Taliglucerase alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Taliglucerase alfa is a hydrolytic lysosomal glucocerebroside-specific enzyme that is FDA approved for the treatment of of type 1 Gaucher disease. Common adverse reactions include pruritus, flushing, headache, arthralgia, pain in extremity, abdominal pain, vomiting, fatigue, back pain, dizziness, nausea and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Taliglucerase alfa is indicated for long-term enzyme replacement therapy (ERT) for adult patients with a confirmed diagnosis of Type 1 Gaucher disease.
Dosage
Recommended Dosage
- Treatment-naïve patients: The recommended dosage for treatment-naïve adult patients is 60 units per kg of body weight administered every other week as a 60 to 120 minute intravenous infusion.
- Patients switching from imiglucerase: Patients currently being treated with imiglucerase for Type 1 Gaucher disease can be switched to taliglucerase alfa. Patients previously treated on a stable dosage of imiglucerase are recommended to begin treatment with ELELYSO at that same dosage when they switch from imiglucerase to taliglucerase alfa dosage adjustments can be made based on achievement and maintenance of each patient's therapeutic goals.
- Taliglucerase alfa should be reconstituted, diluted, and administered under the supervision of a healthcare professional.
DOSAGE FORMS AND STRENGTHS
- For injection: lyophilized powder for reconstitution; 200 units/vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Taliglucerase alfa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Taliglucerase alfa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Taliglucerase alfa is indicated for long-term enzyme replacement therapy (ERT) for pediatric patients with a confirmed diagnosis of Type 1 Gaucher disease.
Dosage
Recommended Dosage
- Treatment-naïve patients: The recommended dosage for treatment of pediatric patients 4 years of age and older is 60 units per kg of body weight administered every other week as a 60 to 120 minute intravenous infusion.
- Patients switching from imiglucerase: Patients currently being treated with imiglucerase for Type 1 Gaucher disease can be switched to taliglucerase alfa. Patients previously treated on a stable dosage of imiglucerase are recommended to begin treatment with taliglucerase alfa at that same dosage when they switch from imiglucerase to taliglucerase alfa dosage adjustments can be made based on achievement and maintenance of each patient's therapeutic goals.
- Taliglucerase alfa should be reconstituted, diluted, and administered under the supervision of a healthcare professional.
DOSAGE FORMS AND STRENGTHS
- For injection: lyophilized powder for reconstitution; 200 units/vial.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Taliglucerase alfa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Taliglucerase alfa in pediatric patients.
Contraindications
- None
Warnings
Hypersensitivity Reactions Including Anaphylaxis
- Serious hypersensitivity reactions, including anaphylaxis, have occurred in some patients treated with taliglucerase alfa. In clinical trials, 2 of 72 (2.8%) patients treated with taliglucerase alfa experienced signs and symptoms consistent with anaphylaxis. Signs and symptoms of these patients included urticaria, hypotension, flushing, wheezing, chest tightness, nausea, vomiting, and dizziness. These reactions occurred during taliglucerase alfa infusion.
- In clinical trials with taliglucerase alfa, 21 of 72 (29%) patients experienced hypersensitivity reactions, including anaphylaxis. Signs and symptoms of hypersensitivity reactions included pruritus, angioedema, flushing, erythema, rash, nausea, vomiting, cough, chest tightness, and throat irritation. These reactions have occurred up to 3 hours after the start of infusion.
- Due to the potential for anaphylaxis, appropriate medical support should be readily available when taliglucerase alfa is administered. Observe patients closely for an appropriate period of time after administration of taliglucerase alfa, taking into account the time to onset of anaphylaxis seen in clinical trials. Inform patients of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs and symptoms occur. If anaphylaxis occurs, taliglucerase alfa should be immediately discontinued, and appropriate medical treatment should be initiated.
- Management of hypersensitivity reactions should be based on the severity of the reaction and include slowing or temporary interruption of the infusion and/or administration of antihistamines, antipyretics, and/or corticosteroids for mild reactions. Pretreatment with antihistamines and/or corticosteroids may prevent subsequent hypersensitivity reactions. Patients were not routinely premedicated prior to infusion of taliglucerase alfa during clinical studies. If severe hypersensitivity reactions occur, immediately stop the infusion of taliglucerase alfa and initiate appropriate treatment.
- Consider the risks and benefits of re-administering taliglucerase alfa in patients who have experienced a severe reaction associated with taliglucerase alfa. Caution should be exercised upon rechallenge, and appropriate medical support should be readily available.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In the clinical trials with taliglucerase alfa, either as initial therapy or as therapy following a switch from imiglucerase (N=72), the most common (≥ 5%) adverse reactions included pruritus, flushing, headache, arthralgia, pain in extremity, abdominal pain, vomiting, fatigue, back pain, dizziness, nausea, and rash.
Clinical Trials of Taliglucerase alfa as Initial Therapy
- Clinical Trial in Patients 19 Years and Older
- The safety of taliglucerase alfa at dosages of either 30 units/kg (n=16) or 60 units/kg (n=16) every other week was assessed in 32 adult treatment-naïve patients (aged 19 to 74 years) with Type 1 Gaucher disease in a 9-month randomized clinical trial.
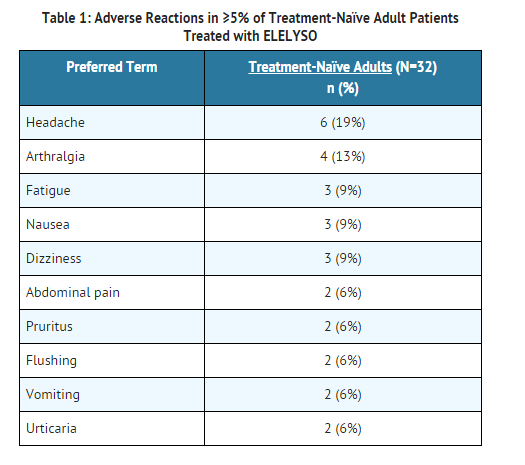
- Similar adverse reactions were observed in patients who continued taliglucerase alfa treatment during the extension trial for up to 24 months. One patient experienced a mild and intermittent Type III immune-mediated fixed drug eruption and continued in the study.
- Clinical Trial in Patients 16 Years and Younger
- The safety of taliglucerase alfa at dosages of either 30 units/kg (n=4) or 60 units/kg (n=5) every other week was assessed in 9 pediatric treatment-naïve patients (aged 2 to 13 years) with Type 1 Gaucher disease in a 12-month randomized clinical trial.
- The most common adverse reaction (≥10%) was vomiting, which occurred in 4 of 9 patients. Two patients developed hypersensitivity reactions; one patient experienced severe vomiting and gastrointestinal inflammation, and 1 experienced mild throat irritation and chest discomfort. Both patients responded to treatment with antihistamines and continued taliglucerase alfa treatment.
Clinical Trial in Patients Switching from Imiglucerase Treatment to Taliglucerase alfa
- The safety of taliglucerase alfa was assessed in 31 patients (26 adult and 5 pediatric patients), ages 6 to 66 years old, with Type 1 Gaucher disease who had previously been receiving treatment with imiglucerase for a minimum of 2 years. Taliglucerase alfa was administered for 9 months at the same number of units as each patient's previous imiglucerase dose.
Immunogenicity
- As with all therapeutic proteins, patients may develop anti-drug antibodies (ADA) to taliglucerase alfa.
- In clinical trials of treatment-naïve adults, 17 (53%) of 32 patients developed ADA during treatment with taliglucerase alfa, and 2 (6%) of 32 patients tested positive for ADA at baseline prior to taliglucerase alfa treatment. Of the 17 patients who developed ADA during taliglucerase alfa treatment, 6 patients (35%) developed hypersensitivity reactions, 2 of whom met criteria for anaphylaxis. Two of the 17 patients who developed ADA during taliglucerase alfa treatment discontinued treatment due to hypersensitivity reactions, one of whom had met criteria for anaphylaxis. Of the 2 patients who tested positive for ADA prior to initiation of taliglucerase alfa treatment, one patient developed a hypersensitivity reaction during the first dose of taliglucerase alfa and withdrew from the study. The second patient did not experience an adverse reaction.
- In a clinical trial of treatment-naïve pediatric patients, 2 (22%) of 9 patients developed ADA during treatment with taliglucerase alfa, and one of 9 patients was ADA-positive prior to initiation of taliglucerase alfa. Two patients (1 who developed ADA during treatment and 1 who was ADA-positive at baseline) experienced hypersensitivity reactions. Both patients continued treatment with taliglucerase alfa.
- In a clinical trial of 31 patients (26 adult and 5 pediatric patients) who switched from imiglucerase to taliglucerase alfa treatment, 4 adults (13% of patients) developed ADA during treatment with taliglucerase alfa. Four additional patients (13%, 2 adults and 2 children) tested positive for ADA at baseline but became ADA-negative after the switch to taliglucerase alfa. Two adult patients (1 patient who developed ADA after the switch and 1 who was ADA positive at baseline) experienced hypersensitivity reactions. Both patients continued treatment with taliglucerase alfa.
- The relationship between ADA and hypersensitivity reactions is not fully understood. Monitoring for ADA to taliglucerase alfa may be useful in ADA positive patients or in patients who have experienced hypersensitivity reactions to taliglucerase alfa or other enzyme replacement therapies.
- Twenty-nine of the 30 adult and pediatric patients who tested positive for ADA were tested for neutralizing antibodies capable of inhibiting the enzymatic activity of ELELYSO. Neutralizing antibodies were detected in 3 (10.3%) of 29 patients, 2 treatment-naïve adult patients and 1 adult patient who switched from imiglucerase. Due to limited available data, it is not possible to determine a relationship between the presence of neutralizing antibodies and therapeutic response with taliglucerase alfa.
- Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to taliglucerase alfa with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of ELELYSO in countries where it is marketed. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Anaphylaxis
Drug Interactions
There is limited information regarding Taliglucerase alfa Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Category B
Risk Summary
- There are no adequate and well-controlled studies of taliglucerase alfa in pregnant women. In animal reproduction studies with taliglucerase alfa in pregnant rats at intravenous doses up to 5 times the recommended human dose (RHD) and in pregnant rabbits at intravenous doses up to 5 times the RHD, there was no evidence of harm to the fetus. Because animal reproduction studies are not always predictive of human response, taliglucerase alfa should be used during pregnancy only if clearly needed.
Clinical Considerations
Disease-associated maternal and embryo-fetal risk
- Women with Type 1 Gaucher disease have an increased risk of spontaneous abortion if disease symptoms are not treated and controlled pre-conception and during a pregnancy. Pregnancy may exacerbate existing Type 1 Gaucher disease symptoms or result in new disease manifestations. Type 1 Gaucher disease manifestations may lead to adverse pregnancy outcomes, including hepatosplenomegaly which can interfere with the normal growth of a fetus and thrombocytopenia which can lead to increased bleeding and possible hemorrhage.
Animal Data
- Reproduction studies have been performed with taliglucerase alfa administered during the period of organogenesis in rats and rabbits. In rats, intravenous doses up to 55 mg/kg/day (about 5 times the RHD of 60 units/kg based on the body surface area) did not cause any adverse effects on embryofetal development. In rabbits, intravenous doses up to 27.8 mg/kg/day (about 5 times the RHD of 60 units/kg based on the body surface area) did not show any embryofetal toxicity.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Taliglucerase alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Taliglucerase alfa during labor and delivery.
Nursing Mothers
- It is not known whether taliglucerase alfa is present in human milk. Because many drugs are present in human milk, caution should be exercised when taliglucerase alfa is administered to a nursing woman.
Pediatric Use
- The use of taliglucerase alfa for treatment of pediatric patients with Type 1 Gaucher disease is supported by evidence of effectiveness from adequate and well-controlled trials of taliglucerase alfa in adults, with additional pharmacodynamic data from 5 pediatric patients and pharmacokinetic data from 9 pediatric patients who participated in clinical trials. Data from 14 pediatric patients were included in the safety evaluation. There are insufficient data to inform dosing in patients less than 4 years of age.
- Pediatric patients experienced a higher frequency of vomiting during taliglucerase alfa treatment (4 of 9 treatment-naïve patients) than adult patients, and this may be a symptom of hypersensitivity reaction. The frequencies of other adverse reactions were similar between pediatric and adult patients.
Geriatic Use
- During clinical trials, 8 patients aged 65 or older were treated with taliglucerase alfa. Clinical trials of taliglucerase alfa did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Taliglucerase alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Taliglucerase alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Taliglucerase alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Taliglucerase alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Taliglucerase alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Taliglucerase alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Administration Instructions
- After reconstitution and dilution, the preparation should be administered via intravenous infusion and filtered through an in-line low protein-binding 0.2 μm filter.
- For pediatric patients: An initial infusion rate of 1 mL/minute should be used. After tolerability to taliglucerase alfa is established, the infusion rate may be increased, but should not exceed the maximum recommended infusion rate of 2 mL/minute. The total volume of the infusion should be delivered over a minimum of 60 minutes.
- For adult patients: An initial infusion rate of 1.2 mL/minute should be used. After tolerability to taliglucerase alfa is established, the infusion rate may be increased, but should not exceed the maximum recommended infusion rate of 2.2 mL/minute. The total volume of the infusion should be delivered over a minimum of 60 minutes.
- As taliglucerase alfa contains no preservative, the product should be used immediately once reconstituted. If immediate use is not possible, the reconstituted product may be stored for up to 24 hours at 2 to 8 °C (36 to 46 °F) under protection from light or up to 4 hours at 20 to 25 °C (68 to 77 °F) without protection from light. The diluted product may be stored for up to 24 hours at 2 to 8 °C (36 to 46 °F) under protection from light. Storage of the reconstituted product and the diluted product should not exceed a total of 24 hours. Do not freeze. Discard any unused product.
Monitoring
There is limited information regarding Monitoring of Taliglucerase alfa in the drug label.
IV Compatibility
Preparation Instructions
- Each vial of taliglucerase alfa provides 200 units of taliglucerase alfa and is intended for single use only. Do not use the vial more than one time. The reconstitution and dilution steps must be completed using aseptic technique.
- Taliglucerase alfa should be reconstituted with Sterile Water for Injection and diluted with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 mL to 200 mL, and delivered by intravenous infusion.
- Prepare taliglucerase alfa according to the following steps. Use aseptic technique.
- Determine the number of vials to be reconstituted based on the patient's weight and the recommended dose of 60 units/kg, using the following calculations (1–3):
- Total dose in units = Patient's weight (kg) × dose (units/kg)
- Total number of vials = Total dose in units divided by 200 units/vial
- Round up to the next whole vial.
- Remove the required number of vials from the refrigerator. Do not leave these vials at room temperature longer than 24 hours prior to reconstitution. Do not heat or microwave these vials.
- Reconstitute each vial of taliglucerase alfa with 5.1 mL of Sterile Water for Injection to yield a reconstituted product volume of 5.3 mL and a withdrawal volume of 5 mL. Upon reconstitution, mix vials gently. DO NOT SHAKE. Prior to further dilution, visually inspect the solution in the vials; the solution should be clear and colorless. Do not use if the solution is discolored or if foreign particulate matter is present.
- Withdraw the calculated dose of drug from the appropriate number of vials and dilute with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 to 200 mL.
- For pediatric patients, a final volume of 100 to 120 mL should be used.
- For adult patients, a final volume of 130 to 150 mL may be used. However, if the volume of reconstituted product alone is equal to or greater than 130 to 150 mL, then the final volume should not exceed 200 mL.
- Mix gently. DO NOT SHAKE. Since this is a protein solution, slight flocculation (described as translucent fibers) occurs occasionally after dilution.
Overdosage
There is limited information regarding Chronic Overdose of Taliglucerase alfa in the drug label.
Pharmacology
Taliglucerase alfa
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 56637.9397 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 18.9-28.7 minutes |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | Intravenous infusion |
Mechanism of Action
- Gaucher disease is an autosomal recessive disorder caused by mutations in the human glucocerebrosidase gene, which results in a reduced activity of the lysosomal enzyme glucocerebrosidase. Glucocerebrosidase catalyzes the conversion of the sphingolipid glucocerebroside into glucose and ceramide. The enzymatic deficiency results in accumulation of substrate glucocerebroside primarily in the lysosomal compartment of macrophages, giving rise to foam cells or "Gaucher cells," which accumulate in the liver, spleen and bone marrow.
- Taliglucerase alfa is a recombinant analog of human lysosomal glucocerebrosidase that catalyzes the hydrolysis of glucocerebroside to glucose and ceramide, reducing the amount of accumulated glucocerebroside. Taliglucerase alfa uptake into cellular lysosomes is mediated by binding of taliglucerase alfa mannose oligosaccharide chains to specific mannose receptors on the cell surface leading to internalization and subsequent transport to the lysosomes.
Structure
- Taliglucerase alfa, a hydrolytic lysosomal glucocerebroside-specific enzyme for intravenous infusion, is a recombinant active form of the lysosomal enzyme, β-glucocerebrosidase, which is expressed in genetically modified carrot plant root cells cultured in a disposable bioreactor system (ProCellEx®). β-Glucocerebrosidase (β-D-glucosyl-N-acylsphingosine glucohydrolase, E.C. 3.2.1.45) is a lysosomal glycoprotein enzyme that catalyzes the hydrolysis of the glycolipid glucocerebroside to glucose and ceramide.
- Taliglucerase alfa is produced by recombinant DNA technology using plant cell culture (carrot). Purified taliglucerase alfa is a monomeric glycoprotein containing 4 N-linked glycosylation sites (Mr = 60,800). Taliglucerase alfa differs from native human glucocerebrosidase by two amino acids at the N terminal and up to 7 amino acids at the C terminal. Taliglucerase alfa is a glycosylated protein with oligosaccharide chains at the glycosylation sites having terminal mannose sugars. These mannose-terminated oligosaccharide chains of taliglucerase alfa are specifically recognized by endocytic carbohydrate receptors on macrophages, the cells that accumulate lipid in Gaucher disease.
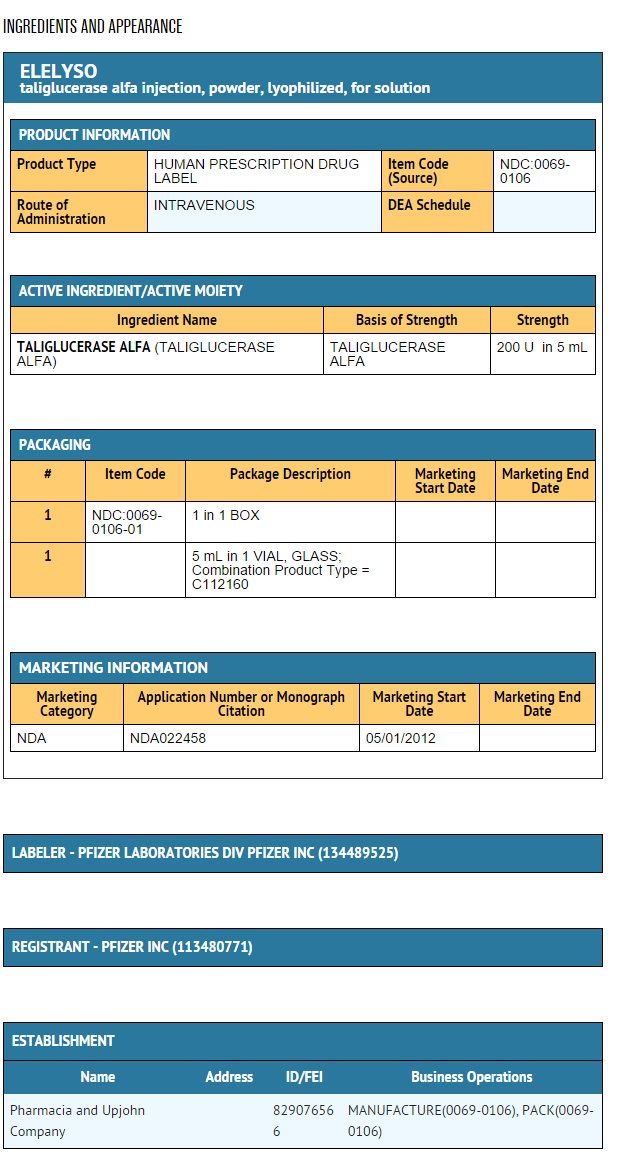
- Taliglucerase alfa is supplied as a sterile, non-pyrogenic, lyophilized product. The quantitative composition of each 200 Unit vial is D-mannitol (206.7 mg), polysorbate 80 (0.56 mg), sodium citrate (30.4 mg), and taliglucerase alfa (212 units). Citric acid may be added to adjust the pH at the time of manufacture.
- A Unit is the amount of enzyme that catalyzes the hydrolysis of 1 micromole of the synthetic substrate para-nitrophenyl-β-D-glucopyranoside (pNP-Glc) per minute at 37°C. After reconstitution with Sterile Water for Injection, taliglucerase alfa concentration is 40 units/mL. Reconstituted solutions have a pH of approximately 6.0.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Taliglucerase alfa in the drug label.
Pharmacokinetics
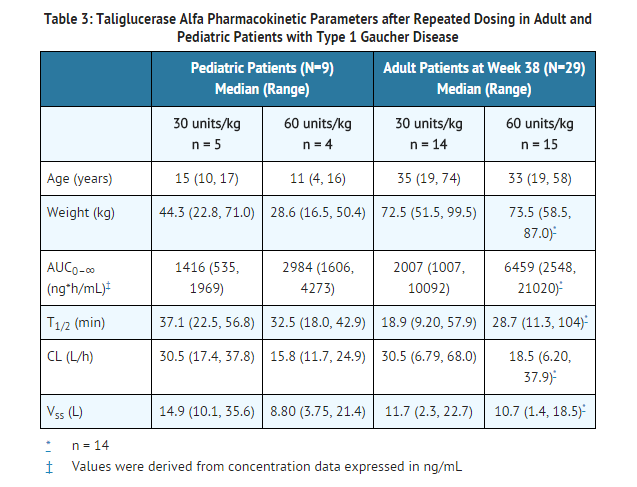
- Pharmacokinetics of taliglucerase alfa were evaluated in 38 patients (29 adult and 9 pediatric patients) who received intravenous infusions of ELELYSO 30 units/kg or 60 units/kg every other week. ELELYSO 30 units/kg is not a recommended dose in treatment-naïve Gaucher disease patients. The pharmacokinetic parameters in adult and pediatric patients are summarized in Table 3.
- In adult Type 1 Gaucher disease patients treated with taliglucerase alfa 30 units/kg or 60 units/kg (N=29) every other week as initial therapy, pharmacokinetics were determined with the first dose and at Week 38 of treatment. The pharmacokinetics of taliglucerase alfa appeared to be nonlinear with a greater than dose-proportional increase in exposure at the doses studied.
- No significant accumulation or change in taliglucerase alfa pharmacokinetics over time from Weeks 1 to 38 was observed with repeated dosages of 30 units/kg or 60 units/kg every other week.Based on the limited data, there were no significant pharmacokinetic differences between male and female patients in this study.
- Pharmacokinetics of taliglucerase alfa were evaluated in 9 pediatric patients 4 to 17 years of age with Type 1 Gaucher disease who were treated with taliglucerase alfa for 10 to 27 months. Six of the 9 patients were treatment-naïve, and 3 patients were switched from imiglucerase. In both the 30 units/kg and 60 units/kg dose groups, clearance values in pediatric patients were similar to those in adult patients. AUC values in pediatric patients were lower than AUC values in adult patients, due to weight-based dosing of taliglucerase alfa and lower body weights in pediatric patients.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with taliglucerase alfa. In a male and female fertility study in rats, taliglucerase alfa did not cause any significant adverse effect on male or female fertility parameters up to a maximum dose of 55 mg/kg/day (about 5 times the recommended human dose of 60 units/kg based on the body surface area).
Clinical Studies
Clinical Trials of Taliglucerase alfa as Initial Therapy
Clinical Trial in Patients 19 Years and Older
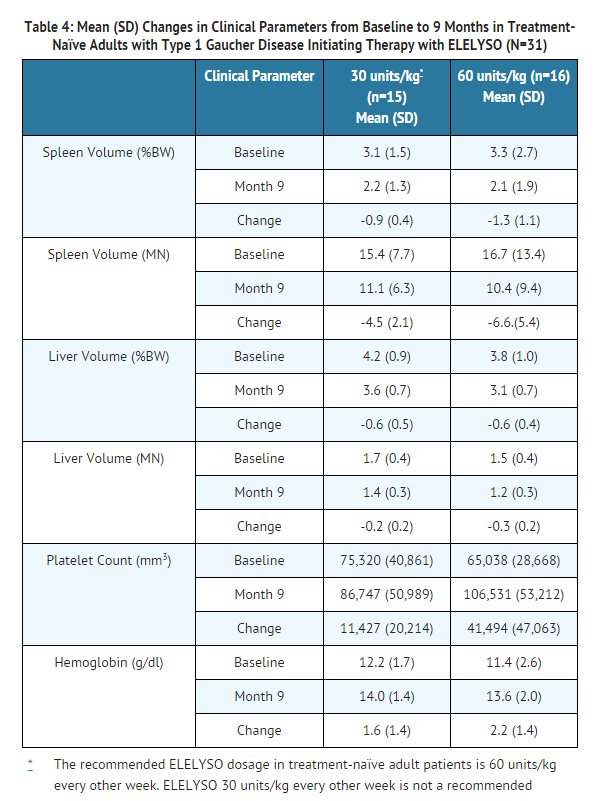
- The safety and efficacy of taliglucerase alfa were assessed in 31 adult patients with Type 1 Gaucher disease. The trial was a 9-month, multi-center, double-blind, randomized trial in patients with Gaucher disease-related enlarged spleens (>8 times normal) and thrombocytopenia (<120,000 /mm3). Sixteen patients had enlarged livers and ten patients had anemia at baseline. All patients were naïve to ERT. Patients with severe neurological symptoms were excluded from the trial. Patients were 19 to 74 years of age (mean age 36 years), and 48% were male. Patients were randomized to receive taliglucerase alfa at a dosage of either 30 units/kg (n=15) or 60 units/kg (n=16) every other week. The recommended dosage in treatment-naïve adult patients is 60 units/kg every other week. Taliglucerase alfa 30 units/kg every other week is not a recommended dosage.
- Table 4 shows the baseline values and mean (±SD) changes in clinical parameters (spleen volume, liver volume, platelet count, and hemoglobin) after 9 months of treatment with taliglucerase alfa. For all clinical trials, liver and spleen volumes were measured by MRI and are reported as percentage of body weight (%BW) and multiples of normal (MN). The observed change from baseline in the primary endpoint, reduction in spleen volume, was considered to be clinically meaningful in light of the natural history of untreated Gaucher disease.

- Twenty-six of the 31 patients in this clinical trial continued blinded treatment with taliglucerase alfa in an extension trial for a total treatment duration of 24 months. The following data are the changes in clinical parameters from baseline to Month 24 for the 30 units/kg (n=17) and 60 units/kg (n=14) dose groups, respectively: mean (SD) spleen volume (%BW) decreased -1.4 (0.6) and -2.0 (2.0); hemoglobin increased 1.3 (0.7) g/dL and 2.4 (2.3) g/dL; liver volume (%BW) decreased -1.1 (0.5) and -1.0 (0.7); and platelet count increased 28,433 (31,996) /mm3 and 72,029 (68,157) /mm3.
Clinical Trial in patients 16 years and younger
- The safety and efficacy of taliglucerase alfa were assessed in 9 pediatric patients with Type 1 Gaucher disease. The trial was a 12-month, multi-center, double-blind, randomized study in treatment-naïve patients. Patients were 2 to 13 years of age (mean age 8.1 years), and 67% were male. Patients were randomized to receive taliglucerase alfa at a dosage of either 30 units/kg (n=4) or 60 units/kg (n=5) every other week. The recommended taliglucerase alfa dosage in treatment-naïve pediatric patients is 60 units/kg every other week. Taliglucerase alfa 30 units/kg every other week is not a recommended dosage.
- Mean (±SD) baseline spleen volume for the 60 units/kg dose group was 29.4 (±24.3) MN, and decreased to 12.9 (±7.2) MN at 12 months. Baseline liver volume for the 60 units/kg dose group was 2.2 (±0.5) MN, and decreased to 1.7 (±0.3) MN at 12 months. Mean (±SD) platelet count for the 60 units/kg dose group was 99,600 (±42,899)/mm3 at baseline, and increased to 172,200 (±89,290)/mm3 at 12 months.
Clinical Trial in Patients Switching from Imiglucerase Treatment to Taliglucerase alfa
- The safety and efficacy of taliglucerase alfa were assessed in 31 patients (26 adult and 5 pediatric patients) with Type 1 Gaucher disease who were switched from imiglucerase to ELELYSO. The trial was a 9-month, multi-center, open-label, single arm study in patients who had been receiving treatment with imiglucerase at dosages ranging from 9.5 units/kg to 60 units/kg every other week for a minimum of 2 years. Patients were required to be clinically stable and have a stable biweekly dose of imiglucerase for at least 6 months prior to enrollment. Patients were 6 to 66 years of age (mean age 42 years, including pediatric patients), and 55% were male. Imiglucerase therapy was stopped, and treatment with taliglucerase alfa was administered every other week at the same number of units as each patient's previous imiglucerase dose. If needed, adjustment of dosage was allowed during the study in order to maintain stability of clinical parameters (i.e., spleen volume, liver volume, platelet count, and hemoglobin).
- Mean (±SD) organ volumes and hematologic values remained stable through 9 months of taliglucerase alfa treatment. At baseline, spleen volume was 5.2 (±0.9) MN, liver volume was 1.0 (±0.1) MN, platelet count was 161,137 (±73,387)/mm3, and hemoglobin was 13.5 (±1.4) g/dL. After 9 months of taliglucerase alfa treatment, spleen volume was 4.8 (±0.9) MN, liver volume was 1.0 (±0.0) MN, platelet count was 161,167 (±80,820)/mm3, and hemoglobin was 13.4 (±1.5) g/dL. Taliglucerase alfa dose remained unchanged in 30 of 31 patients. One patient required a dose increase at Week 24 (from 9.5 units/kg to 19 units/kg) for a platelet count of 92,000/mm3 at Week 22, which subsequently increased to 170,000/mm3 at Month 9.
How Supplied
- Taliglucerase alfa is supplied as a lyophilized powder in single use vials. Each vial contains 200 units of taliglucerase alfa.
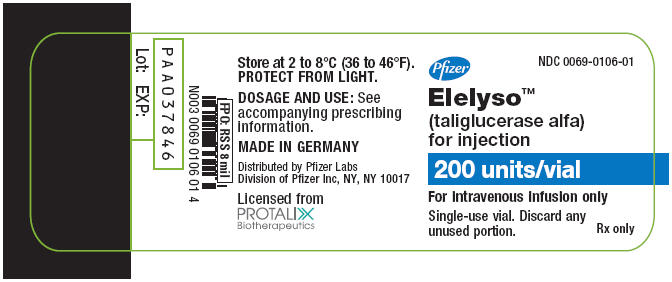
NDC 0069-0106-01, 200 units per vial
Storage
- Store ELELYSO under refrigeration at 2° C to 8° C (36° F to 46° F). Do not freeze. Protect vials from light.
Images
Drug Images
{{#ask: Page Name::Taliglucerase alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Taliglucerase alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hypersensitivity Reactions Including Anaphylaxis
- Advise patients and caregivers that reactions related to administration and infusion may occur during and after ELELYSO treatment, including life-threatening anaphylaxis and severe hypersensitivity reactions. Inform patients of the signs and symptoms of anaphylaxis and hypersensitivity reactions, and have them seek medical care should signs and symptoms occur. Inform patients that they should be carefully re-evaluated for treatment with ELELYSO if serious hypersensitivity reactions, including anaphylaxis, occur. Reduction of the infusion rate and/or pre-treatment with antihistamines, antipyretics and/or corticosteroids may prevent subsequent reactions
Precautions with Alcohol
- Alcohol-Taliglucerase alfa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ELELYSO
Look-Alike Drug Names
- A® — B®[1]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Taliglucerase alfa
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Taliglucerase alfa |Label Name=Teleglucerase image1.jpg
}}
{{#subobject:
|Label Page=Taliglucerase alfa |Label Name=Teleglucerase alpha image.jpg
}}
{{#subobject:
|Label Page=Taliglucerase alfa |Label Name=Teleglucerase alpha ingredients and appearance.png
}}