Reteplase
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Reteplase is a tissue plasminogen activator that is FDA approved for the {{{indicationType}}} of acute myocardial infarction. Common adverse reactions include bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Myocardial Infarction
- Reteplase is indicated for use in the management of acute myocardial infarction (AMI) in adults for the improvement of ventricular function following AMI, the reduction of the incidence of congestive heart failure and the reduction of mortality associated with AMI.
- Treatment should be initiated as soon as possible after the onset of AMI symptoms
- Dosing Information
- First dose: 10 unit IV bolus over 2 minutes
- Second dose (30 minutes after first bolus): 10 unit IV bolus over 2 minutes
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Reteplase in adult patients.
Non–Guideline-Supported Use
Vascular Graft Thrombosis
- Dosing Information
Pulmonary Embolism
- Dosing Information
- First dose: 10 unit IV bolus over 2 minutes
- Second dose (30 minutes after first bolus): 10 unit IV bolus over 2 minutes[2]
Acute Peripheral Ischemia
- Dosing Information
- 0.5-1.0 Units/hour (for approximately 20 hours)[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Reteplase FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Reteplase in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Reteplase in pediatric patients.
Contraindications
- Active internal bleeding
- History of cerebrovascular accident
- Recent intracranial or intraspinal surgery or trauma
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Known bleeding diathesis
- Severe uncontrolled hypertension
Warnings
Bleeding
- The most common complication encountered during reteplase therapy is bleeding. The sites of bleeding include both internal bleeding sites (intracranial, retroperitoneal, gastrointestinal, genitourinary, or respiratory) and superficial bleeding sites (venous cutdowns, arterial punctures, sites of recent surgical intervention).
- The concomitant use of heparin anticoagulation may contribute to bleeding.
- In clinical trials some of the hemorrhage episodes occurred one or more days after the effects of reteplase had dissipated, but while heparin therapy was continuing.
- As fibrin is lysed during reteplase therapy, bleeding from recent puncture sites may occur. Therefore, thrombolytic therapy requires careful attention to all potential bleeding sites (including catheter insertion sites, arterial and venous puncture sites, cutdown sites, and needle puncture sites).
- Noncompressible arterial puncture must be avoided and internal jugular and subclavian venous punctures should be avoided to minimize bleeding from noncompressible sites.
- Should an arterial puncture be necessary during the administration of reteplase, it is preferable to use an upper extremity vessel that is accessible to manual compression.
- Pressure should be applied for at least 30 minutes, a pressure dressing applied, and the puncture site checked frequently for evidence of bleeding.
- Intramuscular injections and nonessential handling of the patient should be avoided during treatment with reteplase.
- Venipunctures should be performed carefully and only as required.
- Should serious bleeding (not controllable by local pressure) occur, concomitant anticoagulant therapy should be terminated immediately.
- In addition, the second bolus of reteplase should not be given if serious bleeding occurs before it is administered.
- Each patient being considered for therapy with reteplase should be carefully evaluated and anticipated benefits weighed against the potential risks associated with therapy.
- In the following conditions, the risks of reteplase therapy may be increased and should be weighed against the anticipated benefits:
- Recent major surgery, e.g., coronary artery bypass graft, obstetrical delivery, organ biopsy
- Previous puncture of noncompressible vessels
- Cerebrovascular disease
- Recent gastrointestinal or genitourinary bleeding
- Recent trauma
- Hypertension: systolic BP ≥ 180 mm Hg and/or diastolic BP ≥ 110 mm Hg
- High likelihood of left heart thrombus, e.g., mitral stenosis with atrial fibrillation
- Acute pericarditis
- Subacute bacterial endocarditis
- Hemostatic defects including those secondary to severe hepatic or renal disease
- Severe hepatic or renal dysfunction
- Pregnancy
- Diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions
- Septic thrombophlebitis or occluded AV cannula at a seriously infected site
- Advanced age
- Patients currently receiving oral anticoagulants, e.g., warfarin sodium
- Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
Cholesterol Embolization
- Cholesterol embolism has been reported rarely in patients treated with thrombolytic agents; the true incidence is unknown.
- This serious condition, which can be lethal, is also associated with invasive vascular procedures (e.g., cardiac catheterization, angiography, vascular surgery) and/or anticoagulant therapy.
- Clinical features of cholesterol embolism may include livedo reticularis, "purple toe syndrome” syndrome, acute renal failure, gangrenous digits, hypertension, pancreatitis,myocardial infarction, cerebral infarction, spinal cord infarction, retinal artery occlusion, bowel infarction, and rhabdomyolysis.
Arrhythmias
- Coronary thrombolysis may result in arrhythmias associated with reperfusion.
- These arrhythmias (such as sinus bradycardia, accelerated idioventricular rhythm, ventricular premature depolarizations, ventricular tachycardia) are not different from those often seen in the ordinary course of acute myocardial infarction and should be managed with standard antiarrhythmic measures.
- It is recommended that antiarrhythmic therapy for bradycardia and/or ventricular irritability be available when reteplase is administered.
Adverse Reactions
Clinical Trials Experience
Bleeding
- The most frequent adverse reaction associated with reteplase is bleeding.
- The types of bleeding events associated with thrombolytic therapy may be broadly categorized as either intracranial hemorrhage or other types of hemorrhage.
Intracranial hemorrhage
- In the INJECT clinical trial the rate of in-hospital, intracranial hemorrhage among all patients treated with reteplase was 0.8% (23 of 2,965 patients).
- As seen with reteplase and other thrombolytic agents, the risk for intracranial hemorrhage is increased in patients with advanced age or with elevated blood pressure.
Other types of hemorrhage
- The incidence of other types of bleeding events in clinical studies of reteplase varied depending upon the use of arterial catheterization or other invasive procedures and whether the study was performed in Europe or the USA.
- The overall incidence of any bleeding event in patients treated with reteplase in clinical studies (n = 3,805) was 21.1%.
- The rates for bleeding events, regardless of severity, for the 10 + 10 unit reteplase regimen from controlled clinical studies are summarized in Table 3.
- In these studies the severity and sites of bleeding events were comparable for reteplase and the comparison thrombolytic agents.
- Should serious bleeding in a critical location (intracranial, gastrointestinal, retroperitoneal, pericardial) occur, any concomitant heparin should be terminated immediately.
- In addition, the second bolus of reteplase should not be given if the serious bleeding occurs before it is administered.
- Death and permanent disability are not uncommonly reported in patients who have experienced stroke (including intracranial bleeding) and other serious bleeding episodes.
- Fibrin which is part of the hemostatic plug formed at needle puncture sites will be lysed during reteplase therapy.
- Therefore, reteplasetherapy requires careful attention to potential bleeding sites (e.g., catheter insertion sites, arterial puncture sites).
Allergic Reactions
- Among the 2,965 patients receiving reteplase in the INJECT trial, serious allergic reactions were noted in 3 patients, with one patient experiencing dyspnea and hypotension.
- No anaphylactoid reactions were observed among the 3,856 patients treated with reteplase in initial clinical trials.
- In an ongoing clinical trial two anaphylactoid reactions have been reported among approximately 2,500 patients receiving reteplase.
Other Adverse Reactions
- Patients administered reteplase as treatment for myocardial infarction have experienced many events which are frequent sequelae of myocardial infarction and may or may not be attributable to reteplase therapy.
- These events includecardiogenic shock, arrhythmias (e.g., sinus bradycardia, accelerated idioventricular rhythm, ventricular premature depolarizations, supraventricular tachycardia, ventricular tachycardia, ventricular fibrillation), AV block, pulmonary edema,heart failure, cardiac arrest, recurrent ischemia, reinfarction, myocardial rupture, mitral regurgitation, pericardial effusion, pericarditis, cardiac tamponade, venous thrombosisand embolism, and electromechanical dissociation.
- These events can be life-threatening and may lead to death.
- Other adverse events have been reported, including nausea and/or vomiting, hypotension, and fever.
Postmarketing Experience
There is limited information regarding Reteplase Postmarketing Experience in the drug label.
Drug Interactions
- The interaction of reteplase with other cardioactive drugs has not been studied.
- In addition to bleeding associated with heparin and vitamin K antagonists, drugs that alter platelet function (such as aspirin, dipyridamole, and abciximab) may increase the risk of bleeding if administered prior to or after reteplase therapy.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Reteplase has been shown to have an abortifacient effect in rabbits when given in doses 3 times the human dose (0.86 units/kg). Reproduction studies performed in rats at doses up to 15 times the human dose (4.31 units/kg) revealed no evidence of fetal anomalies; however, Reteplase administered to pregnant rabbits resulted in hemorrhaging in the genital tract, leading to abortions in mid-gestation. There are no adequate and well-controlled studies in pregnant women. The most common complication of thrombolytic therapy is bleeding and certain conditions, including pregnancy, can increase this risk. Reteplase should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS): C
Drugs which, owing to their pharmacological effects, have caused or may be suspected of causing harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible. Accompanying texts should be consulted for further details.
Labor and Delivery
There is no FDA guidance on use of Reteplase during labor and delivery.
Nursing Mothers
It is not known whether reteplase is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Retavase® is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of reteplase in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Reteplase in geriatric settings.
Gender
There is no FDA guidance on the use of Reteplase with respect to specific gender populations.
Race
There is no FDA guidance on the use of Reteplase with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Reteplase in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Reteplase in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Reteplase in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Reteplase in patients who are immunocompromised.
Administration and Monitoring
Administration
Reteplase is for intravenous administration only. Reteplase is administered as a 10 + 10 unit double-bolus injection. Two 10 unit bolus injections are required for a complete treatment. Each bolus is administered as an intravenous injection over 2 minutes. The second bolus is given 30 minutes after initiation of the first bolus injection. Each bolus injection should be given via an intravenous line in which no other medication is being simultaneously injected or infused. No other medication should be added to the injection solution containing reteplase. There is no experience with patients receiving repeat courses of therapy with reteplase.
Heparin and reteplase are incompatible when combined in solution. 'Do not administer heparin and reteplase simultaneously in the same intravenous line.'
If reteplase is to be injected through an intravenous line containing heparin, a normal saline or 5% dextrose (D5W) solution should be flushed through the line prior to and following the reteplase injection.
Although the value of anticoagulants and antiplatelet drugs during and following administration of reteplase has not been studied, heparin has been administered concomitantly in more than 99% of patients. Aspirin has been given either during and/or following heparin treatment. Studies assessing the safety and efficacy of reteplase without adjunctive therapy with heparin and aspirin have not been performed.
Reconstitution – Reteplase Kit and Reteplase Half-Kit: Reconstitution should be carried out using the diluent and dispensing pin provided with reteplase. It is important that reteplase be reconstituted only with the supplied Sterile Water for Injection, USP (without preservatives). The reconstituted preparation results in a colorless solution containing reteplase 1 unit/mL. Slight foaming upon reconstitution is not unusual; allowing the vial to stand undisturbed for several minutes is usually sufficient to allow dissipation of any large bubbles.
Because reteplase contains no antibacterial preservatives, it should be reconstituted immediately before use. When reconstituted as directed, the solution may be used within 4 hours when stored at 2-30°C (36-86°F). Prior to administration, the product should be visually inspected for particulate matter and discoloration.
Reconstitution Instructions-reteplase Kit and reteplase Half-Kit
Use aseptic technique throughout.
- Step 1: Withdraw 10 mL of Sterile Water for Injection, USP (SWFI) from the supplied vial into a sterile 10 mL syringe.
- Step 2: Open the package containing the dispensing pin. Remove the protective cap from the luer lock port of the dispensing pin and connect the sterile 10mL syringe to the dispensing pin. Remove the protective flip-cap from one vial of reteplase.
- Step 3: Remove the protective cap from the spike end of the dispensing pin, and insert the spike into the vial of reteplase until the security clips lock onto the vial.
Transfer the 10 mL of SWFI through the dispensing pin into the vial of reteplase.
- Step 4: With the dispensing pin and syringe still attached to the vial, swirl the vial gently to dissolve the reteplase. DO NOT SHAKE.
- Step 5: Withdraw 10 mL of reteplase reconstituted solution back into the syringe. A small amount of solution will remain in the vial due to overfill.
- Step 6: Detach the syringe from the dispensing pin, and attach a sterile needle.
- Step 7: The 10 mL bolus dose is now ready for administration. Safely discard all used reconstitution components and the empty reteplase vial according to institutional procedures.
Monitoring
Drug/Laboratory Test Interactions
- Administration of reteplase may cause decreases in plasminogen and fibrinogen. During reteplase therapy, if coagulation tests and/or measurements of fibrinolytic activity are performed, the results may be unreliable unless specific precautions are taken to prevent in vitro artifacts.
- Reteplase is an enzyme that when present in blood in pharmacologic concentrations remains active under in vitro conditions.
- This can lead to degradation of fibrinogen in blood samples removed for analysis.
- Collection of blood samples in the presence of PPACK (chloromethylketone) at 2 µM concentrations was used in clinical trials to prevent in vitro fibrinolytic artifacts.
Use of Antithrombotics
- Heparin and aspirin have been administered concomitantly with and following the administration of reteplase in the management of acute myocardial infarction.
- Because heparin, aspirin, or reteplase may cause bleeding complications, careful monitoring for bleeding is advised, especially at arterial puncture sites.
IV Compatibility
- Heparin and reteplase are incompatible when combined in solution
- Do not administer heparin and reteplase simultaneously in the same intravenous line.
Overdosage
There is limited information regarding Reteplase overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
Reteplase is a recombinant plasminogen activator which catalyzes the cleavage of endogenous plasminogen to generate plasmin. Plasmin in turn degrades the fibrin matrix of the thrombus, thereby exerting its thrombolytic action.1,2In a controlled trial, 36 of 56 patients treated for an acute myocardial infarction (AMI) had a decrease in fibrinogen levels to below 100 mg/dL by 2 hours following the administration of reteplase as a double-bolus intravenous injection (10 + 10 unit) in which 10 units (17.4 mg) was followed 30 minutes later by a second bolus of 10 units (17.4 mg).3 The mean fibrinogen level returned to the baseline value by 48 hours.
Structure
Reteplase is a non-glycosylated deletion mutein of tissue plasminogen activator (tPA), containing the kringle 2 and the protease domains of human tPA. Reteplase contains 355 of the 527 amino acids of native tPA (amino acids 1-3 and 176-527). Reteplase is produced by recombinant DNA technology in E. coli. The protein is isolated as inactive inclusion bodies from E. coli, converted into its active form by an in vitro folding process and purified by chromatographic separation. The molecular weight of Reteplase is 39,571 daltons.
Pharmacodynamics
There is limited information regarding Reteplase Pharmacodynamics in the drug label.
Pharmacokinetics
Based on the measurement of thrombolytic activity, reteplase is cleared from plasma at a rate of 250-450 mL/min, with an effective half-life of 13-16 minutes. Reteplase is cleared primarily by the liver and kidney.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of reteplase.
- Studies to determine mutagenicity, chromosomal aberrations, gene mutations, and micronuclei induction were negative at all concentrations tested.
- Reproductive toxicity studies in rats revealed no effects on fertility at doses up to 15 times the human dose (4.31 units/kg).
Clinical Studies
The safety and efficacy of reteplase were evaluated in three controlled clinical trials in which reteplase was compared to other thrombolytic agents. The INJECT study was designed to assess the relative effects of reteplase or the Streptase® brand of streptokinase upon mortality rates at 35 days following an AMI. The other studies (RAPID 1 and RAPID 2) were arteriographic studies which compared the effect on coronary patency of reteplase to two regimens of alteplase (a tissue plasminogen activator; Activase® in the USA and Actilyse® in Europe) in patients with an AMI. In all three studies, patients were treated with aspirin (initial doses of 160 mg to 350 mg and subsequent doses of 75 mg to 350 mg) and heparin (a 5,000 unit IV bolus prior to the administration of reteplase, followed by a 1000 unit/hour continuous IV infusion for at least 24 hours).3,4,5 The safety and efficacy of reteplase have not been evaluated using antithrombotic or antiplatelet regimens other than those described above.
Reteplase (10 + 10 unit) was compared to streptokinase (1.5 million units over 60 minutes) in a double-blind, randomized, European study (INJECT), which studied 6,010 patients treated within 12 hours of the onset of symptoms of AMI. To be eligible for enrollment, patients had to have chest pain consistent with coronary ischemia and ST segment elevation, or a bundle branch block pattern on the EKG. Patients with known cerebrovascular or other bleeding risks or those with a systolic blood pressure >200 mm Hg or a diastolic blood pressure >100 mm Hg were excluded from enrollment. The results of the primary endpoint (mortality at 35 days), six month mortality and selected other 35 day endpoints are shown in Table 1 for patients receiving study medications.
For mortality, stroke and the combined outcome of mortality or stroke, the 95% confidence intervals in Table 1 reflect the range within which the true difference in outcomes probably lies and includes the possibility of no difference. The incidences of congestive heart failure and of cardiogenic shock were significantly lower among patients treated with reteplase.
The total incidence of stroke was similar between the groups. However, more patients treated with reteplase experienced hemorrhagic strokes than patients treated with streptokinase. An exploratory analysis indicated that the incidence of intracranial hemorrhage was higher among older patients or those with elevated blood pressure. The incidence of intracranial hemorrhage among the 698 patients treated with reteplase who were older than 70 years was 2.2%. Intracranial hemorrhage occurred in 8 of the 332 (2.4%) patients treated with reteplase who had an initial systolic blood pressure >160 mm Hg and in 15 of the 2,629 (0.6%) reteplase patients who had an initial systolic blood pressure <160 mm Hg.
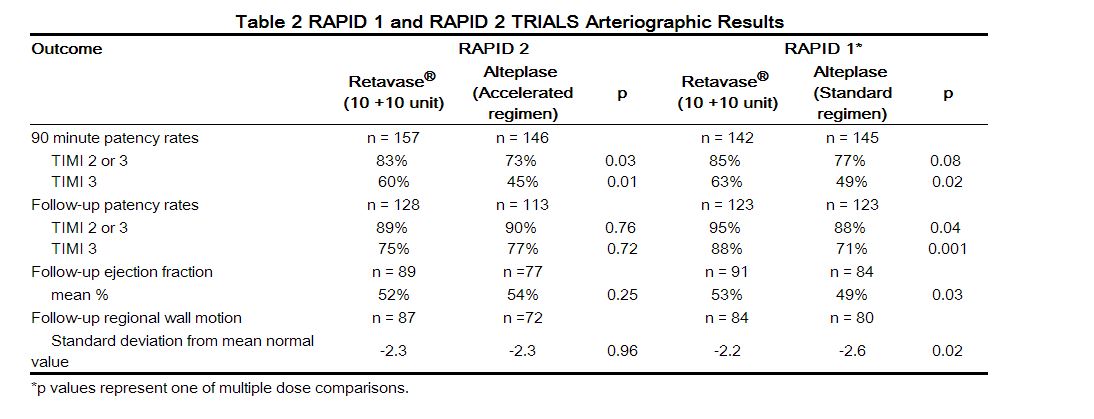
Two arteriographic studies (RAPID 1 and RAPID 2) were performed utilizing open-label administration of the study agents and a blinded review of the arteriograms. In RAPID 1, patients were treated within 6 hours of the onset of symptoms, and in RAPID 2, patients were treated within 12 hours of the onset of symptoms. Both studies evaluated coronary artery perfusion through the infarct-related artery 90 minutes after the initiation of therapy as the primary endpoint. Some patients in each study also had perfusion through the infarct-related artery evaluated at 60 minutes after the initiation of therapy. In RAPID 1, reteplase (in doses of 10 + 10 unit, 15 unit, or 10 + 5 unit) was compared to a 3 hour regimen of alteplase (100 mg administered over 3 hrs).In RAPID 2, reteplase (10 + 10 unit) was compared to an accelerated regimen of alteplase (100 mg administered over 1.5 hrs). The percentages of patients with partial or complete flow (TIMI grades 2 or 3) and complete flow (TIMI grade 3), are shown along with ventricular function assessments in Table 2. The follow-up arteriogram was performed at a median of 8 (RAPID 1) and 5 (RAPID 2) days following the administration of the thrombolytics. In RAPID 1 the best patency results were obtained with the 10 + 10 unit dose. In RAPID 2, the percentage of patients with partial or complete flow and the percentage of patients with complete flow was significantly higher with reteplase than with alteplaseat 90 minutes after the initiation of therapy. In both clinical trials the reocclusion rates were similar for reteplase and alteplase. The relationship between coronary artery patency and clinical efficacy has not been established.
Approximately 70% (RAPID 1) and 78% (RAPID 2) of the patients in the arteriographic studies underwent optional arteriography at 60 minutes following the administration of the study agents. In both trials the percentage of patients with complete flow at 60 minutes was significantly higher with reteplase than with alteplase. Neither RAPID clinical trial was designed nor powered to compare the efficacy or safety of reteplase and alteplase with respect to the outcomes of mortality and stroke.
How Supplied
Reteplase, is supplied as a sterile, preservative-free, lyophilized powder in 10.4 unit (equivalent to 18.1 mg reteplase) vials without a vacuum, in the following packaging configurations:
Reteplase Kit: 2 single-use reteplase vials 10.4 units (18.1 mg), 2 single-use diluent vials for reconstitution (10 mL Sterile Water for Injection, USP), 2 sterile 10 mL syringes, 2 sterile dispensing pins, 4 sterile needles, 2 alcohol swabs and a package insert;
Reteplase Half-Kit: 1 single-use reteplase vial 10.4 units (18.1 mg), 1 single-use diluent vial for reconstitution (10 mL Sterile Water for Injection, USP), a sterile dispensing pin and a package insert.
Storage
Store reteplase at 2-25°C (36-77°F). The box should remain sealed until use to protect the lyophilisate from exposure to light. Do not use beyond the expiration date printed on the box.
Images
Drug Images
{{#ask: Page Name::Reteplase |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Reteplase |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Reteplase in the drug label.
Precautions with Alcohol
Alcohol-Reteplase interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Retavase
Look-Alike Drug Names
There is limited information regarding Reteplase Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ A. Falk, J. Guller, F. S. Nowakowski, H. Mitty, V. Teodorescu, J. Uribarri & J. Vassalotti (2001). "Reteplase in the treatment of thrombosed hemodialysis grafts". Journal of vascular and interventional radiology : JVIR. 12 (11): 1257–1262. PMID 11698623. Unknown parameter
|month=ignored (help) - ↑ U. Tebbe, A. Graf, W. Kamke, R. Zahn, F. Forycki, G. Kratzsch & G. Berg (1999). "Hemodynamic effects of double bolus reteplase versus alteplase infusion in massive pulmonary embolism". American heart journal. 138 (1 Pt 1): 39–44. PMID 10385761. Unknown parameter
|month=ignored (help) - ↑ M. M. Davidian, A. Powell, J. F. Benenati, B. T. Katzen, G. J. Becker & G. Zemel (2000). "Initial results of reteplase in the treatment of acute lower extremity arterial occlusions". Journal of vascular and interventional radiology : JVIR. 11 (3): 289–294. PMID 10735421. Unknown parameter
|month=ignored (help)
{{#subobject:
|Label Page=Reteplase |Label Name=Rets1.JPG
}}
{{#subobject:
|Label Page=Reteplase |Label Name=Reteplase.JPG
}}
{{#subobject:
|Label Page=Reteplase |Label Name=Rets2.jpg
}}
{{#subobject:
|Label Page=Reteplase |Label Name=Rets3.jpg
}}