Solriamfetol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Solriamfetol is a dopamine and norepinephrine reuptake inhibitor (DNRI) that is FDA approved for the treatment of wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea (OSA). Common adverse reactions include headache, nausea, decreased appetite, insomnia, and anxiety..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Solriamfetol is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea (OSA).
Limitations of Use
- Solriamfetol is not indicated to treat the underlying airway obstruction in OSA. Ensure that the underlying airway obstruction is treated (e.g., with continuous positive airway pressure (CPAP)) for at least one month prior to initiating solriamfetol for excessive daytime sleepiness. Modalities to treat the underlying airway obstruction should be continued during treatment with solriamfetol. Solriamfetol is not a substitute for these modalities.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding solriamfetol Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding solriamfetol Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established. Clinical studies of solriamfetol in pediatric patients have not been conducted.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding solriamfetol Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding solriamfetol Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Solriamfetol is contraindicated in patients receiving concomitant treatment with monoamine oxidase (MAO) inhibitors, or within 14 days following discontinuation of monoamine oxidase inhibitor, because of the risk of hypertensive reaction.
Warnings
Blood Pressure and Heart Rate Increases
- Solriamfetol increases systolic blood pressure, diastolic blood pressure, and heart rate in a dose-dependent fashion.
- Epidemiological data show that chronic elevations in blood pressure increase the risk of major adverse cardiovascular events (MACE), including stroke, heart attack, and cardiovascular death. The magnitude of the increase in absolute risk is dependent on the increase in blood pressure and the underlying risk of MACE in the population being treated. Many patients with narcolepsy and OSA have multiple risk factors for MACE, including hypertension, diabetes, hyperlipidemia, and high body mass index (BMI).
- Assess blood pressure and control hypertension before initiating treatment with solriamfetol. Monitor blood pressure regularly during treatment and treat new-onset hypertension and exacerbations of pre-existing hypertension. Exercise caution when treating patients at higher risk of MACE, particularly patients with known cardiovascular and cerebrovascular disease, pre-existing hypertension, and patients with advanced age. Use caution with other drugs that increase blood pressure and heart rate.
- Periodically reassess the need for continued treatment with solriamfetol. If a patient experiences increases in blood pressure or heart rate that cannot be managed with dose reduction of solriamfetol or other appropriate medical intervention, consider discontinuation of solriamfetol.
- Patients with moderate or severe renal impairment may be at a higher risk of increases in blood pressure and heart rate because of the prolonged half-life of solriamfetol.
Psychiatric Symptoms
- Psychiatric adverse reactions have been observed in clinical trials with solriamfetol, including anxiety, insomnia, and irritability.
- Solriamfetol has not been evaluated in patients with psychosis or bipolar disorders. Exercise caution when treating patients with solriamfetol who have a history of psychosis or bipolar disorders.
- Patients with moderate or severe renal impairment may be at a higher risk of psychiatric symptoms because of the prolonged half-life of solriamfetol.
- Patients treated with solriamfetol should be observed for the possible emergence or exacerbation of psychiatric symptoms. If psychiatric symptoms develop in association with the administration of solriamfetol, consider dose reduction or discontinuation of solriamfetol.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of solriamfetol has been evaluated in 930 patients (ages 18 to 75 years) with narcolepsy or OSA. Among these patients, 396 were treated with solriamfetol in the 12-week placebo-controlled trials at doses of 37.5 mg (OSA only), 75 mg, and 150 mg once daily. Information provided below is based on the pooled 12‑week placebo‑controlled studies in patients with narcolepsy or OSA.
Most Common Adverse Reactions
- The most common adverse reactions (incidence ≥ 5% and greater than placebo) reported more frequently with the use of solriamfetol than placebo in either the narcolepsy or OSA populations were headache, nausea, decreased appetite, anxiety, and insomnia.
- Table 1 presents the adverse reactions that occurred at a rate of ≥ 2% and more frequently in solriamfetol-treated patients than in placebo-treated patients in the narcolepsy population.

- Table 2 presents the adverse reactions that occurred at a rate of ≥ 2% and more frequently in solriamfetol-treated patients than in placebo-treated patients in the OSA population.

Other Adverse Reactions Observed During the Premarketing Evaluation of solriamfetol
- Other adverse reactions of < 2% incidence but greater than placebo are shown below. The following list does not include adverse reactions: 1) already listed in previous tables or elsewhere in the labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, or 4) which were not considered to have clinically significant implications.
- Narcolepsy population:
- Psychiatric disorders: agitation, bruxism, irritability
- Respiratory, thoracic and mediastinal disorders: cough
- Skin and subcutaneous tissue disorders: hyperhidrosis
- General disorders and administration site conditions: feeling jittery, thirst, chest discomfort, chest pain
- Investigations: weight decreased
- OSA population:
- Psychiatric disorders: bruxism, restlessness
- Nervous system disorders: disturbances in attention, tremor
- Respiratory, thoracic and mediastinal disorders: cough, dyspnea
- Gastrointestinal disorders: constipation, vomiting
- Investigations: weight decreased
Dose-Dependent Adverse Reactions
- In the 12-week placebo-controlled clinical trials that compared doses of 37.5 mg, 75 mg, and 150 mg daily of solriamfetol to placebo, the following adverse reactions were dose-related: headache, nausea, decreased appetite, anxiety, diarrhea, and dry mouth (Table 3).

Adverse Reactions Resulting in Discontinuation of Treatment
- In the 12-week placebo-controlled clinical trials, 11 of the 396 patients (3%) who received solriamfetol discontinued because of an adverse reaction compared to 1 of the 226 patients (< 1%) who received placebo. The adverse reactions resulting in discontinuation that occurred in more than one solriamfetol-treated patient and at a higher rate than placebo were: anxiety (2/396; < 1%), palpitations (2/396; < 1%), and restlessness (2/396; < 1%).
Increases in Blood Pressure and Heart Rate
- Solriamfetol’s effects on blood pressure and heart rate are summarized below. Table 4 shows maximum mean changes in blood pressure and heart rate recorded at sessions where the Maintenance of Wakefulness Test (MWT) was administered [see Clinical Studies (14)]. Table 5 summarizes 24-hour ambulatory blood pressure monitoring (ABPM) and ambulatory heart rate monitoring performed in the outpatient setting.


Postmarketing Experience
There is limited information regarding Solriamfetol Postmarketing Experience in the drug label.
Drug Interactions
Monoamine Oxidase (MAO) Inhibitors
- Do not administer solriamfetol concomitantly with MAOIs or within 14 days after discontinuing MAOI treatment. Concomitant use of MAO inhibitors and noradrenergic drugs may increase the risk of a hypertensive reaction. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure.
Drugs that Increase Blood Pressure and/or Heart Rate
- Concomitant use of solriamfetol with other drugs that increase blood pressure and/or heart rate has not been evaluated, and such combinations should be used with caution.
Dopaminergic Drugs
- Dopaminergic drugs that increase levels of dopamine or that bind directly to dopamine receptors might result in pharmacodynamic interactions with solriamfetol. Interactions with dopaminergic drugs have not been evaluated with solriamfetol. Use caution when concomitantly administering dopaminergic drugs with solriamfetol.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Exposure Registry
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to solriamfetol during pregnancy. Healthcare providers are encouraged to register pregnant patients, or pregnant women may enroll themselves in the registry by calling 1-877-283-6220 or contacting the company at WWW.solriamfetolPREGNANCYREGISTRY.COM.
Risk Summary
- Available data from case reports are not sufficient to determine drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproductive studies, oral administration of solriamfetol during organogenesis caused maternal and fetal toxicities in rats and rabbits at doses ≥ 4 and 5 times and was teratogenic at doses 19 and ≥ 5 times, respectively, the maximum recommended human dose (MRHD) of 150 mg based on mg/m2 body surface area. Oral administration of solriamfetol to pregnant rats during pregnancy and lactation at doses ≥ 7 times the MRHD based on mg/m2 body surface area resulted in maternal toxicity and adverse effects on fertility, growth, and development in offspring.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Animal Data
- Solriamfetol was administered orally to pregnant rats during the period of organogenesis at 15, 67, and 295 mg/kg/day, which are approximately 1, 4, and 19 times the MRHD based on mg/m2 body surface area. Solriamfetol at ≥ 4 times the MRHD caused maternal toxicity that included hyperactivity, significant decreases in body weight, weight gain, and food consumption. Fetal toxicity at these maternally toxic doses included increased incidence of early resorption and post-implantation loss, and decreased fetal weight. Solriamfetol was teratogenic at 19 times the MRHD; it increased the incidence of fetal malformations that included severe sternebrae mal-alignment, hindlimb rotation, bent limb bones, and situs inversus. This dose was also maternally toxic. The no-adverse-effect level for malformation is 4 times and for maternal and embryofetal toxicity is approximately 1 times the MRHD based on mg/m2 body surface area.
- Solriamfetol was administered orally to pregnant rabbits during the period of organogenesis at 17, 38, and 76 mg/kg/day, which are approximately 2, 5, and 10 times the MRHD based on mg/m2 body surface area. Solriamfetol at 10 times the MRHD caused maternal toxicity of body weight loss and decreased food consumption. Solriamfetol was teratogenic at ≥ 5 times the MRHD, it caused fetal skeletal malformation (slight-to-moderate sternebrae mal-alignment) and decreased fetal weight. The no-adverse-effect level for malformation and fetal toxicity is approximately 2 times and for maternal toxicity is approximately 5 times the MRHD based on mg/m2 body surface area.
- Solriamfetol was administered orally to pregnant rats during the period of organogenesis from gestation day 7 through lactation day 20 post-partum, at 35, 110, and 350 mg/kg/day, which are approximately 2, 7, and 22 times the MRHD based on mg/m2 body surface area. At ≥ 7 times the MRHD, solriamfetol caused maternal toxicity that included decreased body weight gain, decreased food consumption, and hyperpnea. At these maternally toxic doses, fetal toxicity included increased incidence of stillbirth, postnatal pup mortality, and decreased pup weight. Developmental toxicity in offspring after lactation day 20 included decreased body weight, decreased weight gain, and delayed sexual maturation. Mating and fertility of offspring were decreased at maternal doses 22 times the MRHD without affecting learning and memory. The no-adverse-effect level for maternal and developmental toxicity is approximately 2 times the MRHD based on mg/m2 body surface area.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Solriamfetol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Solriamfetol during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data available on the presence of solriamfetol or its metabolites in human milk, the effects on the breastfed infant, or the effect of this drug on milk production.
- Solriamfetol is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for solriamfetol and any potential adverse effects on the breastfed child from solriamfetol or from the underlying maternal condition.
Clinical Considerations
- Monitor breastfed infants for adverse reactions, such as agitation, insomnia, anorexia and reduced weight gain.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established. Clinical studies of solriamfetol in pediatric patients have not been conducted.
Geriatic Use
- Of the total number of patients in the narcolepsy and OSA clinical studies treated with solriamfetol, 13% (123/930) were 65 years of age or over.
- No clinically meaningful differences in safety or effectiveness were observed between elderly and younger patients.
- Solriamfetol is predominantly eliminated by the kidney. Because elderly patients are more likely to have decreased renal function, dosing may need to be adjusted based on eGFR in these patients. Consideration should be given to the use of lower doses and close monitoring in this population.
Gender
There is no FDA guidance on the use of Solriamfetol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Solriamfetol with respect to specific racial populations.
Renal Impairment
- Dosage adjustment is not required for patients with mild renal impairment (eGFR 60‑89 mL/min/1.73 m2). Dosage adjustment is recommended for patients with moderate to severe renal impairment (eGFR 15‑59 mL/min/1.73 m2). Solriamfetol is not recommended for patients with end stage renal disease (eGFR <15 mL/min/1.73 m2)
Hepatic Impairment
There is no FDA guidance on the use of Solriamfetol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Solriamfetol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Solriamfetol in patients who are immunocompromised.
Administration and Monitoring
Administration
Important Considerations Prior to Initiating Treatment
- Prior to initiating treatment with solriamfetol, ensure blood pressure is adequately controlled
General Administration Instructions
- Administer solriamfetol orally upon awakening with or without food. Avoid taking solriamfetol within 9 hours of planned bedtime because of the potential to interfere with sleep if taken too late in the day.
- Solriamfetol 75 mg tablets are functionally scored tablets that can be split in half (37.5 mg) at the score line.
Dosage in Narcolepsy
- Initiate solriamfetol at 75 mg once daily in adults with narcolepsy. The recommended dose range for solriamfetol is 75 mg to 150 mg once daily. Based on efficacy and tolerability, the dosage of solriamfetol may be doubled at intervals of at least 3 days. The maximum recommended dose is 150 mg once daily. Dosages above 150 mg daily do not confer increased effectiveness sufficient to outweigh dose-related adverse reactions.
Dosage in OSA
- Initiate solriamfetol at 37.5 mg once daily in adults with OSA. The recommended dosage range for solriamfetol is 37.5 mg to 150 mg once daily. Based on efficacy and tolerability, the dosage of solriamfetol may be doubled at intervals of at least 3 days. The maximum recommended dosage is 150 mg once daily. Dosages above 150 mg daily do not confer increased effectiveness sufficient to outweigh dose-related adverse reactions.
Dosage Recommendations in Patients with Renal Impairment
- Moderate renal impairment (eGFR 30‑59 mL/min/1.73 m2): Initiate dosing at 37.5 mg once daily. Based on efficacy and tolerability, dose may be increased to a maximum of 75 mg once daily after at least 7 days
- Severe renal impairment (eGFR 15‑29 mL/min/1.73 m2): Administer 37.5 mg once daily. The maximum recommended daily dose is 37.5 mg
- End Stage Renal Disease (eGFR <15 mL/min/1.73 m2): solriamfetol is not recommended for use in patients with ESRD
Monitoring
There is limited information regarding Solriamfetol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Solriamfetol and IV administrations.
Overdosage
- A specific reversal agent for solriamfetol is not available. Hemodialysis removed approximately 21% of a 75 mg dose in end stage renal disease patients. Overdoses should be managed with primarily supportive care, including cardiovascular monitoring.
- Consult with a Certified Poison Control Center at 1-800-222-1222 for latest recommendations.
Pharmacology
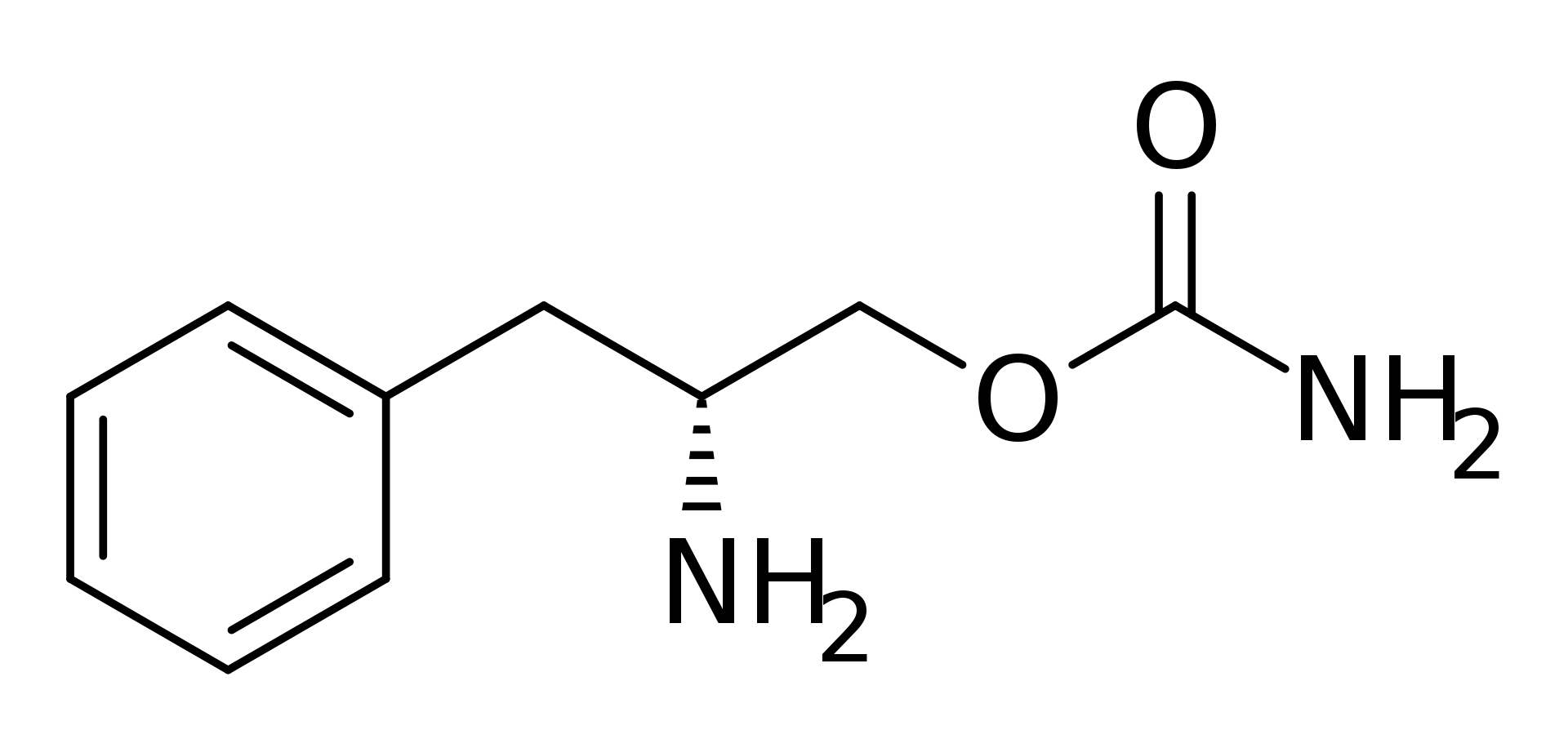
| |
Solriamfetol
| |
| Systematic (IUPAC) name | |
| (2R)-2-Amino-3-phenylpropyl carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | N06 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 194.234 g/mol |
| Synonyms | SKL-N05, ADX-N05, ARL-N05, YKP10A, R228060, and JZP-110; (R)-2-amino-3-phenylpropylcarbamate hydrochloride |
| Pharmacokinetic data | |
| Bioavailability | ~95% |
| Protein binding | 13.3–19.4% |
| Metabolism | negligible |
| Half life | ~7.1 h |
| Excretion | urine (95% unchanged) |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | By mouth |
Mechanism of Action
- The mechanism of action of solriamfetol to improve wakefulness in patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea is unclear. However, its efficacy could be mediated through its activity as a dopamine and norepinephrine reuptake inhibitor (DNRI).
Structure

Pharmacodynamics
- Solriamfetol binds to the dopamine transporter and norepinephrine transporter with low affinity (Ki=14.2 µM and 3.7 µM, respectively), and inhibits the reuptake of dopamine and norepinephrine with low potency (IC50 =2.9 μM and 4.4 μM, respectively). Solriamfetol has no appreciable binding affinity for the serotonin transporter (Ki=81.5 µM) and does not inhibit serotonin reuptake (IC50 > 100 μM). Solriamfetol has no appreciable binding affinity to dopamine, serotonin, norepinephrine, GABA, adenosine, histamine, orexin, benzodiazepine, muscarinic acetylcholine, or nicotinic acetylcholine receptors.
Cardiac Electrophysiology
- The effect of solriamfetol 300 mg and 900 mg (twice and six times the maximum recommended dose, respectively) on the QTc interval was evaluated in a randomized, double-blind, placebo-, and positive-controlled (moxifloxacin 400 mg), 4-period, crossover study in 60 healthy subjects. A large increase in heart rate was observed in both solriamfetol treatment groups (mean change from baseline in HR of 21 and 27 bpm in the 300 and 900 mg groups, respectively, compared with 8 bpm in the placebo group). These heart rate effects impact the interpretability of the QTc effects, particularly in the 900 mg group. In this study, solriamfetol 300 mg did not prolong the QTcF interval to a clinically relevant extent.
Pharmacokinetics
- Solriamfetol exhibits linear kinetics over the dose range of 42 to 1008 mg (approximately 0.28 to 6.7 times the maximum recommended dosage). Steady state is reached in 3 days, and once‑daily administration is expected to result in minimal accumulation (1.06 times single‑dose exposure).
Absorption
- The oral bioavailability of solriamfetol is approximately 95%. Peak plasma concentration of solriamfetol occurs at a median Tmax of 2 hours (range 1.25 to 3.0 hours) post-dose under fasted conditions.
Effect of Food
- Ingestion of solriamfetol with a high-fat meal resulted in minimal change in Cmax and AUCinf; however, a delay of approximately 1 hour in Tmax was observed.
Distribution
- The apparent volume of distribution of solriamfetol is approximately 199 L. Plasma protein binding ranged from 13.3% to 19.4% over solriamfetol concentration range of 0.059 to 10.1 mcg/mL in human plasma. The mean blood‑to‑plasma concentration ratio ranged from 1.16 to 1.29.
Elimination
- Solriamfetol exhibits first‑order elimination after oral administration. The apparent mean elimination half‑life is about 7.1 hours.
Metabolism
- Solriamfetol is minimally metabolized in humans.
Excretion
- Approximately 95% of the dose was recovered in urine as unchanged solriamfetol, and 1% or less of the dose was recovered as the minor inactive metabolite N‑acetyl solriamfetol in a mass balance study. Renal clearance (18.2 L/h) represented the majority of apparent total clearance (19.5 L/h). Active tubular secretion is likely involved in the renal elimination of the parent drug.
Specific Populations
- Population PK analysis indicated that age, gender, and race do not have clinically relevant effects on the pharmacokinetics of solriamfetol. No dose adjustments were made in clinical studies that enrolled patients ages 65 and above.
Patients with Renal Impairment
- Exposures to solriamfetol in patients with renal impairment compared to subjects with normal renal function (eGFR ≥ 90 mL/min/1.73 m2) are summarized in Figure 1. The half‑life of solriamfetol was increased approximately 1.2‑, 1.9‑, and 3.9‑fold in patients with mild (eGFR 60‑89 mL/min/1.73 m2), moderate (eGFR 30–59 mL/min/1.73 m2), or severe (eGFR <30 mL/min/1.73 m2) renal impairment, respectively. Exposure (AUC) and half-life of solriamfetol was significantly increased in patients with ESRD (eGFR <15 mL/min/1.73 m2). An average of 21% of solriamfetol was removed by hemodialysis. In general, median Tmax values were not affected by renal impairment.

Drug Interaction Studies In Vitro Studies
- CYP and UGT Enzymes: Solriamfetol was minimally metabolized in vitro. Solriamfetol is not an inhibitor of CYPs 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4. It does not induce CYP1A2, 2B6, 3A4, or UGT1A1 enzymes at clinically relevant concentrations.
- Transporter Systems: Solriamfetol is a low-avidity substrate of OCT2, MATE1, OCTN1, and OCTN2. Solriamfetol is a weak inhibitor of OCT2 (IC50 of 146 μM) and MATE1 (IC50 of 211 μM), and is not an inhibitor of OCT1, MATE2-K, OCTN1, or OCTN2. Solriamfetol does not appear to be a substrate or inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, OAT1, or OAT3.
- Based on in vitro data, clinically significant PK drug interactions with major CYPs and transporters are not expected in patients taking solriamfetol.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Solriamfetol did not increase the incidence of tumors in rats or mice treated orally for up to 101 and 104 weeks at 35, 80, and 200 mg/kg/day (rat), and 20, 65, and 200 mg/kg/day (mouse), respectively. These doses are approximately 2, 6, and 18 times (rat), and 0.4, 2.6, and 7 times (mouse) the MRHD based on AUC.
Mutagenesis
- Solriamfetol was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or clastogenic in the in vitro mammalian chromosomal aberration assay or in the in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
- Solriamfetol did not affect fertility or sperm parameters when administered orally to male rats for 8 weeks at doses of 35 and 110 mg/kg/day, which are approximately 2 and 7 times the MRHD, based on mg/m2 body surface area. At 350 mg/kg/day, which is approximately 22 times the MRHD based on mg/m2 body surface area, solriamfetol decreased sperm count and sperm concentration without affecting fertility.
- Solriamfetol did not affect fertility when administered orally to female rats for 2 weeks premating, during mating, and through gestation day 7 at 15, 67, and 295 mg/kg/day, which are approximately 1, 4, and 19 times the MRHD, based on mg/m2 body surface area.
Clinical Studies
Narcolepsy
- The efficacy of solriamfetol in improving wakefulness and reducing excessive daytime sleepiness was demonstrated in a 12‑week, multi‑center, randomized, double‑blind, placebo‑controlled, parallel-group study (Study 1; NCT02348593) in adult patients with a diagnosis of narcolepsy according to the ICSD‑3 or DSM‑5 criteria.
- Wakefulness and sleepiness were assessed using the Maintenance of Wakefulness Test (MWT) and the Epworth Sleepiness Scale (ESS). The MWT measures an individual’s ability to remain awake during the daytime in a darkened, quiet environment. Patients were instructed to remain awake for as long as possible during 40‑minute test sessions, and sleep latency was determined as the mean number of minutes patients could remain awake in the first four test sessions. The ESS is an 8‑item questionnaire by which patients rate their perceived likelihood of falling asleep during usual daily life activities. Change in overall symptom severity was assessed using the Patient Global Impression of Change (PGIc) scale. The PGIc is a 7‑point patient-reported scale by which patients rate their symptom change since the beginning of the study. Responses range from “very much improved” to “very much worse.” The co-primary efficacy endpoints were change from baseline in MWT and ESS at Week 12. A pre-specified secondary endpoint was percentage of subjects reported as improved (minimally, much, or very much) at Week 12 by PGIc.
- A total of 239 patients with narcolepsy were randomized to receive solriamfetol 75 mg, 150 mg, or 300 mg (two times the maximum recommended daily dose), or placebo once daily. Patients randomized to the 150-mg dose received 75 mg for the first 3 days before increasing to 150 mg.
- Demographic and baseline disease characteristics were similar for the solriamfetol and placebo groups. Median age was 34 years (range 18 to 70 years), 65% were female, 80% were Caucasian, 14% were African American, and 3% were Asian. Approximately 51% of patients had cataplexy.
- Compared to the placebo group, patients randomized to 150 mg solriamfetol showed statistically significant improvements on the MWT (treatment effect difference: 7.7 minutes, Table 6) and on the ESS (treatment effect difference: 3.8 points, Table 7) at Week 12. These effects were apparent at Week 1 and consistent with the results at Week 12. The change on percentage of subjects reported as improved by PGIc was also statistically significant compared with placebo. There were trends toward improvement in the solriamfetol 75-mg treatment group (Tables 6 and 7); however, these changes were not statistically significant. There was no evidence of differential efficacy in patients with cataplexy and patients without cataplexy. Examination of subgroups by age, race, and sex did not suggest differences in response.
- At Week 12, 150 mg of solriamfetol demonstrated improvements in wakefulness compared to placebo as assessed in test sessions 1 (approximately 1 hour post‑dose) through 5 (approximately 9 hours post‑dose) of the MWT (Figure 2). Nighttime sleep as measured with polysomnography was not affected by the use of solriamfetol in Study 1.

Obstructive Sleep Apnea (OSA)
- The efficacy of solriamfetol in improving wakefulness and reducing excessive daytime sleepiness in patients with OSA was demonstrated in a 12-week multi‑center, randomized, double-blind, placebo‑controlled study (Study 2; NCT02348606) in adults diagnosed with OSA according to ICSD‑3 criteria. The co-primary efficacy endpoints were change from baseline in MWT and ESS at Week 12; a pre-specified secondary endpoint was percentage of subjects reported as improved (minimally, much, or very much) at Week 12 by PGIc.
- A total of 476 patients with OSA were randomized to receive solriamfetol 37.5 mg, 75 mg, 150 mg, or 300 mg (two times the maximum recommended daily dose), or placebo once daily. Patients randomized to the 150-mg dose received 75 mg for the first 3 days before increasing to 150 mg.
- Demographic and baseline disease characteristics were similar for the solriamfetol and placebo groups. Median age was 55 years (range 20 to 75 years), 37% were female, 76% were Caucasian, 19% were African American, and 4% were Asian.
- Compared to the placebo group, patients randomized to 37.5 mg, 75 mg, and 150 mg solriamfetol showed statistically significant improvements on the MWT (treatment effect difference: 4.5 minutes, 8.9 minutes, and 10.7 minutes respectively; Table 6) and ESS (treatment effect difference: 1.9 points, 1.7 points, and 4.5 points respectively; Table 7) at Week 12. These effects were apparent at Week 1 and consistent with the results at Week 12. The change on percentage of subjects reported as improved by PGIc was also statistically significant compared with placebo. Examination of subgroups by age, race, and sex did not suggest differences in response.
- At Week 12, 37.5 mg, 75 mg, and 150 mg of solriamfetol all demonstrated improvements in wakefulness compared to placebo as assessed in test sessions 1 (approximately 1 hour post‑dose) through 5 (approximately 9 hours post‑dose) of the MWT (Figure 3). Nighttime sleep as measured with polysomnography was not affected by the use of solriamfetol in Study 2. Patients’ compliance with a primary OSA therapy device was similar across the placebo and solriamfetol treatment groups at baseline, and did not change during the 12‑week study period in any treatment group.



Maintenance of Efficacy in Narcolepsy and OSA
- The maintenance of effect of solriamfetol in improving wakefulness and reducing excessive daytime sleepiness in patients with narcolepsy and OSA was assessed in two randomized‑withdrawal, placebo‑controlled studies, Study 3 (NCT02348619) and Study 4 (NCT02348632).
- Study 3 was a 6‑week, multi-center, double-blind, placebo‑controlled, randomized‑withdrawal study in 174 adult patients with a diagnosis of OSA. The co-primary efficacy endpoints were change from the beginning to the end of the randomized withdrawal period in MWT and ESS. During a 2‑week, open-label titration phase, patients were started on solriamfetol 75 mg once daily, and were titrated to the maximum tolerable dose between 75 mg and 300 mg per day (two times the maximum recommended daily dose). Patients were continued on this dose for a 2‑week stable-dose phase. At the end of the stable‑dose phase, 124 patients who reported “much” or “very much” improvement on the PGIc and who showed improvements on the MWT and ESS entered a double-blind withdrawal phase and were randomized 1:1 to either continue solriamfetol at the dose received in the stable‑dose phase or switch to placebo. Compared to patients who remained on solriamfetol, patients randomized to placebo experienced statistically significant worsening of sleepiness as measured by the MWT and ESS (Table 8).
- Study 4 was a 52‑week, open-label study in 638 patients with either narcolepsy or OSA who had completed a prior trial. During a 2‑week, open-label titration phase, patients were started on solriamfetol 75 mg once daily, and were titrated to the maximum tolerable dose between 75 mg and 300 mg per day (two times the maximum recommended daily dose). Patients remained on this dose during a subsequent open‑label treatment period of either 38 (for patients previously enrolled in Study 1 or Study 2) or 50 (all others) weeks. A 2‑week randomized‑withdrawal period was incorporated into the study. After 6 months of stable‑dose treatment, 282 patients (79 with narcolepsy; 203 with OSA) entered the randomized‑withdrawal period. Patients were randomized 1:1 to either continue to receive solriamfetol at the dose received in the maintenance phase or to switch to placebo. The primary efficacy endpoint was change from the beginning to the end of the randomized‑withdrawal period in ESS. Compared to patients who remained on solriamfetol, patients randomized to placebo experienced statistically significant worsening of sleepiness as measured by the ESS (Table 8).

How Supplied
- Solriamfetol is packaged in 30‑count and 100‑count white, high density polyethylene (HDPE) bottles.
- Solriamfetol tablets, 75 mg ‑ dark yellow oblong tablet with “75” debossed on one side and a functional score line on the opposite side.
Storage
- Store solriamfetol at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F)
Images
Drug Images
{{#ask: Page Name::Solriamfetol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Solriamfetol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Potential for Abuse and Dependence
- Advise patients that solriamfetol is a federally controlled substance because it has the potential to be abused. Advise patients to keep their medication in a secure place and to dispose of unused solriamfetol as recommended in the Medication Guide.
Primary OSA Therapy Use
- Inform patients that solriamfetol is not indicated to treat the airway obstruction in OSA and they should use a primary OSA therapy, such as CPAP, as prescribed to treat the underlying obstruction. Solriamfetol is not a substitute for primary OSA therapy.
Blood Pressure and Heart Rate Increase
- Instruct patients that solriamfetol can cause elevations of their blood pressure and pulse rate and that they should be monitored for such effects.
Psychiatric Symptoms
- Instruct patients to contact their healthcare provider if they experience, anxiety, insomnia, irritability, agitation, or signs of psychosis or bipolar disorders.
Lactation
- Monitor breastfed infants for adverse reactions such as agitation, insomnia, anorexia, and reduced weight gain.
Medication Guide

Precautions with Alcohol
Alcohol-Solriamfetol interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Solriamfetol Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.