Ramucirumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: HEMORRHAGE
See full prescribing information for complete Boxed Warning.
|
Overview
Ramucirumab is a monoclonal antibody that is FDA approved for the treatment of advanced or metastatic, gastric or gastro-esophageal junction adenocarcinoma with disease progression on or after prior fluoropyrimidine- or platinum-containing chemotherapy and metastatic non-small cell lung cancer (NSCLC) with disease progression on or after platinum-based chemotherapy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose and Schedule
Gastric Cancer
- The recommended dose of Ramucirumab either as a single agent or in combination with weekly paclitaxel is 8 mg/kg every 2 weeks administered as an intravenous infusion over 60 minutes. Continue Ramucirumab until disease progression or unacceptable toxicity.
- When given in combination, administer Ramucirumab prior to administration of paclitaxel.
Non-Small Cell Lung Cancer
- The recommended dose of Ramucirumab is 10 mg/kg administered by intravenous infusion over approximately 60 minutes on day 1 of a 21-day cycle prior to docetaxel infusion. Continue Ramucirumab until disease progression or unacceptable toxicity.
Premedication
- Prior to each Ramucirumab infusion, premedicate all patients with an intravenous histamine H1 antagonist (e.g., diphenhydramine hydrochloride).
- For patients who have experienced a Grade 1 or 2 infusion-related reaction, also premedicate with dexamethasone (or equivalent) and acetaminophen prior to each Ramucirumab infusion.
Dose Modifications
Infusion-Related Reactions (IRR)
- Reduce the infusion rate of Ramucirumab by 50% for Grade 1 or 2 IRRs.
- Permanently discontinue Ramucirumab for Grade 3 or 4 IRRs.
Hypertension
- Interrupt Ramucirumab for severe hypertension until controlled with medical management.
- Permanently discontinue Ramucirumab for severe hypertension that cannot be controlled with antihypertensive therapy
Proteinuria
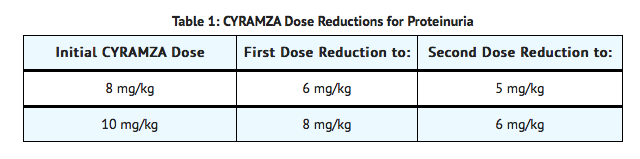
- Interrupt Ramucirumab for urine protein levels ≥2 g/24 hours. Reinitiate treatment at a reduced dose (see TABLE 1) once the urine protein level returns to <2 g/24 hours. If the protein level ≥2 g/24 hours reoccurs, interrupt Ramucirumab and reduce the dose (see TABLE 1) once the urine protein level returns to <2 g/24 hours.
- Permanently discontinue Ramucirumab for urine protein level >3 g/24 hours or in the setting of nephrotic syndrome.
Wound Healing Complications
- Interrupt Ramucirumab prior to scheduled surgery until the wound is fully healed.
Arterial Thromboembolic Events, Gastrointestinal Perforation, or Grade 3 or 4 Bleeding
- Permanently discontinue Ramucirumab.
Preparation for Administration
Inspect vial contents for particulate matter and discoloration prior to dilution. Discard the vial, if particulate matter or discolorations are identified. Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) until time of use. Keep the vial in the outer carton in order to protect from light.
- Calculate the dose and the required volume of Ramucirumab needed to prepare the infusion solution. Vials contain either 100 mg/10 mL or 500 mg/50 mL at a concentration of 10 mg/mL solution of Ramucirumab.
- Withdraw the required volume of Ramucirumab and further dilute with only 0.9% Sodium Chloride Injection in an intravenous infusion container to a final volume of 250 mL. Do not use dextrose containing solutions.
- Gently invert the container to ensure adequate mixing.
- Do not freeze or shake the infusion solution. DO NOT dilute with other solutions or co-infuse with other electrolytes or medications.
- Store diluted infusion for no more than 24 hours at 2°C to 8°C (36°F to 46°F) or 4 hours at room temperature (below 25°C [77°F]).
- Discard vial with any unused portion of Ramucirumab.
Administration
- Visually inspect the diluted solution for particulate matter and discoloration prior to administration. If particulate matter or discolorations are identified, discard the solution.
- Administer diluted Ramucirumab infusion via infusion pump over 60 minutes through a separate infusion line. Use of a protein sparing 0.22 micron filter is recommended. Flush the line with sterile sodium chloride (0.9%) solution for injection at the end of the infusion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ramucirumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ramucirumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ramucirumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ramucirumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ramucirumab in pediatric patients.
Contraindications
None.
Warnings
|
WARNING: HEMORRHAGE
See full prescribing information for complete Boxed Warning.
|
Hemorrhage
- Ramucirumab increased the risk of hemorrhage and gastrointestinal hemorrhage, including severe and sometimes fatal hemorrhagic events. In Study 1, the incidence of severe bleeding was 3.4% for Ramucirumab and 2.6% for placebo. In Study 2, the incidence of severe bleeding was 4.3% for Ramucirumab plus paclitaxel and 2.4% for placebo plus paclitaxel.
- Patients with gastric cancer receiving nonsteroidal anti-inflammatory drugs (NSAIDs) were excluded from enrollment in Studies 1 and 2; therefore, the risk of gastric hemorrhage in Ramucirumab-treated patients with gastric tumors receiving NSAIDs is unknown. Permanently discontinue Ramucirumab in patients who experience severe bleeding.
- In Study 3, the incidence of severe bleeding was 2.4% for Ramucirumab plus docetaxel and 2.3% for placebo plus docetaxel. Patients with NSCLC receiving therapeutic anticoagulation or chronic therapy with NSAIDS or other anti-platelet therapy other than once daily aspirin or with radiographic evidence of major airway or blood vessel invasion or intratumor cavitation were excluded from Study 3; therefore the risk of pulmonary hemorrhage in these groups of patients is unknown.
Arterial Thromboembolic Events
- Serious, sometimes fatal, arterial thromboembolic events (ATEs) including myocardial infarction, cardiac arrest, cerebrovascular accident, and cerebral ischemia occurred in clinical trials including 1.7% of 236 patients who received Ramucirumab as a single agent for gastric cancer in Study 1. Permanently discontinue Ramucirumab in patients who experience a severe ATE.
Hypertension
- An increased incidence of severe hypertension occurred in patients receiving Ramucirumab as a single agent (8%) as compared to placebo (3%) and in patients receiving Ramucirumab plus paclitaxel (15%) as compared to placebo plus paclitaxel (3%) and in patients receiving Ramucirumab plus docetaxel (6%) as compared to placebo plus docetaxel (2%).
- Control hypertension prior to initiating treatment with Ramucirumab. Monitor blood pressure every two weeks or more frequently as indicated during treatment.
- Temporarily suspend Ramucirumab for severe hypertension until medically controlled. Permanently discontinue Ramucirumab if medically significant hypertension cannot be controlled with antihypertensive therapy or in patients with hypertensive crisis or hypertensive encephalopathy.
Infusion-Related Reactions
- Prior to the institution of premedication recommendations across clinical trials of Ramucirumab IRRs occurred in 6 out of 37 patients (16%), including two severe events. The majority of IRRs across trials occurred during or following a first or second Ramucirumab infusion. Symptoms of IRRs included rigors/tremors, back pain/spasms, chest pain and/or tightness, chills, flushing, dyspnea, wheezing, hypoxia, and paresthesia. In severe cases, symptoms included bronchospasm, supraventricular tachycardia, and hypotension.
- Monitor patients during the infusion for signs and symptoms of IRRs in a setting with available resuscitation equipment. Immediately and permanently discontinue Ramucirumab for Grade 3 or 4 IRRs.
Gastrointestinal Perforations
- Ramucirumab is an antiangiogenic therapy that can increase the risk of gastrointestinal perforation, a potentially fatal event. Four of 570 patients (0.7%) who received Ramucirumab as a single agent in clinical trials experienced gastrointestinal perforation. In Study 2, the incidence of gastrointestinal perforations was also increased in patients that received Ramucirumab plus paclitaxel (1.2%) as compared to patients receiving placebo plus paclitaxel (0.3%). In Study 3, the incidence of gastrointestinal perforation was 1% for Ramucirumab plus docetaxel and 0.3% for placebo plus docetaxel. Permanently discontinue Ramucirumab in patients who experience a gastrointestinal perforation.
Impaired Wound Healing
- Ramucirumab has not been studied in patients with serious or non-healing wounds. Ramucirumab is an antiangiogenic therapy with the potential to adversely affect wound healing.
- Withhold Ramucirumab prior to surgery. Resume following the surgical intervention based on clinical judgment of adequate wound healing. If a patient develops wound healing complications during therapy, discontinue Ramucirumab until the wound is fully healed.
Clinical Deterioration in Patients with Child-Pugh B or C Cirrhosis
- Clinical deterioration, manifested by new onset or worsening encephalopathy, ascites, or hepatorenal syndrome was reported in patients with Child-Pugh B or C cirrhosis who received single-agent Ramucirumab. Use Ramucirumab in patients with Child-Pugh B or C cirrhosis only if the potential benefits of treatment are judged to outweigh the risks of clinical deterioration.
Reversible Posterior Leukoencephalopathy Syndrome
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS) has been reported with a rate of <0.1% in clinical studies with Ramucirumab Confirm the diagnosis of RPLS with MRI and discontinue Ramucirumab in patients who develop RPLS. Symptoms may resolve or improve within days, although some patients with RPLS can experience ongoing neurologic sequelae or death
Adverse Reactions
Clinical Trials Experience
The following adverse drug reactions are discussed in greater detail in other sections of the label:
- Hemorrhage
- Arterial Thromboembolic Events
- Hypertension
- Infusion-Related Reactions
- Gastrointestinal Perforation
- Impaired Wound Healing
- Patients with Child-Pugh B or C Cirrhosis
- Reversible Posterior Leukoencephalopathy Syndrome
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Gastric Cancer
- Safety data are presented from two randomized, placebo controlled clinical trials in which patients received Ramucirumab: Study 1, a randomized (2:1), double-blind, clinical trial in which 351 patients received either Ramucirumab 8 mg/kg intravenously every two weeks or placebo every two weeks and Study 2, a double-blind, randomized (1:1) clinical trial in which 656 patients received paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 28-day cycle plus either Ramucirumab 8 mg/kg intravenously every two weeks or placebo every two weeks. Both trials excluded patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or greater, uncontrolled hypertension, major surgery within 28 days, or patients receiving chronic anti-platelet therapy other than once daily aspirin. Study 1 excluded patients with bilirubin ≥1.5 mg/dL and Study 2 excluded patients with bilirubin >1.5 times the upper limit of normal.
Ramucirumab Administered as a Single Agent
- Among 236 patients who received Ramucirumab (safety population) in Study 1, median age was 60 years, 28% were women, 76% were White, and 16% were Asian. Patients in Study 1 received a median of 4 doses of Ramucirumab; the median duration of exposure was 8 weeks, and 32 (14% of 236) patients received Ramucirumab for at least six months.
- In Study 1, the most common adverse reactions (all grades) observed in Ramucirumab-treated patients at a rate of ≥10% and ≥2% higher than placebo were hypertension and diarrhea. The most common serious adverse events with Ramucirumab were anemia (3.8%) and intestinal obstruction (2.1%). Red blood cell transfusions were given to 11% of Ramucirumab-treated patients versus 8.7% of patients who received placebo.
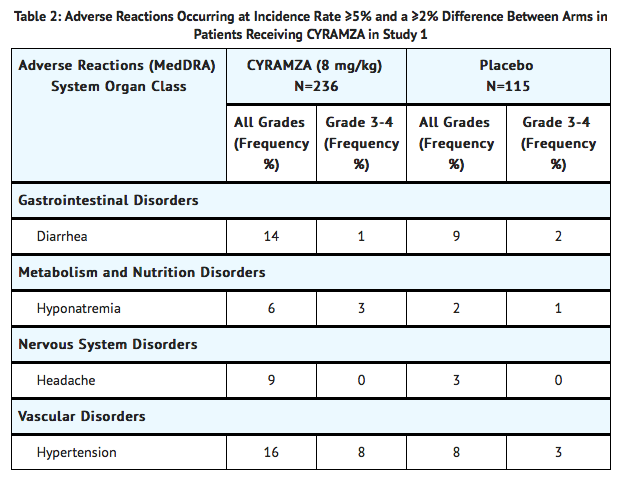
- Clinically relevant adverse reactions reported in ≥1% and <5% of Ramucirumab-treated patients in Study 1 were: neutropenia (4.7% Ramucirumab versus 0.9% placebo), epistaxis (4.7% Ramucirumab versus 0.9% placebo), rash (4.2¢ Ramucirumab versus 1.7% placebo), intestinal obstruction (2.1% Ramucirumab versus 0% placebo), and arterial thromboembolic events (1.7% Ramucirumab versus 0% placebo).
- Across clinical trials of Ramucirumab administered as a single agent, clinically relevant adverse reactions (including Grade ≥3) reported in Ramucirumab-treated patients included proteinuria, gastrointestinal perforation, and infusion-related reactions.
- In Study 1, according to laboratory assessment, 8% of Ramucirumab-treated patients developed proteinuria versus 3% of placebo-treated patients. Two patients discontinued Ramucirumab due to proteinuria. The rate of gastrointestinal perforation in Study 1 was 0.8% and the rate of infusion-related reactions was 0.4%.
Ramucirumab Administered in Combination with Paclitaxel
- Among 327 patients who received Ramucirumab (safety population) in Study 2, median age was 60 years, 31% were women, 63% were White, and 33% were Asian. Patients in Study 2 received a median of 9 doses of Ramucirumab; the median duration of exposure was 18 weeks, and 93 (28% of 327) patients received Ramucirumab for at least six months.
- In Study 2, the most common adverse reactions (all grades) observed in patients treated with Ramucirumab plus paclitaxel at a rate of ≥30% and ≥2% higher than placebo plus paclitaxel were fatigue, neutropenia, diarrhea, and epistaxis. The most common serious adverse events with Ramucirumab plus paclitaxel were neutropenia (3.7%) and febrile neutropenia (2.4%); 19% of patients treated with Ramucirumab plus paclitaxel received granulocyte colony-stimulating factors. Adverse reactions resulting in discontinuation of any component of the Ramucirumab plus paclitaxel combination in 2% or more patients in Study 2 were neutropenia (4%) and thrombocytopenia (3%).
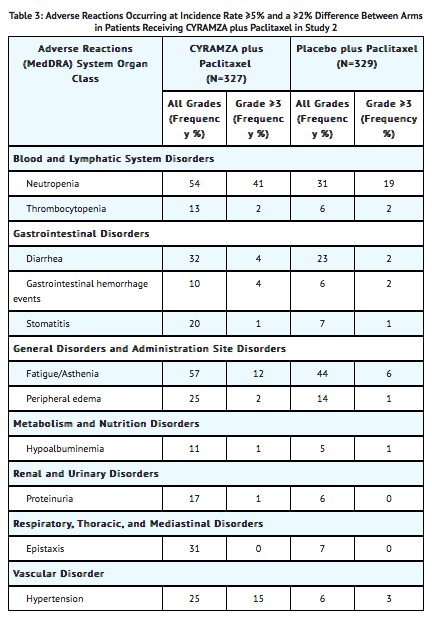
Clinically relevant adverse reactions reported in ≥1% and <5% of the Ramucirumab plus paclitaxel treated patients in Study 2 were sepsis (3.1% Ramucirumab plus paclitaxel versus 1.8% placebo plus paclitaxel) and gastrointestinal perforations (1.2% Ramucirumab plus paclitaxel versus 0.3% for placebo plus paclitaxel).
Non-Small Cell Lung Cancer
Ramucirumab Administered in Combination with Docetaxel
- Study 3 was a multinational, randomized, double-blind study conducted in patients with NSCLC with disease progression on or after one platinum-based therapy for locally advanced or metastatic disease. Patients received either Ramucirumab 10 mg/kg intravenously plus docetaxel 75 mg/m2 intravenously every 3 weeks or placebo plus docetaxel 75 mg/m2 intravenously every 3 weeks. Due to an increased incidence of neutropenia and febrile neutropenia in patients enrolled in East Asian sites, Study 3 was amended and 24 patients (11 Ramucirumab plus docetaxel, 13 placebo plus docetaxel) at East Asian sites received a starting dose of docetaxel at 60 mg/m2 every 3 weeks.
- Study 3 excluded patients with an ECOG PS of 2 or greater, bilirubin greater than the upper limit of normal (ULN), uncontrolled hypertension, major surgery within 28 days, radiographic evidence of major airway or blood vessel invasion by cancer, radiographic evidence of intra-tumor cavitation, or gross hemoptysis within the preceding 2 months, and patients receiving therapeutic anticoagulation or chronic anti-platelet therapy other than once daily aspirin. The study also excluded patients whose only prior treatment for advanced NSCLC was a tyrosine kinase (epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase ALK) inhibitor).
- The data described below reflect exposure to Ramucirumab plus docetaxel in 627 patients in Study 3. Demographics and baseline characteristics were similar between treatment arms. Median age was 62 years; 67% of patients were men; 84% were White and 12% were Asian; 33% had ECOG PS 0; 74% had non-squamous histology and 25% had squamous histology. Patients received a median of 4.5 doses of Ramucirumab; the median duration of exposure was 3.5 months, and 195 (31% of 627) patients received Ramucirumab for at least six months.
- In Study 3, the most common adverse reactions (all grades) observed in Ramucirumab plus docetaxel-treated patients at a rate of ≥30% and ≥2% higher than placebo plus docetaxel were neutropenia, fatigue/asthenia, and stomatitis/mucosal inflammation. Treatment discontinuation due to adverse reactions occurred more frequently in Ramucirumab plus docetaxel-treated patients (9%) than in placebo plus docetaxel-treated patients (5%). The most common adverse events leading to treatment discontinuation of Ramucirumab were infusion-related reaction (0.5%) and epistaxis (0.3%). For patients with non-squamous histology, the overall incidence of pulmonary hemorrhage was 7% and the incidence of ≥Grade 3 pulmonary hemorrhage was 1% for Ramucirumab plus docetaxel compared to 6% overall incidence and 1% for ≥Grade 3 pulmonary hemorrhage for placebo plus docetaxel. For patients with squamous histology, the overall incidence of pulmonary hemorrhage was 10% and the incidence of ≥Grade 3 pulmonary hemorrhage was 2% for Ramucirumab plus docetaxel compared to 12% overall incidence and 2% for ≥Grade 3 pulmonary hemorrhage for placebo plus docetaxel.
- The most common serious adverse events with Ramucirumab plus docetaxel were febrile neutropenia (14%), pneumonia (6%), and neutropenia (5%). The use of granulocyte colony-stimulating factors was 42% in Ramucirumab plus docetaxel-treated patients versus 37% in patients who received placebo plus docetaxel. In patients ≥65 years, there were 18 (8%) deaths on treatment or within 30 days of discontinuation for Ramucirumab plus docetaxel and 9 (4%) deaths for placebo plus docetaxel. In patients <65 years, there were 13 (3%) deaths on treatment or within 30 days of discontinuation for Ramucirumab plus docetaxel and 26 (6%) deaths for placebo plus docetaxel.

Clinically relevant adverse drug reactions reported in ≥1% and <5% of the Ramucirumab plus docetaxel-treated patients in Study 3 were hyponatremia (4.8% Ramucirumab plus docetaxel versus 2.4% for placebo plus docetaxel) and proteinuria (3.3% Ramucirumab plus docetaxel versus 0.8% placebo plus docetaxel).
Postmarketing Experience
There is limited information regarding Ramucirumab Postmarketing Experience in the drug label.
Drug Interactions
- No pharmacokinetic (PK) interactions were observed between ramucirumab and paclitaxel or between ramucirumab and docetaxel.
Use in Specific Populations
Pregnancy
Risk Summary
- Based on its mechanism of action, Ramucirumab may cause fetal harm. Animal models link angiogenesis, VEGF and VEGF Receptor 2 (VEGFR2) to critical aspects of female reproduction, embryofetal development, and postnatal development. There are no adequate or well controlled studies of ramucirumab in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus.
Animal Data
- No animal studies have been specifically conducted to evaluate the effect of ramucirumab on reproduction and fetal development. In mice, loss of the VEGFR2 gene resulted in embryofetal death and these fetuses lacked organized blood vessels and blood islands in the yolk sac. In other models, VEGFR2 signaling was associated with development and maintenance of endometrial and placental vascular function, successful blastocyst implantation, maternal and feto-placental vascular differentiation, and development during early pregnancy in rodents and non-human primates. Disruption of VEGF signaling has also been associated with developmental anomalies including poor development of the cranial region, forelimbs, forebrain, heart, and blood vessels.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ramucirumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ramucirumab during labor and delivery.
Nursing Mothers
- It is not known whether Ramucirumab is excreted in human milk. No studies have been conducted to assess Ramucirumab s impact on milk production or its presence in breast milk. Human IgG is excreted in human milk, but published data suggests that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. Because many drugs are excreted in human milk and because of the potential risk for serious adverse reactions in nursing infants from ramucirumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Ramucirumab in pediatric patients have not been established. In animal studies, effects on epiphyseal growth plates were identified. In cynomolgus monkeys, anatomical pathology revealed adverse effects on the epiphyseal growth plate (thickening and osteochondropathy) at all doses tested (5-50 mg/kg). Ramucirumab exposure at the lowest weekly dose tested in the cynomolgus monkey was 0.2 times the exposure in humans at the recommended dose of ramucirumab as a single agent.
Geriatic Use
- Of the 563 Ramucirumab-treated patients in two randomized gastric cancer clinical studies, 36% were 65 and over, while 7% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
- Of the 1253 patients in Study 3, 455 (36%) were 65 and over and 84 (7%) were 75 and over. Of the 627 patients who received Ramucirumab plus docetaxel in Study 3, 237 (38%) were 65 and over, while 45 (7%) were 75 and over. In an exploratory subgroup analysis of Study 3, the hazard ratio for overall survival in patients less than 65 years old was 0.74 (95% CI: 0.62, 0.87) and in patients 65 years or older was 1.10 (95% CI: 0.89, 1.36).
Gender
There is no FDA guidance on the use of Ramucirumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ramucirumab with respect to specific racial populations.
Renal Impairment
- No dose adjustment is recommended for patients with renal impairment based on population PK analysis.
Hepatic Impairment
- No dose adjustment is recommended for patients with mild hepatic impairment (total bilirubin within upper limit of normal [ULN] and aspartate aminotransferase AST >ULN or total bilirubin >1.0-1.5 times ULN and any AST) based on population PK analysis. Clinical deterioration was reported in patients with Child-Pugh B or C cirrhosis who received single-agent Ramucirumab.
Females of Reproductive Potential and Males
Fertility
- Advise females of reproductive potential that Ramucirumab may impair fertility.
Contraception
- Based on its mechanism of action, Ramucirumab may cause fetal harm. Advise females of reproductive potential to avoid getting pregnant while receiving Ramucirumab and for at least 3 months after the last dose of Ramucirumab.
Immunocompromised Patients
There is no FDA guidance one the use of Ramucirumab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ramucirumab Administration in the drug label.
Monitoring
There is limited information regarding Ramucirumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ramucirumab and IV administrations.
Overdosage
- There are no data on overdose in humans. Ramucirumab was administered at doses up to 10 mg/kg every two weeks without reaching a maximum tolerated dose.
Pharmacology
Mechanism of Action
Ramucirumab is a vascular endothelial growth factor receptor 2 antagonist that specifically binds VEGF Receptor 2 and blocks binding of VEGFR ligands, VEGF-A, VEGF-C, and VEGF-D. As a result, ramucirumab inhibits ligand-stimulated activation of VEGF Receptor 2, thereby inhibiting ligand-induced proliferation, and migration of human endothelial cells. Ramucirumab inhibited angiogenesis in an in vivo animal model.
Structure
There is limited information regarding Ramucirumab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Ramucirumab Pharmacodynamics in the drug label.
Pharmacokinetics
- With the dosing regimen of 8 mg/kg every 2 weeks in patients with advanced gastric or gastro-esophageal junction cancer, the geometric means of the minimum ramucirumab concentrations (Cmin) were 50 μg/mL (6-228 μg/mL) after the third dose and 74 μg/mL (14-234 μg/mL) after the sixth dose. Similar Cmin values of ramucirumab were observed when ramucirumab was administered with paclitaxel. Based on a population PK analysis, the mean (% coefficient of variation [CV%]) volume of distribution at steady state for ramucirumab was 5.5 L (14%), the mean clearance was 0.014 L/hour (30%), and the mean elimination half-life was 15 days (24%).
- With the dosing regimen of 10 mg/kg every 21 days in patients with NSCLC, the geometric means (ranges) of the minimum ramucirumab concentrations (Cmin) were 28 μg/mL (3-108 μg/mL) after the second dose and 38 μg/mL (3-128 μg/mL) after the fourth dose. Based on a population PK analysis in NSCLC patients, the mean (% coefficient of variation [CV%]) clearance for ramucirumab was 0.015 L/hour (27%), the mean volume of distribution at steady state (Vss) was 7.1 L (13%), and the mean elimination half-life was 23 days (24%).
Specific Populations
- Age, sex, and race had no clinically meaningful effect on the PK of ramucirumab based on a population PK analysis.
- Renal Impairment: The effect of renal impairment on the average concentration of ramucirumab at steady state (Css) was evaluated in patients with mild (calculated creatinine clearance CLcr 60-89 mL/min, n=368), moderate (CLcr 30-59 mL/min, n=160) or severe (CLcr 15 -29 mL/min, n=4) renal impairment compared to patients with normal renal function (CLcr ≥90 mL/min, n=360) in a population PK analysis. No clinically meaningful differences in the average Css of ramucirumab were observed between patients with renal impairment and patients with normal renal function.
- Hepatic Impairment: The effect of hepatic impairment on the average Css of ramucirumab was evaluated in patients with mild (total bilirubin within upper limit of normal [ULN] and AST>ULN or total bilirubin >1.0-1.5 times ULN and any AST, n=143) compared to patients with normal hepatic function (total bilirubin and AST ≤ULN, n=735) in a population PK analysis. No clinically meaningful differences in the average Css of ramucirumab were found between patients with mild hepatic impairment and patients with normal hepatic function. No PK data were available from patients with moderate (total bilirubin >1.5-3.0 times ULN and any AST) or severe hepatic dysfunction (total bilirubin >3.0 times ULN and any AST).
- Drug Interaction Studies
- No clinically meaningful changes in paclitaxel exposure or ramucirumab exposure were observed when Ramucirumab 8 mg/kg and paclitaxel 80 mg/m2 were co-administered in patients with solid tumors.
- No clinically meaningful changes in docetaxel exposure were observed when Ramucirumab 10 mg/kg and docetaxel 75 mg/m2 were co-administered in patients with solid tumors. Ramucirumab exposure appeared to be comparable regardless of concomitant docetaxel based on cross study comparisons in patients with solid tumors.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No animal studies have been performed to test ramucirumab for potential carcinogenicity or genotoxicity.
- Inhibition of VEGFR2 signaling in animal models was shown to result in changes to hormone levels critical for pregnancy, and, in monkeys, an increased duration of the follicular cycle. In a 39 week animal study, female monkeys treated with ramucirumab showed dose dependent increases in follicular mineralization of the ovary.
Animal Toxicology and/or Pharmacology
- Adverse effects in the kidney (glomerulonephritis) occurred with doses of 16-50 mg/kg (0.7-5.5 times the exposure in humans at the recommended dose of ramucirumab as a single agent).
- A single dose of ramucirumab resulting in an exposure approximately 10 times the exposure in humans at the recommended dose of ramucirumab as a single agent did not significantly impair wound healing in monkeys using a full-thickness incisional model.
Clinical Studies
Gastric Cancer
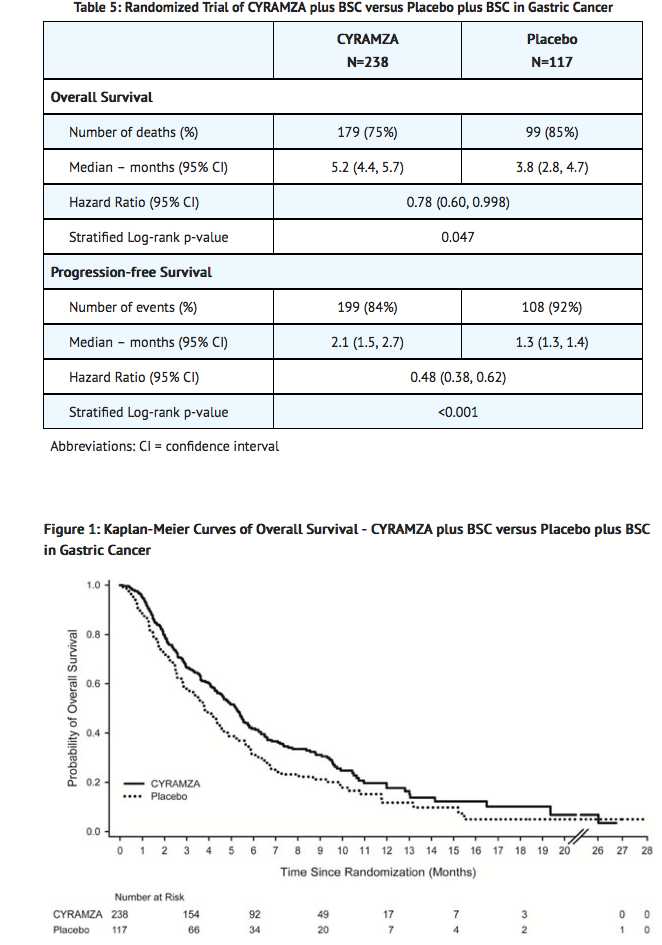
- Study 1 was a multinational, randomized, double-blind, multicenter study of Ramucirumab plus best supportive care (BSC) versus placebo plus BSC that randomized (2:1) 355 patients with locally advanced or metastatic gastric cancer (including adenocarcinoma of the gastro-esophageal junction [GEJ]) who previously received platinum- or fluoropyrimidine-containing chemotherapy. The major efficacy outcome measure was overall survival and the supportive efficacy outcome measure was progression-free survival. Patients were required to have experienced disease progression either within 4 months after the last dose of first-line therapy for locally advanced or metastatic disease or within 6 months after the last dose of adjuvant therapy. Patients were also required to have ECOG PS of 0 or 1. Patients received either an intravenous infusion of Ramucirumab 8 mg/kg (n=238) or placebo solution (n=117) every 2 weeks. Randomization was stratified by weight loss over the prior 3 months (≥10% versus <10%), geographic region, and location of the primary tumor (gastric versus GEJ).
- Demographic and baseline characteristics were similar between treatment arms. Median age was 60 years; 70% of patients were men; 77% were White, 16% Asian; the ECOG PS was 0 for 28% of patients and 1 for 72% of patients; 91% of patients had measurable disease; 75% of patients had gastric cancer; and 25% had adenocarcinoma of the GEJ. The majority of patients (85%) experienced disease progression during or following first-line therapy for metastatic disease. Prior chemotherapy for gastric cancer consisted of platinum/fluoropyrimidine combination therapy (81%), fluoropyrimidine-containing regimens without platinum (15%), and platinum-containing regimens without fluoropyrimidine (4%). In Study 1, patients received a median of 4 doses (range 1-34) of Ramucirumab or a median of 3 doses (range 1-30) of placebo.
- Overall survival and progression-free survival were statistically significantly improved in patients randomized to receive Ramucirumab as compared to patients randomized to receive placebo. Efficacy results are shown in TABLE 5 and FIGURE 1.
- Study 2 was a multinational, randomized, double-blind study of Ramucirumab plus paclitaxel versus placebo plus paclitaxel that randomized (1:1) 665 patients with locally advanced or metastatic gastric cancer (including adenocarcinoma of the gastro-esophageal junction) who previously received platinum- and fluoropyrimidine-containing chemotherapy. Patients were required to have experienced disease progression during, or within 4 months after the last dose of first-line therapy. Patients were also required to have ECOG PS of 0 or 1. Randomization was stratified by geographic region, time to progression from the start of first-line therapy (<6 months versus ≥6 months) and disease measurability.
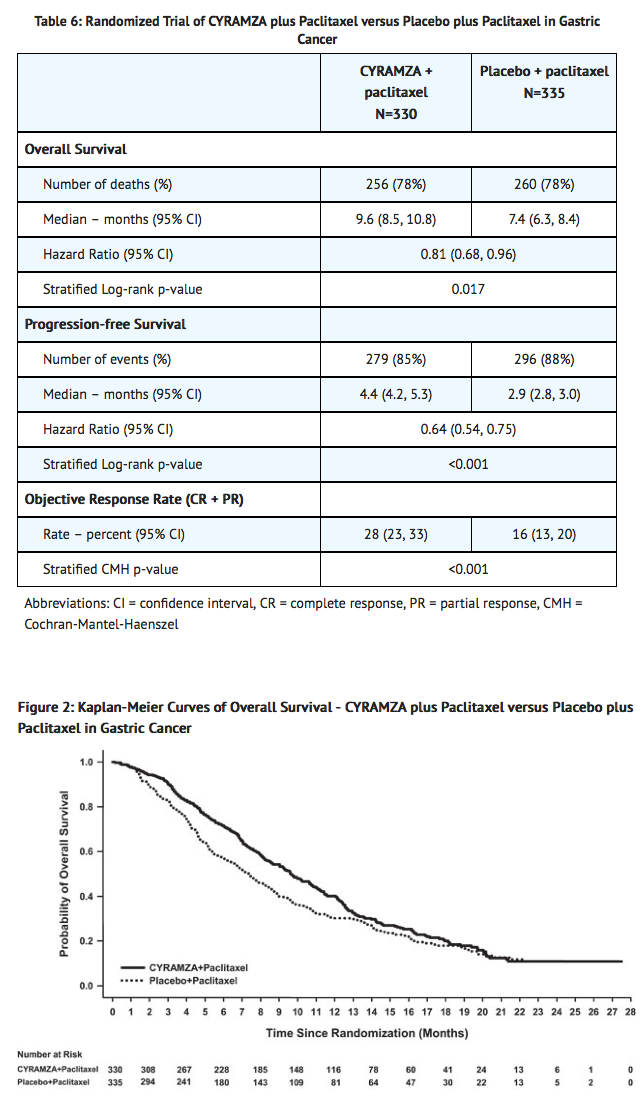
- Patients were randomized to receive either Ramucirumab 8 mg/kg (n=330) or placebo (n=335) as an intravenous infusion every 2 weeks (on days 1 and 15) of each 28-day cycle. Patients in both arms received paclitaxel 80 mg/m2 by intravenous infusion on days 1, 8, and 15 of each 28-day cycle. Prior to administration of each dose of paclitaxel, patients were required to have adequate hematopoietic and hepatic function. The paclitaxel dose was permanently reduced in increments of 10 mg/m2 for a maximum of two dose reductions for Grade 4 hematologic toxicity or Grade 3 paclitaxel-related non-hematologic toxicity. The major efficacy outcome measure was overall survival and the supportive efficacy outcome measures were progression-free survival and objective response rate.
- Demographics and baseline characteristics were similar between treatment arms including the following: Median age was 61 years; 71% of patients were men; 61% were White, 35% Asian; the ECOG PS was 0 for 39% of patients, 1 for 61% of patients; 78% of patients had measurable disease; 79% of patients had gastric cancer; and 21% had adenocarcinoma of the GEJ. Two-thirds of the patients experienced disease progression while on first-line therapy (67%) and 25% of patients received an anthracycline in combination with platinum/fluoropyrimidine combination therapy.
- Overall survival, progression-free survival, and objective response rate were statistically significantly improved in patients randomized to receive Ramucirumab plus paclitaxel compared to patients randomized to receive placebo plus paclitaxel. Efficacy results are shown in TABLE 6 and FIGURE 2.
Non-Small Cell Lung Cancer
Study 3 was a multinational, randomized, double-blind, study of Ramucirumab plus docetaxel versus placebo plus docetaxel, that randomized (1:1) 1253 patients with NSCLC with disease progression on or after one platinum-based therapy for locally advanced or metastatic disease. The major efficacy outcome measure was overall survival and the supportive efficacy outcome measures were progression-free survival and objective response rate. Patients were also required to have ECOG PS 0 or 1. Patients were randomized to receive either Ramucirumab at 10 mg/kg or placebo by intravenous infusion, in combination with docetaxel at 75 mg/m2 every 21 days. Sites in East Asia administered a reduced dose of docetaxel at 60 mg/m2 every 21 days. Patients who discontinued combination therapy because of an adverse event attributed to either Ramucirumab placebo or docetaxel were permitted to continue monotherapy with the other treatment component until disease progression or intolerable toxicity. Randomization was stratified by geographic region, gender, prior maintenance therapy, and ECOG PS.
Demographics and baseline characteristics were similar between treatment arms. Median age was 62 years; 67% of patients were men; 82% were White and 13% were Asian; 32% had ECOG PS 0; 73% had nonsquamous histology and 26% had squamous histology. In addition to platinum chemotherapy (99%), the most common prior therapies were pemetrexed (38%), gemcitabine (25%), taxane (24%), and bevacizumab (14%). Twenty-two percent of patients received prior maintenance therapy. Tumor EGFR status was unknown for the majority of patients (65%). Where tumor EGFR status was known (n=445), 7.5% were positive for EGFR mutation (n=33). No data were collected regarding tumor ALK rearrangement status.
Overall survival and progression-free survival were statistically significantly improved in patients randomized to receive Ramucirumab plus docetaxel compared to patients randomized to receive placebo plus docetaxel. Objective response rate (complete response + partial response) was 23% (95% CI: 20, 26) for Ramucirumab plus docetaxel and 14% (95% CI: 11, 17) for placebo plus docetaxel, p-value of <0.001. Efficacy results are shown in TABLE 7 and FIGURE 3.
How Supplied
Ramucirumab is supplied in single-dose vials as a sterile, preservative-free solution.
- NDC 0002-7669-01: 100 mg/10 mL (10 mg/mL), individually packaged in a carton
- NDC 0002-7678-01: 500 mg/50 mL (10 mg/mL), individually packaged in a carton
Storage
- Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) until time of use. Keep the vial in the outer carton in order to protect from light. Do not freeze or shake the vial.
Images
Drug Images
{{#ask: Page Name::Ramucirumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Ramucirumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients:
- That CYRAMZA can cause severe bleeding. Advise patients to contact their health care provider for bleeding or symptoms of bleeding including lightheadedness.
- Of increased risk of an arterial thromboembolic event.
- To undergo routine blood pressure monitoring and to contact their health care provider if blood pressure is elevated or if symptoms from hypertension occur including severe headache, lightheadedness, or neurologic symptoms.
- To notify their health care provider for severe diarrhea, vomiting, or severe abdominal pain.
- That CYRAMZA has the potential to impair wound healing. Instruct patients not to undergo surgery without first discussing this potential risk with their health care provider.
- Of the potential risk for maintaining pregnancy, risk to the fetus, or risk to postnatal development during and following treatment with CYRAMZA and the need to avoid getting pregnant, including use of adequate contraception, for at least 3 months following the last dose of CYRAMZA.
- To discontinue nursing during CYRAMZA treatment.
Precautions with Alcohol
Alcohol-Ramucirumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cyramza®[1]
Look-Alike Drug Names
There is limited information regarding Ramucirumab Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.