Lipoid pneumonia pathophysiology
|
Lipoid pneumonia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lipoid pneumonia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Lipoid pneumonia pathophysiology |
|
Risk calculators and risk factors for Lipoid pneumonia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ramyar Ghandriz MD[2]
Overview
Lipoid pneumonia pathogenesis is different in its two subtypes: Exogenous form is the result of chronic body reaction to fatty substance in the alveoli. Lipid reaches alveoli by aspiration or inhalation. Some mineral oils can cause lung injuries such as gasoline. Mineral oils can enter the tracheobronchial tree without causing cough reflex which will bother mucociliary transport system chronically. Injected lipids mechanism of further producing lipid pneumonia is more complicated. As the lipid goes inside the alveoli, it is trapped and hard to expectorate, this condition may be worsen by associated neurological and gastrointestinal disorders affecting swallowing or cough. Lipids in alveoli form emulsion and then consumed by macrophages via phagocytosis. Since the alveolar macrophages cannot metabolize consumed fatty substance, oil is repeatedly released into alveoli after death of these macrophages. The oil released, elicits a giant-cell granulomatosis reaction. Pathogenesis of endogenous form is still not well understood however there are plenty of suggested mechanisms, one saying that endogenous lipoid pneumonia can be caused by transbronchial dissemination of cancer cell breakdown products. Poorly differentiated adenocarcinoma cells secreting mucin is the most common neoplastic reason. Another mechanism suggested is anoxic tissue injury stimulating various enzymes such as phospholipase and monooxygenases. Infection changes to endogenous lipid pneumonia is generally localized in airways because the surrounding lung is already consolidated, limiting the spread of bacteria. On gross and microscopic histology will show well circumscribed, firm with prominent lymphatics on lung surface, inflammatory cells, and young fibroblasts. Reactive endarteritis and marked alveolar lining cell hyperplasia may also be seen. Lipid-laden foamy macrophages are part of pathology.
Pathophysiology
Exogenous lipoid pneumonia
The important pathophysiology aspects regarding exogenous lipoid pneumonia include:[1][2]
- It is understood that exogenous lipoid pneumonia is the result of chronic body reaction to fatty substance in the alveoli.
- Lipid reaches alveoli by aspiration or inhalation.
- Some mineral oils can cause lung injuries such as gasoline.
- Mineral oils can enter the tracheobronchial tree without causing cough reflex which will bother mucociliary transport system chronically.
- Injected lipids mechanism of further producing lipid pneumonia is more complicated:
- It is suggested that the lung is the first capillary bed encountered during circulation, bearing the majority of damage.
- As the lipid goes inside the alveoli, it is trapped and hard to expectorate, this condition may be worsen by associated neurological and gastrointestinal disorders affecting swallowing or cough.
- Lipids in alveoli form emulsion and then consumed by macrophages via phagocytosis.
- Since the alveolar macrophages cannot metabolize consumed fatty substance, oil is repeatedly released into alveoli after death of these macrophages.
- The oil released, elicits a giant-cell granulomatosis reaction:
- In fresh lesions, lipid-laden macrophages are seen.
- In advanced lesions larger vacuoles and inflammatory infiltrates are seen in alveolar and bronchial walls and septa.
- In oldest lesions fibrosis and parenchymal destruction around large lipid-containing vacuoles is seen.
- Staining can help demonstrating whether vacuoles are filled with lipid or not:
Endogenous lipoid pneumonia
The pathogenesis of endogenous lipoid pneumonia is still not well understood however there are plenty of suggested mechanisms:[3][4][5]
- Most proven mechanisms are:
- Retained epithelial secretion.
- Cell breakdown.
- Leakage from vessels.
- Prolonged hypoxia.
- Local oxygen and carbon dioxide tension.
- Endogenous lipoid pneumonia can be caused by transbronchial dissemination of cancer cell breakdown products.[6]
- Poorly differentiated adenocarcinoma cells secreting mucin is the most common neoplastic reason.
- Another mechanism suggested is anoxic tissue injury stimulating various enzymes such as phospholipase and mono-oxygenases.[7][8][9]
- These enzymes activation in turn cause modification of LDL cholesterol, enhancing lipid uptake by alveolar macrophages similar to atherogenesis.
- Infection changes to endogenous lipid pneumonia is generally localized in airways because the surrounding lung is already consolidated, limiting the spread of bacteria.[10]
Genetics
There is no genetic predisposition reported with lipoid pneumonia.
Associated Conditions
- Vaping is associated with exogenous lipoid pneumonia.[7][4]
- Fire eating is associated with exogenous lipoid pneumonia.
- Using oily laxative is associated with exogenous lipoid pneumonia.
- Pulmonary adenocarcinoma is associated with endogenous lipid pneumonia.
- Long lasting pulmonary granulomatosis diseases is associated with endogenous lipid pneumonia.
Gross Pathology
- Well circumscribed, firm with prominent lymphatics on lung surface in exogenous type.[11]
- Parenchymal consolidation in endogenous lipoid pneumonia produce a characteristic yellowish discoloration, hence called "golden pneumonia".[12]

Microscopic Pathology
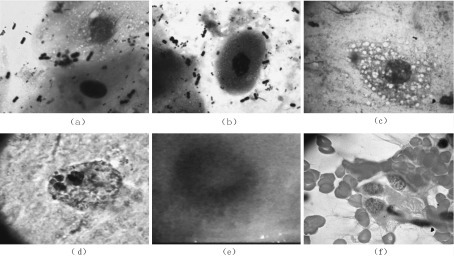
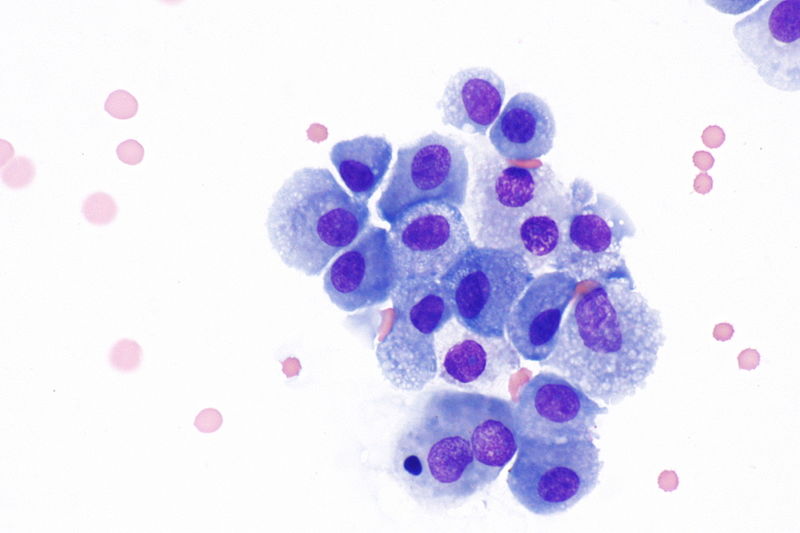
- Lipoid material (or empty spaces), inflammatory cells and young fibroblasts.[14]
- Reactive endarteritis, marked alveolar lining cell hyperplasia.
- Lipid-laden foamy macrophages
|
{{#ev:youtube|https://www.youtube.com/watch?v=nGi0udE5EI8%7C500}} |
References
- ↑ Guerguerian, Anne-Marie; Lacroix, Jacques (2000). "Pulmonary injury after intravenous hydrocarbon injection". Paediatrics & Child Health. 5 (8): 471–472. doi:10.1093/pch/5.8.471. ISSN 1205-7088.
- ↑ Domej, Wolfgang; Mitterhammer, Heike; Stauber, Rudolf; Kaufmann, Peter; Smolle, Karl Heinz (2007). "Successful outcome after intravenous gasoline injection". Journal of Medical Toxicology. 3 (4): 173–177. doi:10.1007/BF03160935. ISSN 1556-9039.
- ↑ Burke, M; Fraser, R (1988). "Obstructive pneumonitis: a pathologic and pathogenetic reappraisal". Radiology. 166 (3): 699–704. doi:10.1148/radiology.166.3.3340764. ISSN 0033-8419.
- ↑ 4.0 4.1 "www.thoracic.org" (PDF).
- ↑ Cohen, Allen B.; Cline, Martin J. (1972). "In VitroStudies of the Foamy Macrophage of Postobstructive Endogenous Lipoid Pneumonia in Man1–3". American Review of Respiratory Disease. 106 (1): 69–78. doi:10.1164/arrd.1972.106.1.69. ISSN 0003-0805.
- ↑ Tamura, A.; Hebisawa, A.; Fukushima, K.; Yotsumoto, H.; Mori, M. (1998). "Lipoid Pneumonia in Lung Cancer: Radiographic and Pathological Features". Japanese Journal of Clinical Oncology. 28 (8): 492–496. doi:10.1093/jjco/28.8.492. ISSN 0368-2811.
- ↑ 7.0 7.1 Taki, Takao; Nakazima, Tomoko; Emi, Yohko; Konishi, Yohichi; Hayashi, Akira; Matsumoto, Makoto (1986). "Accumulation of surfactant phospholipids in lipid pneumonia induced with methylnaphthalene". Lipids. 21 (9): 548–552. doi:10.1007/BF02534050. ISSN 0024-4201.
- ↑ Evans AJ, Sawyez CG, Wolfe BM, Connelly PW, Maguire GF, Huff MW (1993). "Evidence that cholesteryl ester and triglyceride accumulation in J774 macrophages induced by very low density lipoprotein subfractions occurs by different mechanisms". J Lipid Res. 34 (5): 703–17. PMID 8509711.
- ↑ Tölle, Angelika; Kolleck, Ingrid; Schlame, Michael; Wauer, Roland; Stevens, Paul A.; Rüstow, Bernd (1997). "Effect of hyperoxia on the composition of the alveolar surfactant and the turnover of surfactant phospholipids, cholesterol, plasmalogens and vitamin E". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1346 (2): 198–204. doi:10.1016/S0005-2760(97)00036-2. ISSN 0005-2760.
- ↑ Burke, M; Fraser, R (1988). "Obstructive pneumonitis: a pathologic and pathogenetic reappraisal". Radiology. 166 (3): 699–704. doi:10.1148/radiology.166.3.3340764. ISSN 0033-8419.
- ↑ "Pathology Outlines - Lipoid pneumonia".
- ↑ Gaerte, Scott C.; Meyer, Cristopher A.; Winer-Muram, Helen T.; Tarver, Robert D.; Conces, Dewey J. (2002). "Fat-containing Lesions of the Chest". RadioGraphics. 22 (suppl_1): S61–S78. doi:10.1148/radiographics.22.suppl_1.g02oc08s61. ISSN 0271-5333.
- ↑ "Pulmonary Pathology".
- ↑ "Pathology Outlines - Lipoid pneumonia".
- ↑ Han, Chenghong; Liu, Lihai; Du, Shiping; Mei, Jianhua; Huang, Ling; Chen, Min; Lei, Yongliang; Qian, Junwen; Luo, Jianyong; Zhang, Meibian (2016). "Investigation of rare chronic lipoid pneumonia associated with occupational exposure to paraffin aerosol". Journal of Occupational Health. 58 (5): 482–488. doi:10.1539/joh.16-0096-CS. ISSN 1341-9145.