Treprostinil (inhalant)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Treprostinil (inhalant) is a prostacyclin vasodilator that is FDA approved for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Common adverse reactions include cough, headache, nausea, dizziness, flushing, throat irritation, pharyngolaryngeal pain, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Treprostinil is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Studies establishing effectiveness included predominately patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- The effects diminish over the minimum recommended dosing interval of 4 hours; treatment timing can be adjusted for planned activities.
- While there are long-term data on use of treprostinil by other routes of administration, nearly all controlled clinical experience with inhaled treprostinil has been on a background of bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase type 5 inhibitor). The controlled clinical experience was limited to 12 weeks in duration.
Dosage
Usual Dosage in Adults
- Treprostinil is intended for oral inhalation using the treprostinil Inhalation System, which consists of an ultrasonic, pulsed delivery device and its accessories.
- Treprostinil is dosed in 4 separate, equally spaced treatment sessions per day, during waking hours. The treatment sessions should be approximately 4 hours apart.
Initial Dosage:
- Therapy should begin with 3 breaths of treprostinil (18 mcg of treprostinil), per treatment session, 4 times daily. If 3 breaths are not tolerated, reduce to 1 or 2 breaths and subsequently increase to 3 breaths, as tolerated.
Maintenance Dosage:
- Dosage should be increased by an additional 3 breaths at approximately 1-2 week intervals, if tolerated, until the target dose of 9 breaths (54 mcg of treprostinil) is reached per treatment session, 4 times daily. If adverse effects preclude titration to target dose, treprostinil should be continued at the highest tolerated dose.
- If a scheduled treatment session is missed or interrupted, therapy should be resumed as soon as possible at the usual dose.
- The maximum recommended dosage is 9 breaths per treatment session, 4 times daily.
Patients with Hepatic Insufficiency
- Plasma clearance of treprostinil is reduced in patients with hepatic insufficiency. Patients with hepatic insufficiency may therefore be at increased risk of dose-dependent adverse reactions because of an increase in systemic exposure.
Patients with Renal Insufficiency
- Plasma clearance of treprostinil may be reduced in patients with renal insufficiency, since treprostinil and its metabolites are excreted mainly through the urinary route. Patients with renal insufficiency may therefore be at increased risk of dose-dependent adverse reactions.
Administration
- Treprostinil must be used only with the treprostinil Inhalation System. Patients should follow the instructions for use for operation of the treprostinil Inhalation System and for daily cleaning of the device components after the last treatment session of the day. To avoid potential interruptions in drug delivery because of equipment malfunction, patients should have access to a back-up treprostinil Inhalation System device.
- Do not mix treprostinil with other medications in the treprostinil Inhalation System. Compatibility of treprostinil with other medications has not been studied.
- The treprostinil Inhalation System should be prepared for use each day according to the instructions for use. One ampule of treprostinil contains a sufficient volume of medication for all 4 treatment sessions in a single day. Prior to the first treatment session, the patient should twist the top off a single treprostinil ampule and squeeze the entire contents into the medicine cup. Between each of the 4 daily treatment sessions, the device should be capped and stored upright with the remaining medication inside.
- At the end of each day, the medicine cup and any remaining medication must be discarded. The device must be cleaned each day according to the instructions for use.
- Avoid skin or eye contact with treprostinil solution. Do not orally ingest the treprostinil solution.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Treprostinil (inhalant) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Treprostinil (inhalant) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established. Clinical studies of treprostinil did not include patients younger than 18 years to determine whether they respond differently from older patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Treprostinil (inhalant) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Treprostinil (inhalant) in pediatric patients.
Contraindications
- None.
Warnings
Patients with Pulmonary Disease or Pulmonary Infections
- The safety and efficacy of treprostinil have not been established in patients with significant underlying lung disease (e.g., asthma or chronic obstructive pulmonary disease). Patients with acute pulmonary infections should be carefully monitored to detect any worsening of lung disease and loss of drug effect.
Risk of Symptomatic Hypotension
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
Patients with Hepatic or Renal Insufficiency
- Titrate slowly in patients with hepatic or renal insufficiency, because such patients will likely be exposed to greater systemic concentrations relative to patients with normal hepatic or renal function.
Risk of Bleeding
- Since treprostinil inhibits platelet aggregation, there may be an increased risk of bleeding, particularly among patients receiving anticoagulant therapy.
Effect of Other Drugs on Treprostinil
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In a 12-week placebo-controlled study (TRIUMPH I) of 235 patients with PAH (WHO Group 1 and nearly all NYHA Functional Class III), the most commonly reported adverse reactions on treprostinil included: cough and throat irritation; headache, gastrointestinal effects, muscle, jaw or bone pain, flushing and syncope. Table 1 lists the adverse reactions that occurred at a rate of at least 4% and were more frequent in patients treated with treprostinil than with placebo.

- The safety of treprostinil was also studied in a long-term, open-label extension study in which 206 patients were dosed for a mean duration of 2.3 years, with a maximum exposure of 5.4 years. Eighty-nine (89%) percent of patients achieved the target dose of nine breaths, four times daily. Forty-two (42%) percent achieved a dose of 12 breaths four times daily. The adverse events during this chronic dosing study were qualitatively similar to those observed in the 12-week placebo controlled trial.
Adverse Events Associated with Route of Administration
- Adverse events in the treated group during the double-blind and open-label phase reflecting irritation to the respiratory tract included: cough, throat irritation, pharyngeal pain, epistaxis, hemoptysis and wheezing. Serious adverse events during the open-label portion of the study included pneumonia in fifteen subjects. There were three serious episodes of hemoptysis (one fatal) noted during the open-label experience.
Postmarketing Experience
- The following adverse reaction has been identified during the postapproval use of treprostinil. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure:
Drug Interactions
Pharmacokinetic/pharmacodynamic interaction studies have not been conducted with inhaled treprostinil; however, some of such studies have been conducted with orally (treprostinil diolamine) and subcutaneously administered treprostinil (Remodulin®).
Antihypertensive Agents or Other Vasodilators
- Concomitant administration of treprostinil with diuretics, antihypertensive agents or other vasodilators may increase the risk of symptomatic hypotension.
Anticoagulants
- Since treprostinil inhibits platelet aggregation, there may be an increased risk of bleeding, particularly among patients receiving anticoagulants.
Bosentan
- In a human pharmacokinetic study conducted with bosentan (250 mg/day) and an oral formulation of treprostinil, no pharmacokinetic interactions between treprostinil and bosentan were observed.
Sildenafil
- In a human pharmacokinetic study conducted with sildenafil (60 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between treprostinil and sildenafil were observed.
Effect of Cytochrome P450 Inhibitors and Inducers
- In vitro studies of human hepatic microsomes showed that treprostinil does not inhibit cytochrome P450 (CYP) isoenzymes CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A. Additionally, treprostinil does not induce cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor gemfibrozil increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer rifampin decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
Effect of Other Drugs on Treprostinil
- Drug interaction studies have been carried out with treprostinil (oral or subcutaneous) co-administered with acetaminophen (4 g/day), warfarin (25 mg/day), and fluconazole (200 mg/day), respectively in healthy volunteers. These studies did not show a clinically significant effect on the pharmacokinetics of treprostinil. Treprostinil does not affect the pharmacokinetics or pharmacodynamics of warfarin. The pharmacokinetics of R- and S- warfarin and the INR in healthy subjects given a single 25 mg dose of warfarin were unaffected by continuous subcutaneous infusion of treprostinil at an infusion rate of 10 ng/kg/min.
Use in Specific Populations
Pregnancy
- There are no adequate and well controlled studies with treprostinil in pregnant women. Animal reproduction studies have not been conducted with treprostinil administered by the inhalation route. However, studies in pregnant rabbits using continuous subcutaneous (sc) infusions of treprostinil sodium at infusion rates higher than the recommended human sc infusion rate resulted in an increased incidence of fetal skeletal variations associated with maternal toxicity. Animal reproduction studies are not always predictive of human response.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Treprostinil (inhalant) in women who are pregnant.
Labor and Delivery
- No treprostinil treatment-related effects on labor and delivery were seen in animal studies. The effect of treprostinil on labor and delivery in humans is unknown.
Nursing Mothers
- It is not known whether treprostinil is excreted in human milk.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established. Clinical studies of treprostinil did not include patients younger than 18 years to determine whether they respond differently from older patients.
Geriatic Use
- Clinical studies of treprostinil did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
Gender
There is no FDA guidance on the use of Treprostinil (inhalant) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Treprostinil (inhalant) with respect to specific racial populations.
Renal Impairment
- No studies have been performed in patients with renal insufficiency. Since treprostinil and its metabolites are excreted mainly through the urinary route, patients with renal insufficiency may have decreased clearance of the drug and its metabolites and consequently, dose-related adverse outcomes may be more frequent.
Hepatic Impairment
- Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects with mild-to-moderate hepatic insufficiency. Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Treprostinil (inhalant) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Treprostinil (inhalant) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- Patients with acute pulmonary infections should be carefully monitored to detect any worsening of lung disease and loss of drug effect.
IV Compatibility
There is limited information regarding the compatibility of Treprostinil (inhalant) and IV administrations.
Overdosage
- In general, symptoms of overdose with treprostinil include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Provide general supportive care until the symptoms of overdose have resolved.
Pharmacology
Mechanism of Action
- Treprostinil is a prostacyclin analogue. The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation.
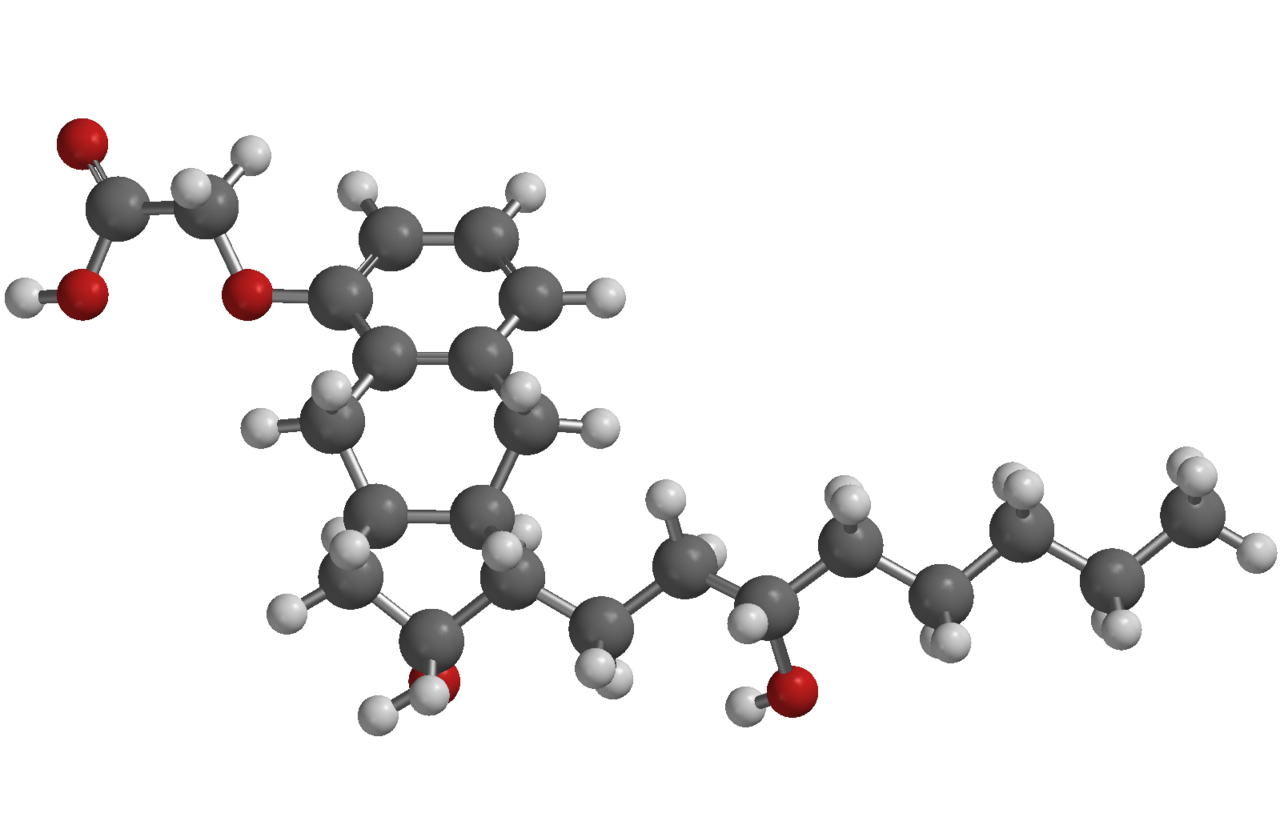
Structure
- Treprostinil is a sterile formulation of treprostinil intended for administration by oral inhalation using the treprostinil Inhalation System. Treprostinil is supplied in 2.9 mL low density polyethylene (LDPE) ampules, containing 1.74 mg treprostinil (0.6 mg/mL). Each ampule also contains 18.9 mg sodium chloride, 18.3 mg sodium citrate, 0.58 mg sodium hydroxide, 11.7 mg 1 N hydrochloric acid, and water for injection. Sodium hydroxide and hydrochloric acid may be added to adjust pH between 6.0 and 7.2.
- Treprostinil is (1R,2R,3aS,9aS)-[2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]inden-5-yl]oxy]acetic acid. Treprostinil has a molecular weight of 390.51 and a molecular formula of C23H34O5.
- The structural formula of treprostinil is:

Pharmacodynamics
- In a clinical trial of 240 healthy volunteers, single doses of treprostinil 54 mcg (the target maintenance dose per session) and 84 mcg (supratherapeutic inhalation dose) prolonged the corrected QTc interval by approximately 10 ms. The QTc effect dissipated rapidly as the concentration of treprostinil decreased.
Pharmacokinetics
- Pharmacokinetic information for single doses of inhaled treprostinil was obtained in healthy volunteers in three separate studies. Treprostinil systemic exposure (AUC and Cmax) post-inhalation was shown to be proportional to the doses administered (18 mcg – 90 mcg).
Absorption and Distribution
- In a three-period crossover study, the bioavailability of two single doses of treprostinil (18 mcg and 36 mcg) was compared with that of intravenous treprostinil in 18 healthy volunteers. Mean estimates of the absolute systemic bioavailability of treprostinil after inhalation were approximately 64% (18 mcg) and 72% (36 mcg).
- Treprostinil plasma exposure data were obtained from two studies at the target maintenance dose, 54 mcg. The mean Cmax at the target dose was 0.91 and 1.32 ng/mL with corresponding mean Tmax of 0.25 and 0.12 hr, respectively. The mean AUC for the 54 mcg dose was 0.81 and 0.97 hr∙ng/mL, respectively.
- Following parenteral infusion, the apparent steady state volume of distribution (Vss) of treprostinil is approximately 14 L/70 kg ideal body weight.
- In vitro treprostinil is 91% bound to human plasma proteins over the 330-10,000 mcg/L concentration range.
Metabolism and Excretion
- Of subcutaneously administered treprostinil, only 4% is excreted unchanged in urine. Treprostinil is substantially metabolized by the liver, primarily by CYP2C8. Metabolites are excreted in urine (79%) and feces (13%) over 10 days. Five apparently inactive metabolites were detected in the urine, each accounting for 10-15% of the dose administered. Four of the metabolites are products of oxidation of the 3-hydroxyloctyl side chain and one is a glucuroconjugated derivative (treprostinil glucuronide).
- The elimination of treprostinil (following subcutaneous administration of treprostinil) is biphasic, with a terminal elimination half-life of approximately 4 hours using a two compartment model.
Special Populations
Hepatic Insufficiency
- Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects presenting with mild-to-moderate hepatic insufficiency. Treprostinil has not been studied in patients with severe hepatic insufficiency.
Renal Insufficiency
- No studies have been performed in patients with renal insufficiency; therefore, since treprostinil and its metabolites are excreted mainly through the urinary route, there is the potential for an increase in both parent drug and its metabolites and an increase in systemic exposure.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- A two-year rat carcinogenicity study was performed with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day. There was no evidence for carcinogenic potential associated with treprostinil inhalation in rats at systemic exposure levels up to 35 times the clinical exposure at the target maintenance dose of 54 mcg. In vitro and in vivo genetic toxicology studies did not demonstrate any mutagenic or clastogenic effects of treprostinil. Treprostinil sodium did not affect fertility or mating performance of male or female rats given continuous subcutaneous (sc) infusions at rates of up to 450 ng treprostinil/kg/min [about 59 times the recommended starting human sc infusion rate (1.25 ng/kg/min) and 8 times the average rate (9.3 ng/kg/min) achieved in clinical trials, on a ng/m2 basis]. In this study, males were dosed from 10 weeks prior to mating and through the 2-week mating period. Females were dosed from 2 weeks prior to mating until gestational day 6.
Developmental Toxicity
- In pregnant rats, continuous sc infusions of treprostinil sodium during organogenesis and late gestational development, at rates as high as 900 ng treprostinil/kg/min (about 117 times the recommended starting human sc infusion rate and about 16 times the average rate achieved in clinical trials, on a ng/m2 basis), resulted in no evidence of harm to the fetus. In pregnant rabbits, effects of continuous sc infusions of treprostinil during organogenesis were limited to an increased incidence of fetal skeletal variations (bilateral full rib or right rudimentary rib on lumbar vertebra 1) associated with maternal toxicity (reduction in body weight and food consumption) at an infusion rate of 150 ng treprostinil/kg/min (about 41 times the starting human sc infusion rate and 5 times the average rate achieved in clinical trials, on a ng/m2 basis).
Inhalational Toxicity
- Rats and dogs that received daily administrations of treprostinil by inhalation for 3 months developed respiratory tract lesions (respiratory epithelial degeneration, goblet cell hyperplasia/hypertrophy, epithelial ulceration, squamous epithelial degeneration and necrosis, and lung hemorrhage). Some of the same lesions seen in animals sacrificed at the end of treatment (larynx, lung and nasal cavity lesions in rats, and lesions of the larynx in dogs) were also observed in animals sacrificed after a 4-week recovery period. Rats also developed cardiac changes (degeneration/fibrosis). A no-effect dose level for these effects was not demonstrated in rats (doses as low as 7 µg/kg/day were administered); whereas 107 µg/kg/day was a no-effect dose level in dogs.
- In a 2-year rat study with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day, there were more deaths (11) in the mid and high dose treprostinil groups during the first 9 weeks of the study, compared to 1 in control groups. At the high dose level, males showed a higher incidence of inflammation in teeth and preputial gland, and females showed higher incidences of inflammation and urothelial hyperplasia in the urinary bladder. The exposures in rats at mid and high dose levels were about 15 and 35 times, respectively, the clinical exposure at the target maintenance dose of 54 mcg.
Clinical Studies
Pulmonary Arterial Hypertension (WHO Group I)
- TRIUMPH I, was a 12-week, randomized, double-blind, placebo-controlled multi-center study of patients with PAH. The study population included 235 clinically stable subjects with pulmonary arterial hypertension (WHO Group 1), nearly all with NYHA Class III (98%) symptoms who were receiving either bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase-5 inhibitor) for at least three months prior to study initiation. Concomitant therapy also could have included anticoagulants, other vasodilators (e.g., calcium channel blockers), diuretics, oxygen, and digitalis, but not a prostacyclin. These patients were administered either placebo or treprostinil in four daily treatment sessions with a target dose of 9 breaths (54 mcg) per session over the course of the 12-week study. Patients were predominantly female (82%), had the origin of PAH as idiopathic/heritable (56%), secondary to connective tissue diseases (33%) or secondary to HIV or previous use of anorexigens (12%); bosentan was the concomitant oral medication in 70% of those enrolled, sildenafil in 30%.
- The primary efficacy endpoint of the trial was the change in six-minute walk distance (6MWD) relative to baseline at 12 weeks. 6MWD was measured at peak exposure (between 10 and 60 minutes after dosing), and 3-5 hours after bosentan or 0.5-2 hours after sildenafil. Patients receiving treprostinil had a placebo-corrected median change from baseline in peak 6MWD of 20 meters at Week 12 (p<0.001). The distribution of these 6MWD changes from baseline at Week 12 were plotted across the range of observed values (Figure 1). 6MWD measured at trough exposure (defined as measurement of 6MWD at least 4 hours after dosing) improved by 14 meters. There were no placebo-controlled 6MWD assessments made after 12 weeks.

Figure 1: Distributions of 6MWD Changes from Baseline at Week 12 during Peak Plasma Concentration of treprostinil
- The placebo-corrected median treatment effect on 6MWD was estimated (using the Hodges-Lehmann estimator) within various subpopulations defined by age quartile, gender, geographic region of the study site, disease etiology, baseline 6MWD quartile, and type of background therapy (Figure 2).

Figure 2. Placebo Corrected Median Treatment Effect (Hodges-Lehmann estimate with 95% CI) on 6MWD Change from Baseline at Week 12 During Peak Plasma Concentration of treprostinil for Various Subgroups
Long-term Treatment of PAH
- In long-term follow-up of patients who were treated with treprostinil in the pivotal study and the open-label extension (N=206), Kaplan-Meier estimates of survival at 1, 2, and 3 years were 97%, 91%, and 82%, respectively. These uncontrolled observations do not allow comparison with a control group not given treprostinil and cannot be used to determine the long-term effect of treprostinil on mortality.
How Supplied
- Treprostinil (treprostinil) inhalation solution is supplied in 2.9 mL clear LDPE ampules packaged as four ampules in a foil pouch.Treprostinil is a clear colorless to slightly yellow solution containing 1.74 mg treprostinil per ampule at a concentration of 0.6 mg/mL.
Storage
- Ampules of treprostinil are stable until the date indicated when stored in the unopened foil pouch at 25°C (77°F), with excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Once the foil pack is opened, ampules should be used within 7 days. Because treprostinil is light-sensitive, unopened ampules should be stored in the foil pouch.
Images
Drug Images
{{#ask: Page Name::Treprostinil (inhalant) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Treprostinil (inhalant) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be properly trained in the administration process for Tyvaso, including dosing, Tyvaso Inhalation System set up, operation, cleaning, and maintenance, according to the instructions for use.
- To avoid potential interruptions in drug delivery because of equipment malfunction, patients should have access to a back-up Tyvaso Inhalation System device.
- In the event that a scheduled treatment session is missed or interrupted, therapy should be resumed as soon as possible.
- Patients should avoid skin or eye contact with Tyvaso. If Tyvaso comes in contact with the skin or eyes, instruct patients to rinse immediately with water.
Precautions with Alcohol
- Alcohol-Treprostinil (inhalant) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TYVASO ®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.