Voglibose
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
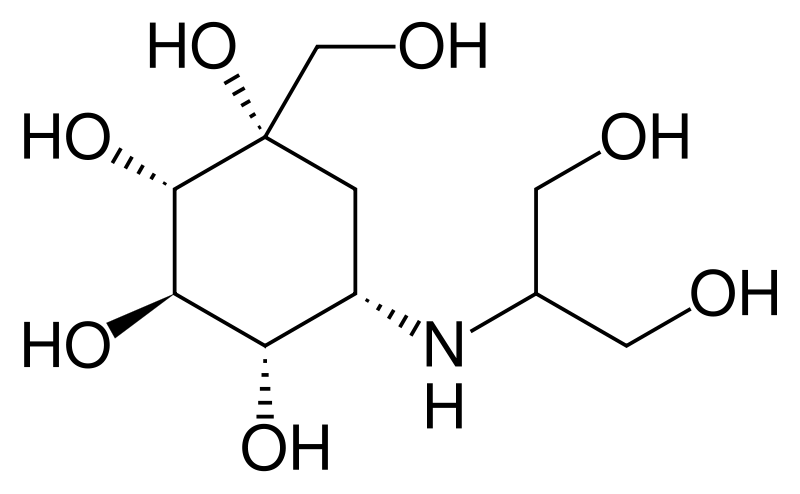
| Formula | C10H21NO7 |
| Molar mass | 267.28 g/mol |
|
WikiDoc Resources for Voglibose |
|
Articles |
|---|
|
Most recent articles on Voglibose |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Voglibose at Clinical Trials.gov Clinical Trials on Voglibose at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Voglibose
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Voglibose Discussion groups on Voglibose Directions to Hospitals Treating Voglibose Risk calculators and risk factors for Voglibose
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Voglibose |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Voglibose (INN and USAN) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. It is made in India by Ranbaxy Labs and sold under the trade name Volix.
Glenmark is also producing voglibose under brand name vocarb.
Diabetes is chronic metabolic disorder characterised by hyperglycemia which is due to relative or absolute deficiency of insulin or insulin resistance.
PPHG is termed as Post Prandial Hyperglycemia which is primarily due to first phase insulin secretion. Alpha glucosidase inhibitor is one agent which delays the glucose absorption at the intestine level and thereby prevents sudden surge of glucose post meal.
There are three molecules which belong to this class namely, Acarbose, Miglitol and Voglibose. Voglibose is the latest molecule in this class. Voglibose scores over both Acarbose and MIglitol in terms of potency and side effect profile.
There are several trials supporting the use of Voglibose in the management of PPHG. Also, it has been established that it is PPHG not FPG which is marker of cardiovascular disorders associated with diabetes. So, controlling PPHG is imperative and Voglibose is indicated for the management of PPHG.
Sun Pharmaceuticals launched Voglibose 0.2 / 0.3 mg under the brand name VOLIBO.
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Alpha-glucosidase inhibitors
- Endocrinology