travis | first2 = WD. | last3 = Colby | first3 = TV. | last4 = Toews | first4 = GB. | last5 = Kazerooni | first5 = EA. | last6 = Gross | first6 = BH. | last7 = Jain | first7 = A. | last8 = Strawderman | first8 = RL. | last9 = Flint | first9 = A. | title = Histopathologic variability in usual and nonspecific interstitial pneumonias. | journal = Am J Respir Crit Care Med | volume = 164 | issue = 9 | pages = 1722-7 | month = Nov | year = 2001 | doi = 10.1164/ajrccm.164.9.2103074| PMID = 11719316 }}</ref> | |||
Features differentiating NSIP and UIP include: | |||
=== | :* Irregular [[fibrosis]] | ||
:* Honeycombing | |||
:* Fibroblast predominant foci | |||
:* Fibroblasts secreting transforming growth factor–Beta (TGF-β) and fibronectin<ref name="Miki-2000">{{Cite journal | last1 = Miki | first1 = H. | last2 = Mio | first2 = T. | last3 = Nagai | first3 = S. | last4 = Hoshino | first4 = Y. | last5 = Nagao | first5 = T. | last6 = Kitaichi | first6 = M. | last7 = Izumi | first7 = T. | title = Fibroblast contractility: usual interstitial pneumonia and nonspecific interstitial pneumonia. | journal = Am J Respir Crit Care Med | volume = 162 | issue = 6 | pages = 2259-64 | month = Dec | year = 2000 | doi = 10.1164/ajrccm.162.6.9812029 | PMID = 11112149 }}</ref> | |||
===Respiratory Bronchiolitis-Interstitial Lung Disease === | |||
*[[Cigarette smoking]] could be one of the major causative agent of Respiratory Bronchiolitis-Interstitial Lung Disease (RB-ILD). A relation between the duration and the intensity of cigarette smoking and visualization of opacities on chest radiographs was reported in a few studies.<ref name="Carilli-1973">{{Cite journal | last1 = Carilli | first1 = AD. | last2 = Kotzen | first2 = LM. | last3 = Fischer | first3 = MJ. | title = The chest roentgenogram in smoking females. | journal = Am Rev Respir Dis | volume = 107 | issue = 1 | pages = 133-6 | month = Jan | year = 1973 | doi = | PMID = 4683317 }}</ref><ref name="Weiss-1984">{{Cite journal | last1 = Weiss | first1 = W. | title = Cigarette smoke, asbestos, and small irregular opacities. | journal = Am Rev Respir Dis | volume = 130 | issue = 2 | pages = 293-301 | month = Aug | year = 1984 | doi = | PMID = 6380358 }}</ref><ref name="Weiss-1991">{{Cite journal | last1 = Weiss | first1 = W. | title = Cigarette smoking and small irregular opacities. | journal = Br J Ind Med | volume = 48 | issue = 12 | pages = 841-4 | month = Dec | year = 1991 | doi = | PMID = 1772799 }}</ref><ref name="Dick-1992">{{Cite journal | last1 = Dick | first1 = JA. | last2 = Morgan | first2 = WK. | last3 = Muir | first3 = DF. | last4 = Reger | first4 = RB. | last5 = Sargent | first5 = N. | title = The significance of irregular opacities on the chest roentgenogram. | journal = Chest | volume = 102 | issue = 1 | pages = 251-60 | month = Jul | year = 1992 | doi = | PMID = 1623762 }}</ref> | |||
* The pathology is seen in the lumen of the bronchiole. Sometimes the bronchioles, alveolar ducts and the peribronchiolar alveolar spaces may show clusters of dusty brown macrophages.<ref name="Myers-1987">{{Cite journal | last1 = Myers | first1 = JL. | last2 = Veal | first2 = CF. | last3 = Shin | first3 = MS. | last4 = Katzenstein | first4 = AL. | title = Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. | journal = Am Rev Respir Dis | volume = 135 | issue = 4 | pages = 880-4 | month = Apr | year = 1987 | doi = | PMID = 3565934 }}</ref><ref name="Yousem-1989">{{Cite journal | last1 = Yousem | first1 = SA. | last2 = Colby | first2 = TV. | last3 = Gaensler | first3 = EA. | title = Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. | journal = Mayo Clin Proc | volume = 64 | issue = 11 | pages = 1373-80 | month = Nov | year = 1989 | doi = | PMID = 2593722 }}</ref><ref name="Niewoehner-1974">{{Cite journal | last1 = Niewoehner | first1 = DE. | last2 = Kleinerman | first2 = J. | last3 = Rice | first3 = DB. | title = Pathologic changes in the peripheral airways of young cigarette smokers. | journal = N Engl J Med | volume = 291 | issue = 15 | pages = 755-8 | month = Oct | year = 1974 | doi = 10.1056/NEJM197410102911503 | PMID = 4414996 }}</ref><ref name="Churg-2010">{{Cite journal | last1 = Churg | first1 = A. | last2 = Müller | first2 = NL. | last3 = Wright | first3 = JL. | title = Respiratory bronchiolitis/interstitial lung disease: fibrosis, pulmonary function, and evolving concepts. | journal = Arch Pathol Lab Med | volume = 134 | issue = 1 | pages = 27-32 | month = Jan | year = 2010 | doi = 10.1043/1543-2165-134.1.27 | PMID = 20073602 }}</ref><ref name="Cosio-1980">{{Cite journal | last1 = Cosio | first1 = MG. | last2 = Hale | first2 = KA. | last3 = Niewoehner | first3 = DE. | title = Morphologic and morphometric effects of prolonged cigarette smoking on the small airways. | journal = Am Rev Respir Dis | volume = 122 | issue = 2 | pages = 265-21 | month = Aug | year = 1980 | doi = | PMID = 7416603 }}</ref><ref name="Colby-1998">{{Cite journal | last1 = Colby | first1 = TV. | title = Bronchiolitis. Pathologic considerations. | journal = Am J Clin Pathol | volume = 109 | issue = 1 | pages = 101-9 | month = Jan | year = 1998 | doi = | PMID = 9426525 }}</ref> | |||
*Granular golden brown particles having plenty of cytoplasm may be seen. These particles are PAS-positive and Prussian blue–positive which implies increased iron content in the alveolar macrophages. This increased iron content could be associated with smoking.<ref name="Niewoehner-1974">{{Cite journal | last1 = Niewoehner | first1 = DE. | last2 = Kleinerman | first2 = J. | last3 = Rice | first3 = DB. | title = Pathologic changes in the peripheral airways of young cigarette smokers. | journal = N Engl J Med | volume = 291 | issue = 15 | pages = 755-8 | month = Oct | year = 1974 | doi = 10.1056/NEJM197410102911503 | PMID = 4414996 }}</ref><ref name="Cosio-1980">{{Cite journal | last1 = Cosio | first1 = MG. | last2 = Hale | first2 = KA. | last3 = Niewoehner | first3 = DE. | title = Morphologic and morphometric effects of prolonged cigarette smoking on the smallairways. | journal = Am Rev Respir Dis | volume = 122 | issue = 2 | pages = 265-21 | month = Aug | year = 1980 | doi = | PMID = 7416603 }}</ref><ref name="Churg-2010">{{Cite journal | last1 = Churg | first1 = A. | last2 = Müller | first2 = NL. | last3 = Wright | first3 = JL. | title = Respiratory bronchiolitis/interstitial lung disease: fibrosis, pulmonary function, and evolving concepts. | journal = Arch Pathol Lab Med | volume = 134 | issue = 1 | pages = 27-32 | month = Jan | year = 2010 | doi = 10.1043/1543-2165-134.1.27 | PMID = 20073602 }}</ref> | |||
*A common feature of histology of DIP and respiratory bronchiolitis is a mixture of alveolar septal thickening, epithelial hyperplasia and pigmented macrophages in the lumen. There are lymphocytes and histiocytes deposited in an irregular way in the submucosa. Similar to the black pigment in the [[macrophages]], a dark black anthracotic pigment can be seen in the histiocytes.<ref name="Yousem-1989">{{Cite journal | last1 = Yousem | first1 = SA. | last2 = Colby | first2 = TV. | last3 = Gaensler | first3 = EA. | title = Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. | journal = Mayo Clin Proc | volume = 64 | issue = 11 | pages = 1373-80 | month = Nov | year = 1989 | doi = | PMID = 2593722 }}</ref><ref name="Colby-1998">{{Cite journal | last1 = Colby | first1 = TV. | title = Bronchiolitis. Pathologic considerations. | journal = Am J Clin Pathol | volume = 109 | issue = 1 | pages = 101-9 | month = Jan | year = 1998 | doi = | PMID = 9426525 }}</ref> Type 2 hyperplastic cells and cuboidal bronchiolar type epithelium line the fibrosis around the bronchioles. | |||
===Desquamative Interstitial Pneumonia === | |||
* Desquamative interstitial pneumonia (DIP) lacks the typical patchy appearance of UIP. | |||
* In DIP alveolar walls are lined with chronic inflammatory cells and dense connective tissue and the alveolar spaces are filled with macrophages. | |||
* In DIP mild fibrosis without honeycomb changes are present occasionally. | |||
* Eosinophilic and plasma cell infiltration are also seen. | |||
* Mononuclear changes within the most distal spaces is a key finding in DIP. These mononuclear cells appear as finely granular brown pigment with mottled tiny black particles. These cells are known as smoker’s macrophages, which are different from the desquamated pneumocytes. | |||
* Some of these changes overlap in both DIP and respiratory bronchiolitis.<ref name="Moon-1999">{{Cite journal | last1 = Moon | first1 = J. | last2 = du Bois | first2 = RM. | last3 = Colby | first3 = TV. | last4 = Hansell | first4 = DM. | last5 = Nicholson | first5 = AG. | title = Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. | journal = Thorax | volume = 54 | issue = 11 | pages = 1009-14 | month = Nov | year = 1999 | doi = | PMID = 10525560 }}</ref><ref name="Yousem-1989">{{Cite journal | last1 = Yousem | first1 = SA. | last2 = Colby | first2 = TV. | last3 = Gaensler | first3 = EA. | title = Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. | journal = Mayo Clin Proc | volume = 64 | issue = 11 | pages = 1373-80 | month = Nov | year = 1989 | doi = | PMID = 2593722 }}</ref> | |||
=== | ===Cryptogenic-Organizing Pneumonia=== | ||
Cryptogenic organizing pneumonia (COP) is caused by disorganization of the alveolar epithelium. COP is characterized by: | |||
*Plasma protein leakage, fibroblast migration and fibrin deposition inside the lumen | |||
*Involvement of the [[vascular endothelial growth factor]] and matrix metalloproteinases.<ref name="Qiu-2013">{{Cite journal | last1 = Qiu | first1 = YY. | last2 = Miao | first2 = LY. | last3 = Cai | first3 = HR. | last4 = Xiao | first4 = YL. | last5 = Ye | first5 = Q. | last6 = Meng | first6 = FQ. | last7 = Feng | first7 = AN. | title = [The clinicopathological features of acute fibrinous and organizing pneumonia]. | journal = Zhonghua Jie He He Hu Xi Za Zhi | volume = 36 | issue = 6 | pages = 425-30 | month = Jun | year = 2013 | doi = | PMID = 24103205 }}</ref> | |||
*Accumulation of [[fibroblasts]] and [[myofibroblasts]] in the alveolar ducts and alveoli | |||
*Involvement of polyps in the bronchial lumen in some patients | |||
*Excess of granulation tissue deposition; the pattern of extension sometimes appearing like a butterfly | |||
Some recent studies show that COP can be a rare extra-intestinal manifestation of [[Crohn's Disease]].<ref name="Dinneen-2013">{{Cite journal | last1 = Dinneen | first1 = HS. | last2 = Samiullah | first2 = S. | last3 = Lenza | first3 = C. | title = Cryptogenic organizing pneumonia: A rare extra-intestinal manifestation of Crohn's disease. | journal = J Crohns Colitis | volume = | issue = | pages = | month = Oct | year = 2013 | doi = 10.1016/j.crohns.2013.09.006 | PMID = 24090908 }}</ref> | |||
===Acute Interstitial pneumonia (Hamman-Rich Syndrome) === | |||
AIP | Acute Interstitial Pneumonia (AIP) has a similar appearance to Diffuse Alveolar Damage (DAD). Shown below is a table summarizing the pathological features of the three different stages of AIP. It should be noted that similar lesions belonging to the same stage are seen in AIP whereas lesions of different ages are noted in UIP in the absence of a specific unified pattern at a given point of time. <ref name="Olson-1990">{{Cite journal | last1 = Olson | first1 = J. | last2 = Colby | first2 = TV. | last3 = Elliott | first3 = CG. | title = Hamman-Rich syndrome revisited. | journal = Mayo Clin Proc | volume = 65 | issue = 12 | pages = 1538-48 | month = Dec | year = 1990 | doi = | PMID = 2255216 }}</ref><ref name="Katzenstein-1986">{{Cite journal | last1 = Katzenstein | first1 = AL. | last2 = Myers | first2 = JL. | last3 = Mazur | first3 = MT. | title = Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. | journal = Am J Surg Pathol | volume = 10 | issue = 4 | pages = 256-67 | month = Apr | year = 1986 | doi = | PMID = 3706612 }}</ref> | ||
=== | {| {{table}} | ||
| align="center" style="background:#f0f0f0" | '''Stage''' | |||
| align="center" style="background:#f0f0f0 " | '''Pathological Feature ''' | |||
|- | |||
| Exudative stage|| Histology specimen is never obtained since patient presents late. | |||
|- | |||
| Proliferative stage|| Most commonly seen stage.<br> Inflammatory infiltration causes septal destruction and hyaline membrane formation leading to thickening of the septa and the interstitium. | |||
|- | |||
| Chronic or healed phase||Diffuse scarring is seen. | |||
|- | |||
|} | |||
*Release of [[tumor necrosis factor alpha]], interleukin 1β, monocyte chemoattractant factor and neutrophils cause further damage. This damage in turn causes release of toxic oxygen radicals and proteases. Overall it leads to an exudate formation and cellular damage. | |||
*A fibroblast proliferation and differentiation into myofibroblasts leads to collagen formation which widens the septa. Later hyaline membrane decreases and there is a rise in the number of type II epithelial cells. | |||
* A few patients resolve after this stage whereas a majority progress to the next stage i.e fibrosis. | |||
AIP shows prominent myofibroblastic proliferation whereas this finding is only seen in a few cases of [[ARDS]] secondary to infection or drug toxicity.<ref name="Kang-2009">{{Cite journal | last1 = Kang | first1 = D. | last2 = Nakayama | first2 = T. | last3 = Togashi | first3 = M. | last4 = Yamamoto | first4 = M. | last5 = Takahashi | first5 = M. | last6 = Kunugi | first6 = S. | last7 = Ishizaki | first7 = M. | last8 = Fukuda | first8 = Y. | title = Two forms of diffuse alveolar damage in the lungs of patients with acute respiratory distress syndrome. | journal = Hum Pathol | volume = 40 | issue = 11 | pages = 1618-27 | month = Nov | year = 2009 | doi = 10.1016/j.humpath.2009.04.019 | PMID = 19647854 }}</ref><ref name="Pache-1998">{{Cite journal | last1 = Pache | first1 = JC. | last2 = Christakos | first2 = PG. | last3 = Gannon | first3 = DE. | last4 = Mitchell | first4 = JJ. | last5 = Low | first5 = RB. | last6 = Leslie | first6 = KO. | title = Myofibroblasts in diffuse alveolar damage of the lung. | journal = Mod Pathol | volume = 11 | issue = 11 | pages = 1064-70 | month = Nov | year = 1998 | doi = | PMID = 9831203 }}</ref> | |||
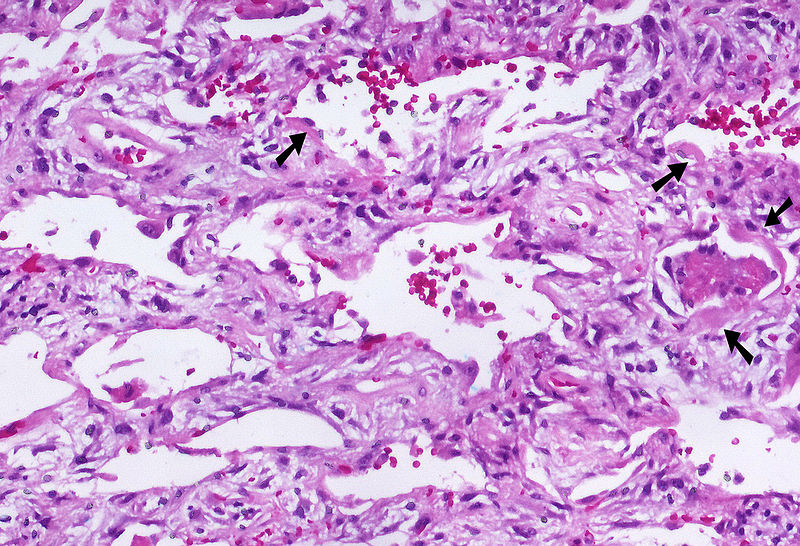
Shown below is an image depicting AIP: | |||
[[File:800px-Acute interstitial pneumonia (AIP) Idiopathic DAD 3.jpg|400px|Acute Interstitial Pneumonia]] | |||
===Lymphoid Interstitial Pneumonia === | |||
*Characteristic findings of Lymphoid Interstitial Pneumonia (LIP) are lymphoid and chronic cell infiltration predominantly seen in the alveolar septa but sometimes also seen around bronchi and vessels. | |||
*[[Granulomas]] specially non–caseating, fibrotic changes, honeycombing and loss of normal lung tissue is also seen as the disease progresses.<ref name="-2002">{{Cite journal | title = American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. | journal = Am J Respir Crit Care Med | volume = 165 | issue = 2 | pages = 277-304 | month = Jan | year = 2002 | doi = 10.1164/ajrccm.165.2.ats01 | PMID = 11790668 }}</ref><ref name="Koss-1987">{{Cite journal | last1 = Koss | first1 = MN. | last2 = Hochholzer | first2 = L. | last3 = Langloss | first3 = JM. | last4 = Wehunt | first4 = WD. | last5 = Lazarus | first5 = AA. | title = Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 cases. | journal = Pathology | volume = 19 | issue = 2 | pages = 178-85 | month = Apr | year = 1987 | doi = | PMID = 3453998 }}</ref> | |||
*In LIP, B cell polyclonality differentiates it from pulmonary lymphoma whereas in HIV, T cells are more predominant. However, sometimes no specific cell types are seen.<ref name="Koss-1987">{{Cite journal | last1 = Koss | first1 = MN. | last2 = Hochholzer | first2 = L. | last3 = Langloss | first3 = JM. | last4 = Wehunt | first4 = WD. | last5 = Lazarus | first5 = AA. | title = Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 cases. | journal = Pathology | volume = 19 | issue = 2 | pages = 178-85 | month = Apr | year = 1987 | doi = | PMID = 3453998 }}</ref><ref name="Travis-1992">{{Cite journal | last1 = Travis | first1 = WD. | last2 = Fox | first2 = CH. | last3 = Devaney | first3 = KO. | last4 = Weiss | first4 = LM. | last5 = O'Leary | first5 = TJ. | last6 = Ognibene | first6 = FP. | last7 = Suffredini | first7 = AF. | last8 = Rosen | first8 = MJ. | last9 = Cohen | first9 = MB. | title = Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis. | journal = Hum Pathol | volume = 23 | issue = 5 | pages = 529-41 | month = May | year = 1992 | doi = | PMID = 1314778 }}</ref><ref name="Lin-1988">{{Cite journal | last1 = Lin | first1 = RY. | last2 = Gruber | first2 = PJ. | last3 = Saunders | first3 = R. | last4 = Perla | first4 = EN. | title = Lymphocytic interstitial pneumonitis in adult HIV infection. | journal = N Y State J Med | volume = 88 | issue = 5 | pages = 273-6 | month = May | year = 1988 | doi = | PMID = 3288914 }}</ref> | |||
*A Bcl-6 gene mutation has been associated with mucosa-associated lymphoid tissue (MALT), [[HIV]] and [[EBV]] and other virus negative LIP.<ref name="Kurosu-2004">{{Cite journal | last1 = Kurosu | first1 = K. | last2 = Weiden | first2 = MD. | last3 = Takiguchi | first3 = Y. | last4 = Rom | first4 = WN. | last5 = Yumoto | first5 = N. | last6 = Jaishree | first6 = J. | last7 = Nakata | first7 = K. | last8 = Kasahara | first8 = Y. | last9 = Tanabe | first9 = N. | title = BCL-6 mutations in pulmonary lymphoproliferative disorders: demonstration of an aberrant immunological reaction in HIV-related lymphoid interstitial pneumonia. | journal = J Immunol | volume = 172 | issue = 11 | pages = 7116-22 | month = Jun | year = 2004 | doi = | PMID = 15153535 }}</ref> | |||
*Features like monoclonality, hilar involvement, pleural involvement, bronchial wall involvement all point towards a malignant transformation.<ref name="Turner-1984">{{Cite journal | last1 = Turner | first1 = RR. | last2 = Colby | first2 = TV. | last3 = Doggett | first3 = RS. | title = Well-differentiated lymphocytic lymphoma. A study of 47 patients with primary manifestation in the lung. | journal = Cancer | volume = 54 | issue = 10 | pages = 2088-96 | month = Nov | year = 1984 | doi = | PMID = 6386139 }}</ref> | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Disease]] | |||
[[Category:Pulmonology]] | |||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 00:54, 3 December 2013
|
Idiopathic Interstitial Pneumonia Microchapters |
|
Differentiating Idiopathic interstitial pneumonia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Idiopathic interstitial pneumonia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Idiopathic interstitial pneumonia pathophysiology |
|
Idiopathic interstitial pneumonia pathophysiologyin the news |
|
Directions to Hospitals Treating Idiopathic interstitial pneumonia |
|
Risk calculators and risk factors for Idiopathic interstitial pneumonia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Overview
Idiopathic interstitial pneumonia (IIP) is a disease entity that can be histologically classified into different categories. Idiopathic pulmonary fibrosis has the same features as that of usual interstitial pneumonia (UIP) whereas no specific pattern or common feature is noted among the other types of IIP. The pathophysiology of IIP can be summarized in the following three stages: recruitment of inflammatory cells, abnormal collagen deposition and fibroblastic proliferation and lastly progression to fibrosis.
Pathophysiology
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) has often been considered an autoimmune disease. However, it is perhaps better characterized as an abnormal and excessive deposition of fibrotic tissue in the pulmonary interstitium with minimal associated inflammation.[1] Autoantibodies, a hallmark of autoimmune diseases, are found in a minority of patients with true idiopathic pulmonary fibrosis. Moreover, many autoimmune diseases that are associated with pulmonary fibrosis such as scleroderma, are more frequently associated with a related but more inflammatory disease, nonspecific interstitial pneumonitis.[2] IPF is associated with smoking[3] and exhibits some dependency on the amount of smoking.[4]
Shown below is an image depicting IPF:
Idiopathic Non-specific Interstitial Pneumonia
As the name implies, idiopathic non-specific interstitial pneumonia (NSIP) has very inconsistent and non-specific findings.[5][6][7] Changes similar to other cases of interstitial pneumonia are seen which are migration of inflammatory cells in the alveolar septa and its widening with or without fibrosis. NSIP can be divided into three groups based on histopathological changes.[8] Shown below is a table summarizing the pathological findings in the three groups of NSIP:
| Stage | Pathological Feature |
| Group I | Inflammatory cells predominant stage |
| Group II | Accompanying fibrosis |
| Group III | Fibrosis prevalent |
Bronchoalveloar lavage (BAL) reveals the presence of lymphocytes in the alveolar septum which is an evidence of the involvement of the immune system.[6][9][10][11] A greater number of dendritic cells (DC), which help in antigen presentation, are visualized in close association with CD4 and CD8 lymphocytes in the biopsy of NSIS patients than in UIP.[12] Fibroblasts are the key cells involved in fibrotic lung diseases.[13]
The pathological mechanism of NSIP involves:
- Epithelial injury and dysregulated repair (major role)[14]
- Cytokines
- Proteins like epimorphin (a cell surface associated protein)[15] matrix metalloproteinases,[16] heat shock protein,[17] surfactant protein C[18][19][20][21][22]
- The coagulation system[23][24]
- Intercellular adhesion molecules-1,[25] IL-4, IL-13, IL-18[26]
- Interferon-gamma
- Pro fibrotic chemokines[27]
- CCL7, and CCL5
- Lymphocytes[28]
- Dendritic cells
- Fibroblasts
Some common associations between Idiopathic Non-specific Interstitial Pneumonia (NSIP) and Usual Interstitial Pneumonia (UIP) have been noted. Histologically patients can manifest lesions of both UIP and NSIP simultaneously. The reason for this presentation is still unknown but environmental exposures and genetic mutations could be some of the causes.[29][5]
Features differentiating NSIP and UIP include:
Respiratory Bronchiolitis-Interstitial Lung Disease
- Cigarette smoking could be one of the major causative agent of Respiratory Bronchiolitis-Interstitial Lung Disease (RB-ILD). A relation between the duration and the intensity of cigarette smoking and visualization of opacities on chest radiographs was reported in a few studies.[31][32][33][34]
- The pathology is seen in the lumen of the bronchiole. Sometimes the bronchioles, alveolar ducts and the peribronchiolar alveolar spaces may show clusters of dusty brown macrophages.[35][36][37][38][39][40]
- Granular golden brown particles having plenty of cytoplasm may be seen. These particles are PAS-positive and Prussian blue–positive which implies increased iron content in the alveolar macrophages. This increased iron content could be associated with smoking.[37][39][38]
- A common feature of histology of DIP and respiratory bronchiolitis is a mixture of alveolar septal thickening, epithelial hyperplasia and pigmented macrophages in the lumen. There are lymphocytes and histiocytes deposited in an irregular way in the submucosa. Similar to the black pigment in the macrophages, a dark black anthracotic pigment can be seen in the histiocytes.[36][40] Type 2 hyperplastic cells and cuboidal bronchiolar type epithelium line the fibrosis around the bronchioles.
Desquamative Interstitial Pneumonia
- Desquamative interstitial pneumonia (DIP) lacks the typical patchy appearance of UIP.
- In DIP alveolar walls are lined with chronic inflammatory cells and dense connective tissue and the alveolar spaces are filled with macrophages.
- In DIP mild fibrosis without honeycomb changes are present occasionally.
- Eosinophilic and plasma cell infiltration are also seen.
- Mononuclear changes within the most distal spaces is a key finding in DIP. These mononuclear cells appear as finely granular brown pigment with mottled tiny black particles. These cells are known as smoker’s macrophages, which are different from the desquamated pneumocytes.
- Some of these changes overlap in both DIP and respiratory bronchiolitis.[41][36]
Cryptogenic-Organizing Pneumonia
Cryptogenic organizing pneumonia (COP) is caused by disorganization of the alveolar epithelium. COP is characterized by:
- Plasma protein leakage, fibroblast migration and fibrin deposition inside the lumen
- Involvement of the vascular endothelial growth factor and matrix metalloproteinases.[42]
- Accumulation of fibroblasts and myofibroblasts in the alveolar ducts and alveoli
- Involvement of polyps in the bronchial lumen in some patients
- Excess of granulation tissue deposition; the pattern of extension sometimes appearing like a butterfly
Some recent studies show that COP can be a rare extra-intestinal manifestation of Crohn's Disease.[43]
Acute Interstitial pneumonia (Hamman-Rich Syndrome)
Acute Interstitial Pneumonia (AIP) has a similar appearance to Diffuse Alveolar Damage (DAD). Shown below is a table summarizing the pathological features of the three different stages of AIP. It should be noted that similar lesions belonging to the same stage are seen in AIP whereas lesions of different ages are noted in UIP in the absence of a specific unified pattern at a given point of time. [44][45]
| Stage | Pathological Feature |
| Exudative stage | Histology specimen is never obtained since patient presents late. |
| Proliferative stage | Most commonly seen stage. Inflammatory infiltration causes septal destruction and hyaline membrane formation leading to thickening of the septa and the interstitium. |
| Chronic or healed phase | Diffuse scarring is seen. |
- Release of tumor necrosis factor alpha, interleukin 1β, monocyte chemoattractant factor and neutrophils cause further damage. This damage in turn causes release of toxic oxygen radicals and proteases. Overall it leads to an exudate formation and cellular damage.
- A fibroblast proliferation and differentiation into myofibroblasts leads to collagen formation which widens the septa. Later hyaline membrane decreases and there is a rise in the number of type II epithelial cells.
- A few patients resolve after this stage whereas a majority progress to the next stage i.e fibrosis.
AIP shows prominent myofibroblastic proliferation whereas this finding is only seen in a few cases of ARDS secondary to infection or drug toxicity.[46][47]
Shown below is an image depicting AIP:
Lymphoid Interstitial Pneumonia
- Characteristic findings of Lymphoid Interstitial Pneumonia (LIP) are lymphoid and chronic cell infiltration predominantly seen in the alveolar septa but sometimes also seen around bronchi and vessels.
- Granulomas specially non–caseating, fibrotic changes, honeycombing and loss of normal lung tissue is also seen as the disease progresses.[48][49]
- In LIP, B cell polyclonality differentiates it from pulmonary lymphoma whereas in HIV, T cells are more predominant. However, sometimes no specific cell types are seen.[49][50][51]
- A Bcl-6 gene mutation has been associated with mucosa-associated lymphoid tissue (MALT), HIV and EBV and other virus negative LIP.[52]
- Features like monoclonality, hilar involvement, pleural involvement, bronchial wall involvement all point towards a malignant transformation.[53]
References
- ↑ Selman, Moisés (2001). "Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy". Annals of Internal Medicine. 134 (2): 136–51. Unknown parameter
|coauthors=ignored (help) - ↑ King, Jr., Talmadge E. (2005). "Centennial review: clinical advances in the diagnosis and therapy of the interstitial lung diseases". American Journal of Respiratory and Critical Care Medicine. 172 (3): 268–79.
- ↑ Nagai, Sonoko (2000). "Smoking-related interstitial lung diseases". Current Opinion in Pulmonary Medicine. 6 (5): 415–9. PMID 10958232. Unknown parameter
|coauthors=ignored (help) - ↑ Baumgartner, KB (1997). "Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis". American Journal of Respiratory and Critical Care Medicine. 155 (1): 242–248. PMID 9001319. Unknown parameter
|coauthors=ignored (help) - ↑ 5.0 5.1 Flaherty, KR.; Travis, WD.; Colby, TV.; Toews, GB.; Kazerooni, EA.; Gross, BH.; Jain, A.; Strawderman, RL.; Flint, A. (2001). "Histopathologic variability in usual and nonspecific interstitial pneumonias". Am J Respir Crit Care Med. 164 (9): 1722–7. doi:10.1164/ajrccm.164.9.2103074. PMID 11719316. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 Cottin, V.; Donsbeck, AV.; Revel, D.; Loire, R.; Cordier, JF. (1998). "Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients". Am J Respir Crit Care Med. 158 (4): 1286–93. doi:10.1164/ajrccm.158.4.9802119. PMID 9769293. Unknown parameter
|month=ignored (help) - ↑ Katzenstein, AL.; Myers, JL. (2000). "Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: classification and diagnostic criteria". Am J Surg Pathol. 24 (1): 1–3. PMID 10632482. Unknown parameter
|month=ignored (help) - ↑ Katzenstein, AL.; Fiorelli, RF. (1994). "Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance". Am J Surg Pathol. 18 (2): 136–47. PMID 8291652. Unknown parameter
|month=ignored (help) - ↑ Fujita, J.; Yamadori, I.; Suemitsu, I.; Yoshinouchi, T.; Ohtsuki, Y.; Yamaji, Y.; Kamei, T.; Kobayashi, M.; Nakamura, Y. (1999). "Clinical features of non-specific interstitial pneumonia". Respir Med. 93 (2): 113–8. PMID 10464862. Unknown parameter
|month=ignored (help) - ↑ Nagai, S.; Kitaichi, M.; Itoh, H.; Nishimura, K.; Izumi, T.; Colby, TV. (1998). "Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP". Eur Respir J. 12 (5): 1010–9. PMID 9863989. Unknown parameter
|month=ignored (help) - ↑ Park, CS.; Jeon, JW.; Park, SW.; Lim, GI.; Jeong, SH.; Uh, ST.; Park, JS.; Choi, DL.; Jin, SY. (1996). "Nonspecific interstitial pneumonia/fibrosis: clinical manifestations, histologic and radiologic features". Korean J Intern Med. 11 (2): 122–32. PMID 8854648. Unknown parameter
|month=ignored (help) - ↑ Shimizu, S.; Yoshinouchi, T.; Ohtsuki, Y.; Fujita, J.; Sugiura, Y.; Banno, S.; Yamadori, I.; Eimoto, T.; Ueda, R. (2002). "The appearance of S-100 protein-positive dendritic cells and the distribution of lymphocyte subsets in idiopathic nonspecific interstitial pneumonia". Respir Med. 96 (10): 770–6. PMID 12412975. Unknown parameter
|month=ignored (help) - ↑ Selman, M.; King, TE.; Pardo, A. (2001). "Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy". Ann Intern Med. 134 (2): 136–51. PMID 11177318. Unknown parameter
|month=ignored (help) - ↑ Ishii, H.; Mukae, H.; Kadota, J.; Fujii, T.; Abe, K.; Ashitani, J.; Kohno, S. "Increased levels of interleukin-18 in bronchoalveolar lavage fluid of patients with idiopathic nonspecific interstitial pneumonia". Respiration. 72 (1): 39–45. doi:10.1159/000083399. PMID 15753633.
- ↑ Terasaki, Y.; Fukuda, Y.; Ishizaki, M.; Yamanaka, N. (2000). "Increased expression of epimorphin in bleomycin-induced pulmonary fibrosis in mice". Am J Respir Cell Mol Biol. 23 (2): 168–74. doi:10.1165/ajrcmb.23.2.3973. PMID 10919982. Unknown parameter
|month=ignored (help) - ↑ Suga, M.; Iyonaga, K.; Okamoto, T.; Gushima, Y.; Miyakawa, H.; Akaike, T.; Ando, M. (2000). "Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias". Am J Respir Crit Care Med. 162 (5): 1949–56. doi:10.1164/ajrccm.162.5.9906096. PMID 11069839. Unknown parameter
|month=ignored (help) - ↑ Kakugawa, T.; Mukae, H.; Hayashi, T.; Ishii, H.; Nakayama, S.; Sakamoto, N.; Yoshioka, S.; Sugiyama, K.; Mine, M. (2005). "Expression of HSP47 in usual interstitial pneumonia and nonspecific interstitial pneumonia". Respir Res. 6: 57. doi:10.1186/1465-9921-6-57. PMID 15955241.
- ↑ Thomas, AQ.; Lane, K.; Phillips, J.; Prince, M.; Markin, C.; Speer, M.; Schwartz, DA.; Gaddipati, R.; Marney, A. (2002). "Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred". Am J Respir Crit Care Med. 165 (9): 1322–8. doi:10.1164/rccm.200112-123OC. PMID 11991887. Unknown parameter
|month=ignored (help) - ↑ Brasch, F.; Griese, M.; Tredano, M.; Johnen, G.; Ochs, M.; Rieger, C.; Mulugeta, S.; Müller, KM.; Bahuau, M. (2004). "Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene". Eur Respir J. 24 (1): 30–9. PMID 15293602. Unknown parameter
|month=ignored (help) - ↑ Nogee, LM.; Dunbar, AE.; Wert, SE.; Askin, F.; Hamvas, A.; Whitsett, JA. (2001). "A mutation in the surfactant protein C gene associated with familial interstitial lung disease". N Engl J Med. 344 (8): 573–9. doi:10.1056/NEJM200102223440805. PMID 11207353. Unknown parameter
|month=ignored (help) - ↑ Stevens, PA.; Pettenazzo, A.; Brasch, F.; Mulugeta, S.; Baritussio, A.; Ochs, M.; Morrison, L.; Russo, SJ.; Beers, MF. (2005). "Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene". Pediatr Res. 57 (1): 89–98. doi:10.1203/01.PDR.0000147567.02473.5A. PMID 15557112. Unknown parameter
|month=ignored (help) - ↑ Chibbar, R.; Shih, F.; Baga, M.; Torlakovic, E.; Ramlall, K.; Skomro, R.; Cockcroft, DW.; Lemire, EG. (2004). "Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis". Mod Pathol. 17 (8): 973–80. doi:10.1038/modpathol.3800149. PMID 15133475. Unknown parameter
|month=ignored (help) - ↑ Eitzman, DT.; McCoy, RD.; Zheng, X.; Fay, WP.; Shen, T.; Ginsburg, D.; Simon, RH. (1996). "Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene". J Clin Invest. 97 (1): 232–7. doi:10.1172/JCI118396. PMID 8550840. Unknown parameter
|month=ignored (help) - ↑ Kim, KK.; Flaherty, KR.; Long, Q.; Hattori, N.; Sisson, TH.; Colby, TV.; Travis, WD.; Martinez, FJ.; Murray, S. "A plasminogen activator inhibitor-1 promoter polymorphism and idiopathic interstitial pneumonia". Mol Med. 9 (1–2): 52–6. PMID 12765340.
- ↑ Takehara, H.; Tada, S.; Kataoka, M.; Matsuo, K.; Ueno, Y.; Ozaki, S.; Miyake, T.; Fujimori, Y.; Yamadori, I. (2001). "Intercellular adhesion molecule-1 in patients with idiopathic interstitial pneumonia". Acta Med Okayama. 55 (4): 205–11. PMID 11512562. Unknown parameter
|month=ignored (help) - ↑ Jakubzick, C.; Choi, ES.; Kunkel, SL.; Evanoff, H.; Martinez, FJ.; Puri, RK.; Flaherty, KR.; Toews, GB.; Colby, TV. (2004). "Augmented pulmonary IL-4 and IL-13 receptor subunit expression in idiopathic interstitial pneumonia". J Clin Pathol. 57 (5): 477–86. PMID 15113854. Unknown parameter
|month=ignored (help) - ↑ Choi, ES.; Jakubzick, C.; Carpenter, KJ.; Kunkel, SL.; Evanoff, H.; Martinez, FJ.; Flaherty, KR.; Toews, GB.; Colby, TV. (2004). "Enhanced monocyte chemoattractant protein-3/CC chemokine ligand-7 in usual interstitial pneumonia". Am J Respir Crit Care Med. 170 (5): 508–15. doi:10.1164/rccm.200401-002OC. PMID 15191918. Unknown parameter
|month=ignored (help) - ↑ Keogh, KA.; Limper, AH. (2005). "Characterization of lymphocyte populations in nonspecific interstitial pneumonia". Respir Res. 6: 137. doi:10.1186/1465-9921-6-137. PMID 16287509.
- ↑ Monaghan, H.; Wells, AU.; Colby, TV.; du Bois, RM.; Hansell, DM.; Nicholson, AG. (2004). "Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias". Chest. 125 (2): 522–6. PMID 14769733. Unknown parameter
|month=ignored (help) - ↑ Miki, H.; Mio, T.; Nagai, S.; Hoshino, Y.; Nagao, T.; Kitaichi, M.; Izumi, T. (2000). "Fibroblast contractility: usual interstitial pneumonia and nonspecific interstitial pneumonia". Am J Respir Crit Care Med. 162 (6): 2259–64. doi:10.1164/ajrccm.162.6.9812029. PMID 11112149. Unknown parameter
|month=ignored (help) - ↑ Carilli, AD.; Kotzen, LM.; Fischer, MJ. (1973). "The chest roentgenogram in smoking females". Am Rev Respir Dis. 107 (1): 133–6. PMID 4683317. Unknown parameter
|month=ignored (help) - ↑ Weiss, W. (1984). "Cigarette smoke, asbestos, and small irregular opacities". Am Rev Respir Dis. 130 (2): 293–301. PMID 6380358. Unknown parameter
|month=ignored (help) - ↑ Weiss, W. (1991). "Cigarette smoking and small irregular opacities". Br J Ind Med. 48 (12): 841–4. PMID 1772799. Unknown parameter
|month=ignored (help) - ↑ Dick, JA.; Morgan, WK.; Muir, DF.; Reger, RB.; Sargent, N. (1992). "The significance of irregular opacities on the chest roentgenogram". Chest. 102 (1): 251–60. PMID 1623762. Unknown parameter
|month=ignored (help) - ↑ Myers, JL.; Veal, CF.; Shin, MS.; Katzenstein, AL. (1987). "Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases". Am Rev Respir Dis. 135 (4): 880–4. PMID 3565934. Unknown parameter
|month=ignored (help) - ↑ 36.0 36.1 36.2 Yousem, SA.; Colby, TV.; Gaensler, EA. (1989). "Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia". Mayo Clin Proc. 64 (11): 1373–80. PMID 2593722. Unknown parameter
|month=ignored (help) - ↑ 37.0 37.1 Niewoehner, DE.; Kleinerman, J.; Rice, DB. (1974). "Pathologic changes in the peripheral airways of young cigarette smokers". N Engl J Med. 291 (15): 755–8. doi:10.1056/NEJM197410102911503. PMID 4414996. Unknown parameter
|month=ignored (help) - ↑ 38.0 38.1 Churg, A.; Müller, NL.; Wright, JL. (2010). "Respiratory bronchiolitis/interstitial lung disease: fibrosis, pulmonary function, and evolving concepts". Arch Pathol Lab Med. 134 (1): 27–32. doi:10.1043/1543-2165-134.1.27. PMID 20073602. Unknown parameter
|month=ignored (help) - ↑ 39.0 39.1 Cosio, MG.; Hale, KA.; Niewoehner, DE. (1980). "Morphologic and morphometric effects of prolonged cigarette smoking on the small airways". Am Rev Respir Dis. 122 (2): 265–21. PMID 7416603. Unknown parameter
|month=ignored (help) - ↑ 40.0 40.1 Colby, TV. (1998). "Bronchiolitis. Pathologic considerations". Am J Clin Pathol. 109 (1): 101–9. PMID 9426525. Unknown parameter
|month=ignored (help) - ↑ Moon, J.; du Bois, RM.; Colby, TV.; Hansell, DM.; Nicholson, AG. (1999). "Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease". Thorax. 54 (11): 1009–14. PMID 10525560. Unknown parameter
|month=ignored (help) - ↑ Qiu, YY.; Miao, LY.; Cai, HR.; Xiao, YL.; Ye, Q.; Meng, FQ.; Feng, AN. (2013). "[The clinicopathological features of acute fibrinous and organizing pneumonia]". Zhonghua Jie He He Hu Xi Za Zhi. 36 (6): 425–30. PMID 24103205. Unknown parameter
|month=ignored (help) - ↑ Dinneen, HS.; Samiullah, S.; Lenza, C. (2013). "Cryptogenic organizing pneumonia: A rare extra-intestinal manifestation of Crohn's disease". J Crohns Colitis. doi:10.1016/j.crohns.2013.09.006. PMID 24090908. Unknown parameter
|month=ignored (help) - ↑ Olson, J.; Colby, TV.; Elliott, CG. (1990). "Hamman-Rich syndrome revisited". Mayo Clin Proc. 65 (12): 1538–48. PMID 2255216. Unknown parameter
|month=ignored (help) - ↑ Katzenstein, AL.; Myers, JL.; Mazur, MT. (1986). "Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study". Am J Surg Pathol. 10 (4): 256–67. PMID 3706612. Unknown parameter
|month=ignored (help) - ↑ Kang, D.; Nakayama, T.; Togashi, M.; Yamamoto, M.; Takahashi, M.; Kunugi, S.; Ishizaki, M.; Fukuda, Y. (2009). "Two forms of diffuse alveolar damage in the lungs of patients with acute respiratory distress syndrome". Hum Pathol. 40 (11): 1618–27. doi:10.1016/j.humpath.2009.04.019. PMID 19647854. Unknown parameter
|month=ignored (help) - ↑ Pache, JC.; Christakos, PG.; Gannon, DE.; Mitchell, JJ.; Low, RB.; Leslie, KO. (1998). "Myofibroblasts in diffuse alveolar damage of the lung". Mod Pathol. 11 (11): 1064–70. PMID 9831203. Unknown parameter
|month=ignored (help) - ↑ "American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001". Am J Respir Crit Care Med. 165 (2): 277–304. 2002. doi:10.1164/ajrccm.165.2.ats01. PMID 11790668. Unknown parameter
|month=ignored (help) - ↑ 49.0 49.1 Koss, MN.; Hochholzer, L.; Langloss, JM.; Wehunt, WD.; Lazarus, AA. (1987). "Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 cases". Pathology. 19 (2): 178–85. PMID 3453998. Unknown parameter
|month=ignored (help) - ↑ Travis, WD.; Fox, CH.; Devaney, KO.; Weiss, LM.; O'Leary, TJ.; Ognibene, FP.; Suffredini, AF.; Rosen, MJ.; Cohen, MB. (1992). "Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis". Hum Pathol. 23 (5): 529–41. PMID 1314778. Unknown parameter
|month=ignored (help) - ↑ Lin, RY.; Gruber, PJ.; Saunders, R.; Perla, EN. (1988). "Lymphocytic interstitial pneumonitis in adult HIV infection". N Y State J Med. 88 (5): 273–6. PMID 3288914. Unknown parameter
|month=ignored (help) - ↑ Kurosu, K.; Weiden, MD.; Takiguchi, Y.; Rom, WN.; Yumoto, N.; Jaishree, J.; Nakata, K.; Kasahara, Y.; Tanabe, N. (2004). "BCL-6 mutations in pulmonary lymphoproliferative disorders: demonstration of an aberrant immunological reaction in HIV-related lymphoid interstitial pneumonia". J Immunol. 172 (11): 7116–22. PMID 15153535. Unknown parameter
|month=ignored (help) - ↑ Turner, RR.; Colby, TV.; Doggett, RS. (1984). "Well-differentiated lymphocytic lymphoma. A study of 47 patients with primary manifestation in the lung". Cancer. 54 (10): 2088–96. PMID 6386139. Unknown parameter
|month=ignored (help)