Pegaspargase
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pegaspargase is an asparginase that is FDA approved for the treatment of acute lymphoblastic leukemia (ALL) and acute lymphoblastic leukemia and hypersensitivity to asparaginase. Common adverse reactions include allergic reactions (including anaphylaxis), central nervous system thrombosis, coagulopathy, elevated transaminases, hyperbilirubinemia, hyperglycemia, and pancreatitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose
- The recommended dose of Pegaspargase is 2,500 International Units/m2 intramuscularly or intravenously. Pegaspargase should be administered no more frequently than every 14 days.
Instructions for Administration
- When Pegaspargase is administered intramuscularly, the volume at a single injection site should be limited to 2 mL. If the volume to be administered is greater than 2 mL, multiple injection sites should be used. Pegaspargase does not contain a preservative. Use only one dose per vial; discard unused product.
- When administered intravenously, Pegaspargase should be given over a period of 1 to 2 hours in 100 mL of sodium chloride or dextrose injection 5%, through an infusion that is already running. After the solution is diluted for intravenous use, the solution should be used immediately. If immediate use is not possible, the diluted solution should be stored refrigerated at 2°C to 8°C (36°F to 46°F). Storage after dilution should not exceed 48 hours from the time of preparation to completion of administration. Protect infusion bags from direct sunlight.
Preparation and Handling Precautions
Do not administer Pegaspargase if drug has been:
- frozen
- stored at room temperature 15° to 25°C (59° to 77°F) for more than 48 hours
- shaken or vigorously agitated
Parenteral drug products should be inspected visually for particulate matter, cloudiness, or discoloration prior to administration, whenever solution and container permit. If any of these are present, discard the vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pegaspargase in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pegaspargase in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pegaspargase FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pegaspargase in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pegaspargase in pediatric patients.
Contraindications
- History of serious allergic reactions to Pegaspargase.
- History of serious thrombosis with prior L-asparaginase therapy.
- History of pancreatitis with prior L-asparaginase therapy.
- History of serious hemorrhagic events with prior L-asparaginase therapy.
Warnings
Anaphylaxis and Serious Allergic Reactions
- Anaphylaxis and serious allergic reactions can occur in patients receiving Pegaspargase. The risk of serious allergic reactions is higher in patients with known hypersensitivity to other forms of L-asparaginase. Observe patients for 1 hour after administration of Pegaspargase in a setting with resuscitation equipment and other agents necessary to treat anaphylaxis (for example, epinephrine, oxygen, intravenous steroids, antihistamines). Discontinue Pegaspargase in patients with serious allergic reactions.
Thrombosis
- Serious thrombotic events, including sagittal sinus thrombosis can occur in patients receiving Pegaspargase. Discontinue Pegaspargase in patients with serious thrombotic events.
Pancreatitis
- Pancreatitis can occur in patients receiving Pegaspargase. Evaluate patients with abdominal pain for evidence of pancreatitis. Discontinue Pegaspargase in patients with pancreatitis.
Glucose Intolerance
- Glucose intolerance can occur in patients receiving Pegaspargase. In some cases, glucose intolerance is irreversible. Monitor serum glucose.
Coagulopathy
- Increased prothrombin time, increased partial thromboplastin time, and hypofibrinogenemia can occur in patients receiving Pegaspargase Monitor coagulation parameters at baseline and periodically during and after treatment. Initiate treatment with fresh-frozen plasma to replace coagulation factors in patients with severe or symptomatic coagulopathy.
Hepatotoxicity and Abnormal Liver Function
- Hepatotoxicity and abnormal liver function, including elevations of AST (SGOT), ALT (SGPT), alkaline phosphatase, bilirubin (direct and indirect), and depression of serum albumin, and plasma fibrinogen can occur. Perform appropriate monitoring.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are described in greater detail in other sections of the label:
- Anaphylaxis and serious allergic reactions.
- Serious thrombosis.
- Pancreatitis.
- Glucose intolerance.
- Coagulopathy.
- Hepatotoxicity and abnormal liver function.
The most common adverse reactions with Pegaspargase are allergic reactions (including anaphylaxis), hyperglycemia, pancreatitis, central nervous system (CNS) thrombosis, coagulopathy, hyperbilirubinemia, and elevated transaminases. Hyperlipidemia (hypercholesterolemia and hypertriglyceridemia) has been reported in patients exposed to Pegaspargase.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
First-Line ALL
- The data presented below are derived from 2 studies in patients with standard-risk ALL who received Pegaspargase as a component of first-line multi-agent chemotherapy. Study 1 was a randomized (1:1), active-controlled study that enrolled 118 patients, with a median age of 4.7 years (1.1-9.9 years), of whom 54% were males and 65% White, 14% Hispanic, 8% Black, 8% Asian, and 6% other. Of the 59 patients in Study 1 who were randomized to Pegaspargase 48 patients (81%) received all 3 planned doses of Pegaspargase 6 (10%) received 2 doses, 4 (7%) received 1 dose, and 1 patient (2%) did not receive the assigned treatment. Study 2 is an ongoing, multi-factorial design study in which all patients received Pegaspargase as a component of various multi-agent chemotherapy regimens; interim safety data are available for 2,770 patients. Study participants had a median age of 4 years (1-10 years), and were 55% male, 68% White, 18% Hispanic, 4% Black, 3% Asian, and 7% other. Per protocol, the schedule of Pegaspargase varied by treatment arm, with intermittent doses of Pegaspargase for up to 10 months.
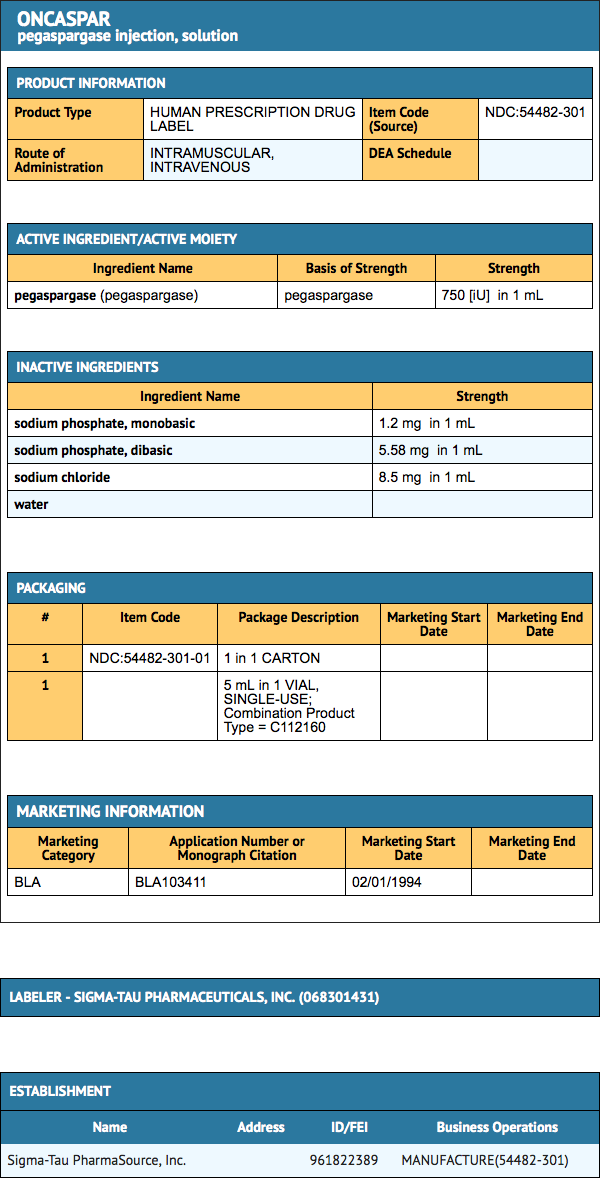
- In Study 1, detailed safety information was collected for pre-specified adverse reactions identified as asparaginase-induced adverse reactions and for grade 3 and 4 non-hematologic adverse reactions according to the Children’s Cancer Group (CCG) Toxicity and Complication Criteria. The per-patient incidence, by treatment arm, for these selected adverse reactions occurring at a severity of grade 3 or 4 are presented in TABLE 1 below:
- Safety data were collected in Study 2 only for National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2.0, grade 3 and 4 non-hematologic toxicities. In this study, the per-patient incidence for the following adverse reactions occurring during treatment courses in which patients received Pegaspargase were: elevated transaminases, 11%; coagulopathy, 7%; hyperglycemia, 5%; CNS thrombosis/hemorrhage, 2%; pancreatitis, 2%; clinical allergic reaction, 1%; and hyperbilirubinemia, 1%. There were 3 deaths due to pancreatitis.
Previously Treated ALL
- Adverse reaction information was obtained from 5 clinical trials that enrolled a total of 174 patients with relapsed ALL who received Pegaspargase as a single agent or in combination with multi-agent chemotherapy. The toxicity profile of Pegaspargase in patients with previously treated relapsed ALL is similar to that reported above with the exception of clinical allergic reactions (see TABLE 2). The most common adverse reactions of Pegaspargase were clinical allergic reactions, elevated transaminases, hyperbilirubinemia, and coagulopathies. The most common serious adverse events due to Pegaspargase treatment were thrombosis (4%), hyperglycemia requiring insulin therapy (3%), and pancreatitis (1%).
Allergic Reactions
Allergic reactions include the following: bronchospasm, hypotension, laryngeal edema, local erythema or swelling, systemic rash, and urticaria.
First-Line ALL
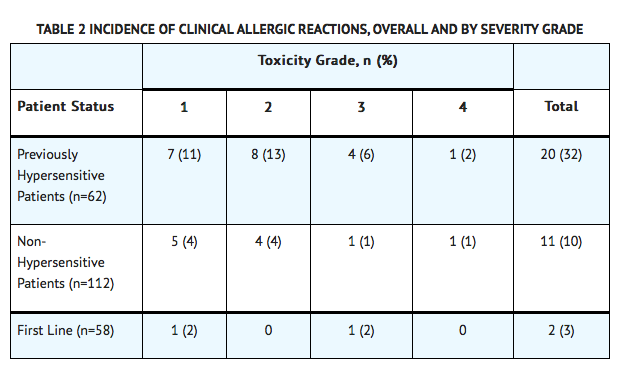
- Among 58 Pegaspargase-treated patients enrolled in Study 1, clinical allergic reactions were reported in 2 patients (3%). One patient experienced a grade 1 allergic reaction and the other grade 3 hives; both occurred during the first delayed intensification phase of the study (see TABLE 2).
Previously Treated ALL
- Among 62 patients with relapsed ALL and prior hypersensitivity reactions to asparaginase, 35 patients (56%) had a history of clinical allergic reactions to native Escherichia (E.) coli L-asparaginase, and 27 patients (44%) had history of clinical allergic reactions to both native E. coli and native Erwinia L-asparaginase. Twenty (32%) of these 62 patients experienced clinical allergic reactions to Pegaspargase (see TABLE 2).
- Among 112 patients with relapsed ALL with no prior hypersensitivity reactions to asparaginase, 11 patients (10%) experienced clinical allergic reactions to Pegaspargase (see TABLE 2).
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity, defined as development of binding and/or neutralizing antibodies to the product.
- In Study 1, Pegaspargase-treated patients were assessed for evidence of binding antibodies using an enzyme-linked immunosorbent assay (ELISA) method. The incidence of protocol-specified “high-titer” antibody formation was 2% in Induction (n=48), 10% in Delayed Intensification 1 (n=50), and 11% in Delayed Intensification 2 (n=44). There is insufficient information to determine whether the development of antibodies is associated with an increased risk of clinical allergic reactions, altered pharmacokinetics, or loss of anti-leukemic efficacy.
- The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay, and the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, concomitant medications, and underlying disease. Therefore, comparison of the incidence of antibodies to Pegaspargase with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Pegaspargase Postmarketing Experience in the drug label.
Drug Interactions
- No formal drug interaction studies, between Pegaspargase and other drugs, have been performed.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with Pegaspargase It is also not known whether Pegaspargase can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pegaspargase should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pegaspargase in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pegaspargase during labor and delivery.
Nursing Mothers
- It is not known whether Pegaspargase is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Pegaspargase, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Pegaspargase in pediatric settings.
Geriatic Use
- Clinical studies of Pegaspargase did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects.
Gender
There is no FDA guidance on the use of Pegaspargase with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pegaspargase with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pegaspargase in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pegaspargase in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pegaspargase in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pegaspargase in patients who are immunocompromised.
Others
Administration and Monitoring
Administration
There is limited information regarding Pegaspargase Administration in the drug label.
Monitoring
There is limited information regarding Pegaspargase Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pegaspargase and IV administrations.
Overdosage
- Three patients received 10,000 International Units/m2 of Pegaspargase as an intravenous infusion. One patient experienced a slight increase in liver enzymes. A second patient developed a rash 10 minutes after the start of the infusion, which was controlled with the administration of an antihistamine and by slowing down the infusion rate. A third patient did not experience any adverse reactions.
Pharmacology
Pegaspargase
| |
| Systematic (IUPAC) name | |
| Pegylated E. coli L-asparagine amidohydrolase | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 31731.9 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- The mechanism of action of Pegaspargase is thought to be based on selective killing of leukemic cells due to depletion of plasma asparagine. Some leukemic cells are unable to synthesize asparagine due to a lack of asparagine synthetase and are dependent on an exogenous source of asparagine for survival. Depletion of asparagine, which results from treatment with the enzyme L-asparaginase, kills the leukemic cells. Normal cells, however, are less affected by the depletion due to their ability to synthesize asparagine.
Structure
- Pegaspargase (pegaspargase) is L-asparaginase (L-asparagine amidohydrolase) that is covalently conjugated to monomethoxypolyethylene glycol (mPEG). L-asparaginase is a tetrameric enzyme that is produced endogenously by E. coli and consists of identical 34.5 kDa subunits. Approximately 69 to 82 molecules of mPEG are linked to L-asparaginase; the molecular weight of each mPEG molecule is about 5 kDa. Pegaspargase activity is expressed in International Units. One International Unit of L-asparaginase is defined as the amount of enzyme required to generate 1 micromole of ammonia per minute at pH 7.3 and 37°C.
Pharmacodynamics
- In Study 1, pharmacodynamics were assessed in 57 newly diagnosed pediatric patients with standard-risk ALL who received three intramuscular doses of Pegaspargase (2,500 International Units/m2), one each during induction and two delayed intensification treatment phases. Pharmacodynamic activity was assessed through serial measurements of asparagine in sera (n=57) and cerebrospinal fluid (CSF) (n=50). The data for asparagine depletion are presented in clinical studies.
Pharmacokinetics
- Pharmacokinetic assessments were based on an enzymatic assay measuring asparaginase activity. Serum pharmacokinetics were assessed in 34 newly diagnosed pediatric patients with standard-risk ALL in Study 1 following intramuscular administration of 2,500 International Units/m2. The elimination half-life of Pegaspargase was approximately 5.8 days during the induction phase. Similar elimination half-lives were observed during Delayed Intensification 1 and Delayed Intensification 2. Concentrations greater than 0.1 International Units/mL were observed in over 90% of the samples from patients treated with Pegaspargase during induction, Delayed Intensification 1, and Delayed Intensification 2 for approximately 20 days.
- In 3 pharmacokinetic studies, 37 patients with relapsed ALL received Pegaspargase at 2,500 International Units/m2 intramuscularly every 2 weeks. The plasma half-life of Pegaspargase was 3.2 ± 1.8 days in 9 patients who were previously hypersensitive to native E. coli L-asparaginase and 5.7 ± 3.2 days in 28 non-hypersensitive patients. The area under the plasma concentration-time curve (AUC) was 9.5 ± 4.0 International Units/mL/day in the previously hypersensitive patients and 9.8 ± 6.0 International Units/mL/day in the non-hypersensitive patients.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term carcinogenicity studies in animals have been performed with Pegaspargase.
- No relevant studies addressing mutagenic potential have been conducted. Pegaspargase did not exhibit a mutagenic effect when tested against Salmonella typhimurium strains in the Ames assay.
- No studies have been performed on impairment of fertility.
Clinical Studies
There is limited information regarding Pegaspargase Clinical Studies in the drug label.
How Supplied
- Pegaspargase (pegaspargase) is supplied as a sterile solution in Type I single-use vials containing 3,750 International Units of L-asparaginase per 5 mL solution (NDC 54482-301-01).
Storage
- Store Pegaspargase under refrigeration at 2°C to 8ºC (36°F to 46°F). Do not shake or freeze product. Protect from light. Do not use Pegaspargase after the expiration date on the vial.
Images
Drug Images
{{#ask: Page Name::Pegaspargase |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pegaspargase |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pegaspargase Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pegaspargase interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Pegaspargase Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.