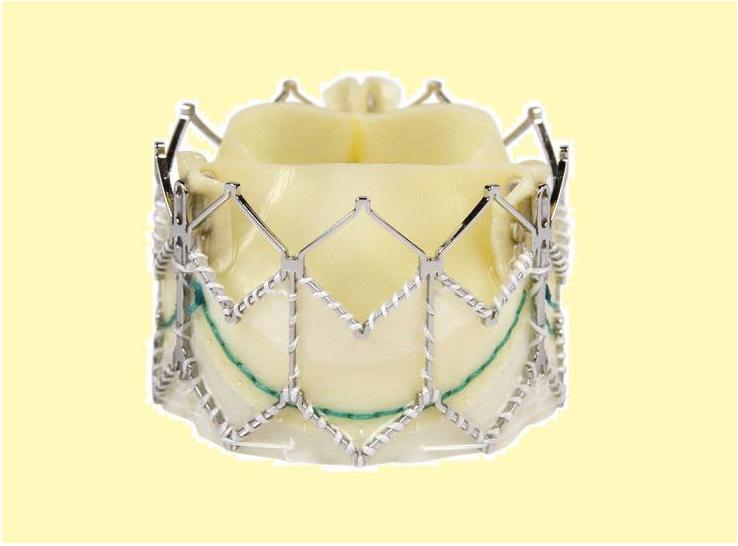
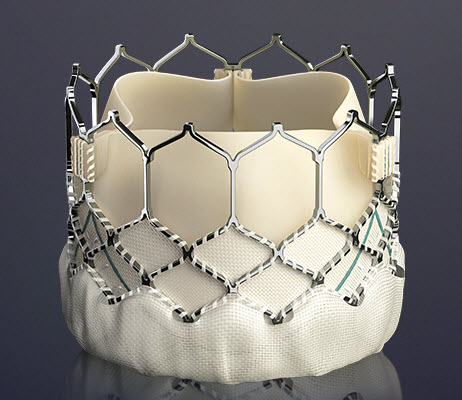
TAVR Valve types
|
Aortic Stenosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Percutaneous Aortic Balloon Valvotomy (PABV) or Aortic Valvuloplasty |
|
Transcatheter Aortic Valve Replacement (TAVR) |
|
Case Studies |
|
TAVR Valve types On the Web |
|
American Roentgen Ray Society Images of TAVR Valve types |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1],Associate Editor(s)-in-Chief: Seyedmahdi Pahlavani, M.D. [2]Tarek Nafee, M.D. [3]
Overview
There are currently 8 prosthetic transcatheter valve types which are available. Two of these are FDA approved in the United States.[1] The CHOICE trial is a small study which compared the most prominent two valves (CORE and SAPIEN) and no major significant differences were found in primary clinical endpoints between both valves. Nevertheless, there are situations, anatomical variability, and operator preferences that continue to make one valve more preferable to the other, depending on the situation. There are currently three ongoing major trials comparing newer generation valves to the aforementioned devices.
Valve types
Synopsis
| Valve | Type of Expansion | Device Placement | Year of Introduction | FDA approval | Available Sizes (in mm) |
|---|---|---|---|---|---|
| Core valve | Self expanding | TF | 2005 | Yes | 23, 26, 29 |
| Edwards Sapien valves | Balloon expandable | TF | 2007 | Yes | 20, 23, 26, 29 |
| Acurate neo valve[2] | Self expanding | TF, TA | 2011 | No | S, M, L † |
| Jena Valve | Self expanding | TF, TA | 2011 | No | 23, 25, 27 |
| St. Jude Medical Portico | Self expanding | TF | 2014 | No | 23, 25, 27, 29 |
| Direct Flow Medical Valve | Self expanding | TF | 2013 | No | 23, 25, 27, 29 |
| Medtronic Engager Valve | Self expanding | TF | 2013 | No | 23, 26 |
| Boston Scientific Lotus Valve | Mechanically Expanded | TF | 2013 | No | 23,25,27 |
TF: Trasfemoral, TA: Transapical
† They claimed that can cover aortic annulus diameters from 21 to 27 mm.
Description of Valves
Core Valve
The Core-Valve device was first inserted in 2005.[3][4] It consists of three leaflets of bioprosthetic pericardial valve tissue mounted on a self-expendable nitinol stent, which expands from the left ventricular outflow tract (LVOT) to the ascending aorta. The Core Valve frame is currently available in 3 sizes (23 mm, 26 mm and 29 mm). The multilevel nitinol frame was designed for optimal functionality,stability,and durability. The inflow portion of the frame exerts high radial expansive force to provide proper support of the frame within the annular location.[5] The design of this portion of the frame with its radial strength prevents annular recoil, allowing the frame to partially conform to the non circular shape of the aortic annulus. The center portion of the frame has very high hoop strength that resists size and shape deformation which is a very important part of the device since it contains the valve leaflets, which are supra-annular. This center portion of the frame is concave to allow normal flow of blood through the coronary arteries and coronary cannulation after implantation. The largest part of the frame is the outflow portion that exerts low radial forces and allow optimal flow of blood through the valve. For the tissue in the valves, porcine (pig) pericardium was selected due to its lower profile (compared with bovine (cow) pericardium) and its durability. The trileaflet valve is made of six individual pieces of porcine pericardium, with three pieces used to make a skirt at the inflow section of the valve thus preventing aortic regurgitation and three leaflet elements that are constructed with long commissures to distribute the aortic pressure load to the valve leaflets and the commissural posts.
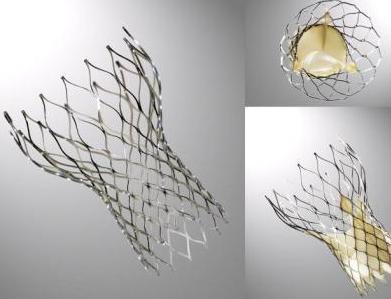
The Edwards SAPIEN Valves
SAPIEN XT
This prosthesis is considered the second generation of the Cribier-Edwards valve.[6] It is a balloon-expendable valve made of a stainless steel frame covered by a Dacron skirt where three leaflets of pericardium are sutured. The device is placed in a subcoronary position during rapid ventricular pacing, via anterograde, transapical or a retrograde transfemoral approach. It is available in two sizes (23mm and 26 mm). In the first generation the leaflets were made of equine (horse) pericardium; in the second generation they are made of bovine (cow) pericardium with improvements made in the frame suture and an increase in the skirt length to decrease the risk of aortic regurgitation.
SAPIEN 3
The Edwards SAPIEN 3 Transcatheter Heart Valve is comprised of a balloon-expandable, radiopaque, cobalt-chromium frame, trileaflet bovine pericardial tissue valve, and polyethylene terephthalate (PET) fabric skirt. The leaflets are treated according to the Carpentier-Edwards ThermaFix process. It is available in 4 sizes (20mm, 23mm, 26mm and 29mm).
-
The Edwards SAPIEN XT
-
The Edwards SAPIEN 3
St. Jude Portico valve
The valve stent is made from nitinol, a nickel-titanium alloy that has self-expanding properties and is radiopaque. The valve cuff is made from porcine pericardium that is sutured to the stent frame. The cuff provides the sealing area for implantation. The valve orifice is made by suturing three valve leaflets, each made from a single layer of bovine pericardium, into a tri-leaflet configuration on the stent frame. It is available in 4 sizes (23mm, 25mm, 27mm and 29mm).
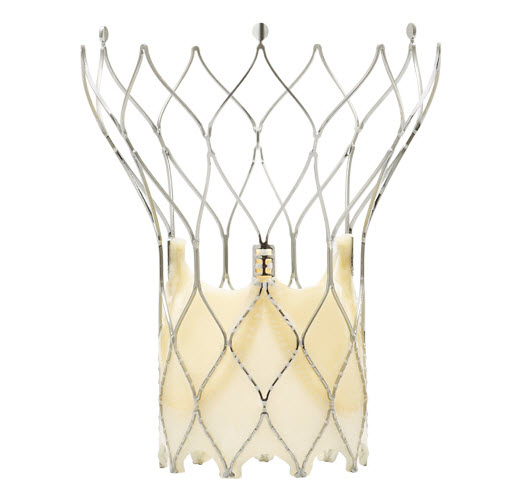
Direct Flow Medical valve
It features a metal-free frame. The Direct Flow Medical System incorporates a polymer frame, which is expanded using pressurized saline and contrast for placement, assessment and repositioning. The saline/contrast solution is exchanged for a quick-curing polymer that solidifies and secures the valve in place once optimal positioning is reached. It is available in 4 sizes(23 mm, 25 mm, 27 mm, 29 mm).

Symetis Acurate neo valve
Acurate neo is composed of a porcine pericardial tissue valve sutured within a self-expanding nitinol stent covered by a pericardial skirt on the outer and inner surface of the stent body.It is a nitinol-based valve that incorporate features that facilitate positioning and anatomic orientation in relation to the native valve commissures and coronaries. The valve is currently implanted only transapically. It is available in three sizes (S, M, L) and its delivery system boasts an 18F outer diameter and it cover the aortic annulus diameters from 21 to 27 mm.
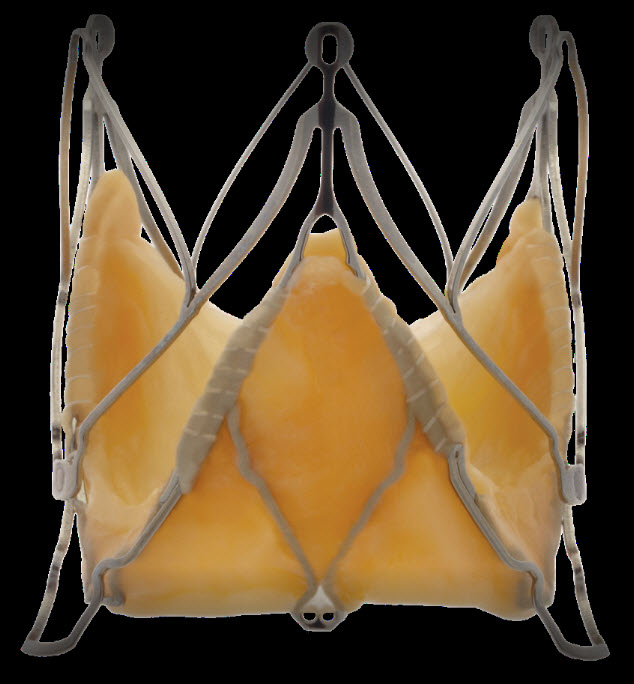
Medtronic Engager valve
It has a self expanding nitinol frame and polyester skirt and bovine pericardial tissue. It is available in 2 sizes(23 mm, 26 mm)
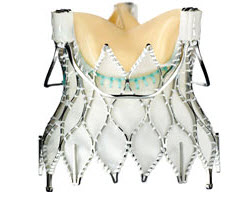
Lotus edge valve
Lotus valve consisting of a pre-attached, stent-mounted tissue valve prosthesis and catheter delivery system for guidance and percutaneous placement of the valve. It is the first device of its kind that offers controlled mechanical expansion, which allows the valve to be fully deployed, assessed and then released, providing unparalleled control during the procedure. It is available in 3 sizes (23 mm, 25 mm and 27 mm).

Jena valve
The transapical JenaValve prosthesis consists of a natural aortic porcine root bioprosthesis fitted with an outer porcine pericardial patch, a so-called skirt. The JenaValve is available in 3 sizes, 23mm, 25mm and 27mm. It is not commercially available now and is waiting for approval.

References
- ↑ Vahl TP, Kodali SK, Leon MB (2016). "Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure". J. Am. Coll. Cardiol. 67 (12): 1472–87. doi:10.1016/j.jacc.2015.12.059. PMID 27012409.
- ↑ Schäfer U, Conradi L, Diemert P, Deuschl F, Schofer N, Seiffert M, Lubos E, Schirmer J, Reichenspurner H, Blankenberg S, Treede H (2015). "Symetis ACURATE TAVI: review of the technology, developments and current data with this self-expanding transcatheter heart valve". Minerva Cardioangiol. 63 (5): 359–69. PMID 26198875.
- ↑ Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation. 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help) - ↑ Heart Mirror J 2010; 157-159.
- ↑ CARDIAC INTERVENTIONS TODAY,TAVI using the CoreValve revalving system,July.August 2010
- ↑ Heart Mirror J 2010; 157-159.