Nitric oxide synthase
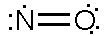
The heme moiety of the enzyme is colored.
Overview
The nitric oxide synthase (NOS; EC 1.14.13.39) is an enzyme in the body that contributes to transmission from one neuron to another, to the immune system and to dilating blood vessels. It does so by synthesis of nitric oxide (NO) from the terminal nitrogen atom of L-arginine in the presence of NADPH and dioxygen (O2). NOS is the only known enzyme that binds flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme, tetrahydrobiopterin (BH4) and calmodulin. NOS activates Cyclic GMP, which induces smooth muscle relaxation by multiple mechanisms including: 1. increased intracellular cGMP, which inhibits calcium entry into the cell, and decreases intracellular calcium concentrations 2. activates K+ channels, which leads to hyperpolarization and relaxation 3. stimulates a cGMP-dependent protein kinase that activates myosin light chain phosphatase, the enzyme that dephosphorylates myosin light chains, which leads to smooth muscle relaxation.
Classification
NOS was first identified by Furchgott (1980) who experimented on the aortas of rabbits.[1] Since then, the different forms of NO synthase have been classified as follows:
| Name | Gene(s) | Location | Function |
| Neuronal NOS (nNOS or NOS1) | NOS1 |
| |
| Inducible NOS (iNOS or NOS2) | NOS2A, NOS2B, NOS2C |
| |
| Endothelial NOS (eNOS or NOS3 or cNOS) | NOS3 |
nNOS
Neuronal NOS (nNOS) produces NO in nervous tissue in both the central and peripheral nervous system. Neuronal NOS also performs a role in cell communication and is associated with plasma membranes. nNOS action can be inhibited by NPA (N-propyl-L-arginine).
iNOS
Inducible NOS (iNOS) can be found in the immune system but is also found in the cardiovascular system. It uses the oxidative stress of NO (a free radical) to be used by macrophages in immune defence against pathogens.
eNOS
Endothelial NOS (eNOS), also known as nitric oxide synthase 3 (NOS3) or constitutive NOS (cNOS), generates NO in blood vessels and is involved with regulating vascular function. A constitutive Ca2+ dependent NOS provides a basal release of NO. eNOS is associated with plasma membranes surrounding cells and the membranes of Golgi bodies within cells.
Chemical reaction
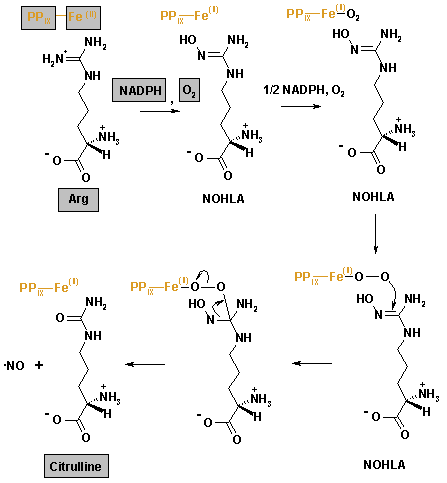
Nitric oxide synthase produces NO by catalysing a five-electron oxidation of a guanidino nitrogen of L-arginine (L-Arg). Oxidation of L-Arg to L-citrulline occurs via two successive monooxygenation reactions producing Nω-hydroxy-L-arginine (NOHLA) as an intermediate. 2 mol of O2 and 1.5 mol of NADPH are consumed per mole of NO formed.
- L-Arg + NADPH + H+ + O2 → NOHLA + NADP+ + H2O
- NOHLA + ½ NADPH + ½ H+ + O2 → L-citrulline + ½ NADP+ + NO + H2O
Structure
All three isoforms (each of which is presumed to function as a homodimer during activation) share a carboxyl-terminal reductase domain homologous to the cytochrome P450 reductase. They also share an amino-terminal oxygenase domain containing a heme prosthetic group, which is linked in the middle of the protein to a calmodulin-binding domain. Binding of calmodulin appears to act as a "molecular switch" to enable electron flow from flavin prosthetic groups in the reductase domain to heme. This facilitates the conversion of O2 and L-arginine to NO and L-citrulline. The oxygenase domain of each NOS isoform also contains an BH4 prosthetic group, which is required for the efficient generation of NO. Unlike other enzymes where BH4 is used as a source of reducing equivalents and is recycled by dihydrobiopterin reductase (EC 1.5.1.33), BH4 activates heme-bound O2 by donating a single electron, which is then recaptured to enable nitric oxide release.
The first nitric oxide synthase to be identified was found in neuronal tissue (NOS1 or nNOS); the endothelial NOS (eNOS or NOS3) was the third to be identified. They were originally classified as "constituitvely expressed" and "Ca2+ sensitive" but it is now known that they are present in many different cell types and that expression is regulated under specific physiological conditions.
In NOS1 and NOS3, physiological concentrations of Ca2+ in cells regulate the binding of calmodulin to the "latch domains", thereby initiating electron transfer from the flavins to the heme moieties. In contrast, calmodulin remains tightly bound to the inducible and Ca2+-insensitive isoform (iNOS or NOS2) even at a low intracellular Ca2+ activity, acting essentially as a subunit of this isoform.
Nitric oxide may itself regulate NOS expression and activity. Specifically, NO has been shown to play an important negative feedback regulatory role on NOS3, and therefore vascular endothelial cell function. This process, known formally as S-nitrosation (and referred to by many in the field as S-nitrosylation), has been shown to reversibly inhibit NOS3 activity in vascular endothelial cells. This process may be important because it is regulated by cellular redox conditions and may thereby provide a mechanism for the association between "oxidative stress" and endothelial dysfunction. In addition to NOS3, both NOS1 and NOS2 have been found to be S-nitrosated, but the evidence for dynamic regulation of those NOS isoforms by this process is less complete. In addition, both NOS1 and NOS2 have been shown to form ferrous-nitrosyl complexes in their heme prosthetic groups that may act partially to self-inactivate these enzymes under certain conditions. The rate-limiting step for the production of nitric oxide may well be the availability of L-arginine in some cell types. This may be particularly important after the induction of NOS2.
References
External links
- InterPro: IPR004030 - InterPro entry for NOS
- PDB: 1DWX - X-ray structure of murine iNOS oxygenase domain in complex with NOHLA and BH4
- PDB: 1F20 - X-ray structure of rat nNOS reductase FAD/NADP+ domain
- The Nobel Prize in Physiology or Medicine 1998
- University of Edinburgh, School of Chemistry - NO Synthase
- Nitric+oxide+synthase at the US National Library of Medicine Medical Subject Headings (MeSH)