Pulmonary atresia pathophysiology
|
Pulmonary atresia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Pulmonary atresia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Pulmonary atresia pathophysiology |
|
Risk calculators and risk factors for Pulmonary atresia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Muhammad Waqas, M.D.[2]
Overview
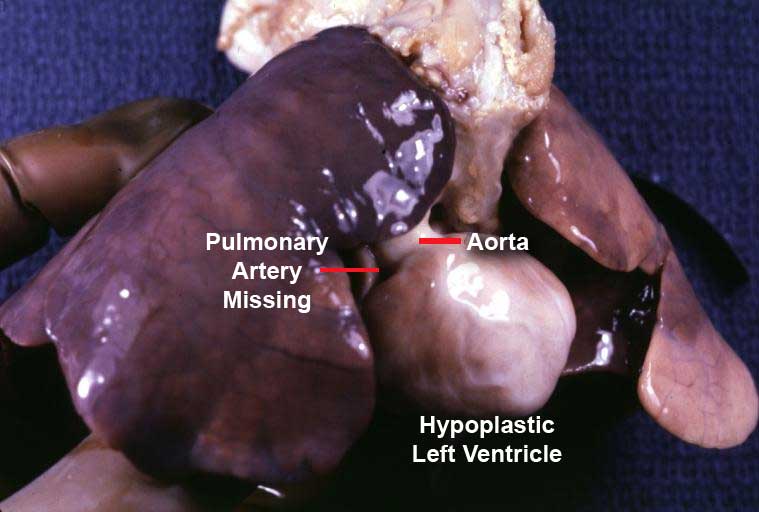
In a normal heart, the opening in the pulmonary valve has three flaps to open and close. In a patient with pulmonary atresia, the flap-like openings are covered by a layer of tissue. This significantly impacts the ability for the heart to shunt blood to the lungs for oxygenation.
Pathophysiology
The pulmonary valve is located on the right side of the heart between the right ventricle and pulmonary artery. [1] In a normal functioning heart, the opening to the pulmonary valve has three flaps that open and close like one way doors. As these flaps open and close they force blood to flow forward into the pulmonary artery and backward into the right ventricle then forward again to the lungs where the blood becomes oxygenated. With the disease pulmonary atresia, the flap-like openings are completely covered by a layer of tissue, thus preventing the ability of blood flow to the lungs to become oxygenated. The body requires oxygenated blood for survival.[2] [1] Pulmonary atresia is not threatening to a developing fetus however, because the mother's placenta provides the needed oxygen since the baby's lungs are not yet functional. Once the baby is born its lungs must now provide the oxygen needed for survival, but with Pulmonary atresia there is no opening on the pulmonary valve for blood to get to the lungs and become oxygenated. Due to this, the newborn baby is blue in color and pulmonary atresia can usually be diagnosed within hours or minutes after birth. [1] .[3][4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18]
References
- ↑ 1.0 1.1 1.2 Schmoldt A, Benthe HF, Haberland G, Raffle A, Gray J, MacDonald HR, Ehrhart IC, Parker PE, Weidner WJ, Dabney JM, Scott JB, Haddy FJ, Gatzy JT (September 1975). "Digitoxin metabolism by rat liver microsomes". Biochem. Pharmacol. 24 (17): 1639–41. doi:10.1136/bmj.1.6001.93-a. PMC 5922622. PMID 10.
- ↑ Pierpont ME, Basson CT, Benson DW, Gelb BD, Giglia TM, Goldmuntz E; et al. (2007). "Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics". Circulation. 115 (23): 3015–38. doi:10.1161/CIRCULATIONAHA.106.183056. PMID 17519398.
- ↑ Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F; et al. (2003). "Acquired von Willebrand syndrome in aortic stenosis". N Engl J Med. 349 (4): 343–9. doi:10.1056/NEJMoa022831. PMID 12878741.
- ↑ Tamura T, Horiuchi H, Imai M, Tada T, Shiomi H, Kuroda M; et al. (2015). "Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index". J Atheroscler Thromb. 22 (11): 1115–23. doi:10.5551/jat.30809. PMID 26269004.
- ↑ Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Circulation. 118 (15): e523–661. doi:10.1161/CIRCULATIONAHA.108.190748. PMID 18820172.
- ↑ Carabello BA (2002). "Clinical practice. Aortic stenosis". N Engl J Med. 346 (9): 677–82. doi:10.1056/NEJMcp010846. PMID 11870246.
- ↑ "15 Years in TAVI".
- ↑ "Harold on History: The Evolution of Transcatheter Aortic Valve Replacement - American College of Cardiology".
- ↑ Vaslef SN, Roberts WC (1993). "Early descriptions of aortic valve stenosis". Am Heart J. 125 (5 Pt 1): 1465–74. doi:10.1016/0002-8703(93)91036-e. PMID 8480616.
- ↑ Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkilä J, Tilvis R (1994). "Factors associated with calcific aortic valve degeneration in the elderly". Eur Heart J. 15 (7): 865–70. PMID 7925504.
- ↑ Aronow WS, Schwartz KS, Koenigsberg M (1987). "Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients". Am J Cardiol. 59 (9): 998–9. PMID 3565291.
- ↑ Pawade TA, Newby DE, Dweck MR (2015). "Calcification in Aortic Stenosis: The Skeleton Key". J Am Coll Cardiol. 66 (5): 561–77. doi:10.1016/j.jacc.2015.05.066. PMID 26227196.
- ↑ Lugiano, CA. (2013). "Aortic stenosis". JAAPA. 26 (11): 46–7. doi:10.1097/01.JAA.0000436518.69169.8e. PMID 24153092. Unknown parameter
|month=ignored (help) - ↑ Mylonakis E, Calderwood SB (2001). "Infective endocarditis in adults". N Engl J Med. 345 (18): 1318–30. doi:10.1056/NEJMra010082. PMID 11794152.
- ↑ Siu SC, Silversides CK (2010). "Bicuspid aortic valve disease". J Am Coll Cardiol. 55 (25): 2789–800. doi:10.1016/j.jacc.2009.12.068. PMID 20579534.
- ↑ Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS; et al. (2011). "Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study". J Am Coll Cardiol. 58 (3): 291–7. doi:10.1016/j.jacc.2010.11.073. PMC 3147295. PMID 21737022.
- ↑ Gotoh T, Kuroda T, Yamasawa M, Nishinaga M, Mitsuhashi T, Seino Y; et al. (1995). "Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study)". Am J Cardiol. 76 (12): 928–32. PMID 7484833.
- ↑ Hull MC, Morris CG, Pepine CJ, Mendenhall NP (2003). "Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy". JAMA. 290 (21): 2831–7. doi:10.1001/jama.290.21.2831. PMID 14657067.