Ketorolac tromethamine (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ketorolac tromethamine (ophthalmic) is a analgesic that is FDA approved for the treatment of ocular itching due to seasonal allergic conjunctivitis. Common adverse reactions include edema, hypertension, pruritus, rash, sweating, abdominal pain, constipation, diarrhea, flatulence, heartburn, indigestion, nausea, stomatitis, vomiting, anemia, purpura, dizziness, headache, somnolence, iritis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Ketorolac tromethamine ophthalmic solution, 0.5%, is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. Ketorolac tromethamine ophthalmic solution, 0.5% is also indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction.
Dosage
- The recommended dose of ketorolac tromethamine ophthalmic solution, 0.5%, is one drop (0.25 mg) four times a day for relief of ocular itching due to seasonal allergic conjunctivitis.
- For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ketorolac tromethamine ophthalmic solution, 0.5%, should be applied to the affected eye(s) four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period.
- Ketorolac tromethamine ophthalmic solution, 0.5% has been safely administered in conjunction with other ophthalmic medications such as antibiotics, beta blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ketorolac tromethamine (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ketorolac tromethamine (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Ketorolac tromethamine (ophthalmic) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ketorolac tromethamine (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ketorolac tromethamine (ophthalmic) in pediatric patients.
Contraindications
- Ketorolac tromethamine ophthalmic solution, 0.5% is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation.
Warnings
- There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other nonsteroidal anti-inflammatory agents. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
- With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
Adverse Reactions
Clinical Trials Experience
- The most frequent adverse events reported with the use of ketorolac tromethamine ophthalmic solutions have been transient stinging and burning on instillation. These events were reported by up to 40% of patients participating in clinical trials.
- Other adverse events occurring approximately 1 to 10% of the time during treatment with ketorolac tromethamine ophthalmic solutions included allergic reactions, corneal edema, iritis, ocular inflammation, ocular irritation, superficial keratitis, and superficial ocular infections.
- Other adverse events reported rarely with the use of ketorolac tromethamine ophthalmic solutions included: corneal infiltrates, corneal ulcer, eye dryness, headaches, and visual disturbance (blurry vision).
- Clinical Practice: The following events have been identified during postmarketing use of ketorolac tromethamine ophthalmic solution, 0.5% in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical ketorolac tromethamine ophthalmic solution, 0.5%, or a combination of these factors, include corneal erosion, corneal perforation, corneal thinning, and epithelial breakdown
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ketorolac tromethamine (ophthalmic) in the drug label.
Drug Interactions
There is limited information regarding Ketorolac tromethamine (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Teratogenic Effects: Pregnancy Category C.
- Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits or rats at oral doses up to 109 times and 303 times the maximum recommended human topical ophthalmic dose, respectively, on a mg/kg basis assuming 100% absorption in humans and animals. When administered to rats after Day 17 of gestation at oral doses up to 45 times the maximum recommended human topical ophthalmic dose, respectively, on a mg/kg basis, assuming 100% absorption in humans and animals, ketorolac tromethamine resulted in dystocia and increased pup mortality. There are no adequate and well-controlled studies in pregnant women. Ketorolac tromethamine ophthalmic solution, 0.5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Nonteratogenic Effects:
- Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of ketorolac tromethamine ophthalmic solution, 0.5% during late pregnancy should be avoided.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ketorolac tromethamine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ketorolac tromethamine (ophthalmic) during labor and delivery.
Nursing Mothers
- Caution should be exercised when ketorolac tromethamine ophthalmic solution, 0.5% is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) with respect to pediatric patients.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ketorolac tromethamine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ketorolac tromethamine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Ketorolac tromethamine (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ketorolac tromethamine (ophthalmic) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Ketorolac tromethamine (ophthalmic) in the drug label.
Pharmacology
Mechanism of Action
- Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis. Ketorolac tromethamine given systemically does not cause pupil constriction.Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed in animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increased vascular permeability, leukocytosis, and increased intraocular pressure. Prostaglandins also appear to play a role in the miotic response produced during ocular surgery by constricting the iris sphincter independently of cholinergic mechanisms
Structure
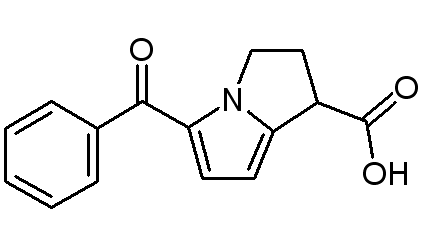
- Ketorolac tromethamine ophthalmic solution, 0.5% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) and has the following structure:
- Ketorolac tromethamine ophthalmic solution, 0.5% is supplied as a sterile isotonic aqueous 0.5% solution, with a pH of 7.4. Ketorolac tromethamine ophthalmic solution, 0.5% is a racemic mixture of R-( + )- and S-(-)- ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The molecular weight of ketorolac tromethamine is 376.41. The osmolality of ketorolac tromethamine ophthalmic solution, 0.5% is 290 mOsmol/kg.
- Each mL of ketorolac tromethamine ophthalmic solution, 0.5% contains: Active: ketorolac tromethamine 0.5%. Inactives: benzalkonium chloride 0.01%; edetate disodium 0.1%; octoxynol 40; purified water; sodium chloride; hydrochloric acid and/or sodium hydroxide to adjust pH.
Pharmacodynamics
- Two drops (0.1 mL) of 0.5% ketorolac tromethamine ophthalmic solution, instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved measurable levels in 8 of 9 patients' eyes (mean ketorolac concentration 95 ng/mL aqueous humor, range 40 to 170 ng/mL). Ocular administration of ketorolac tromethamine reduces prostaglandin E2 (PGE2) levels in aqueous humor. The mean concentration of PGE2 was 80 pg/mL in the aqueous humor of eyes receiving vehicle and 28 pg/mL in the eyes receiving ketorolac tromethamine 0.5% ophthalmic solution.
- One drop (0.05 mL) of 0.5% ketorolac tromethamine ophthalmic solution, was instilled into one eye and one drop of vehicle into the other eye TID in 26 normal subjects. Only 5 of 26 subjects had a detectable amount of ketorolac in their plasma (range 10.7 to 22.5 ng/mL) at Day 10 during topical ocular treatment. When ketorolac tromethamine 10 mg is administered systemically every 6 hours, peak plasma levels at steady state are around 960 ng/mL.
- Two controlled clinical studies showed that ketorolac tromethamine ophthalmic solution, was significantly more effective than its vehicle in relieving ocular itching caused by seasonal allergic conjunctivitis.
- Two controlled clinical studies showed that patients treated for two weeks with ketorolac tromethamine ophthalmic solution, were less likely to have measurable signs of inflammation (cell and flare) than patients treated with its vehicle.
- Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure; however, changes in intraocular pressure may occur following cataract surgery.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ketorolac tromethamine (ophthalmic) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ketorolac tromethamine (ophthalmic) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ketorolac tromethamine (ophthalmic) in the drug label.
How Supplied
- Ketorolac tromethamine ophthalmic solution, 0.5% is supplied sterile in a white LDPE plastic DROP-TAINER* bottle, a natural dropper tip and a gray polypropylene cap as follows:
- 5 mL in 8 mL bottle NDC 61314-126-05
- 10 mL in 10 mL bottle NDC 61314-126-10
- Storage: Store at room temperature 15°-30°C (59°-86°F) with protection from light.
- FOR TOPICAL OPHTHALMIC USE ONLY
- DROP-TAINER is a registered trademark of Alcon Research, Ltd.
Storage
There is limited information regarding Ketorolac tromethamine (ophthalmic) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ketorolac tromethamine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ketorolac tromethamine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Ketorolac tromethamine (ophthalmic) in the drug label.
Precautions with Alcohol
- Alcohol-Ketorolac tromethamine (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ketorolac tromethamine (ophthalmic) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ketorolac tromethamine (ophthalmic) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ketorolac tromethamine (ophthalmic) |Label Name=Ketorolac tromethamine (ophthalmic)06.png
}}
{{#subobject:
|Label Page=Ketorolac tromethamine (ophthalmic) |Label Name=Ketorolac tromethamine (ophthalmic)07.png
}}