Ivacaftor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ivacaftor is a cystic fibrosis transmembrane conductance regulator that is FDA approved for the treatment of cystic fibrosis. Common adverse reactions include headache, oropharyngeal pain, upper respiratory tract infection, nasal congestion, abdominal pain, nasopharyngitis, diarrhea, rash, nausea, and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cystic Fibrosis
- Dosage: One 150 mg tablet taken orally every 12 hours (300 mg total daily dose) with fat-containing food. Examples of appropriate fat-containing foods include eggs, butter, peanut butter, cheese pizza,
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivacaftor in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivacaftor in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Cystic Fibrosis
- Dosage: One 150 mg tablet taken orally every 12 hours (300 mg total daily dose) with fat-containing food. Examples of appropriate fat-containing foods include eggs, butter, peanut butter, cheese pizza,
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivacaftor in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivacaftor in pediatric patients.
Contraindications
None
Warnings
Transaminase (ALT or AST) Elevations
- Elevated transaminases have been reported in patients with CF receiving Ivacaftor. It is recommended that ALT and AST be assessed prior to initiating Ivacaftor every 3 months during the first year of treatment, and annually thereafter. Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Dosing should be interrupted in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming Ivacaftor dosing.
Concomitant Use with CYP3A Inducers
- Use of Ivacaftor with strong CYP3A inducers, such as rifampin, substantially decreases the exposure of ivacaftor, which may reduce the therapeutic effectiveness of Ivacaftor Therefore, co-administration of Ivacaftor with strong CYP3A inducers (e.g., rifampin, St. John's Wort) is not recommended.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The overall safety profile of Ivacaftor is based on pooled data from three placebo-controlled clinical trials conducted in 353 patients with CF who had a G551D mutation in the CFTR gene (Trials 1 and 2) or were homozygous for the F508del mutation (Trial 3). In addition, an 8-week crossover design trial (Trial 4) involving 39 patients with a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene was conducted. Patients treated with Ivacaftor in these trials were between the ages of 6 and 57 years.
- Of the 353 patients included in the pooled analyses of patients with CF who had either a G551D mutation or were homozygous for the F508del mutation in the CFTR gene, 50% of patients were female and 97% were Caucasian; 221 received Ivacaftor and 132 received placebo from 16 to 48 weeks. The proportion of patients who prematurely discontinued study drug due to adverse reactions was 2% for Ivacaftor-treated patients and 5% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in Ivacaftor-treated patients included abdominal pain, increased hepatic enzymes, and hypoglycemia.
- The most common adverse reactions in the 221 patients treated with Ivacaftor were headache (17%), upper respiratory tract infection (16%), nasal congestion (16%), nausea]] (10%), rash (10%), rhinitis (6%), dizziness (5%), arthralgia (5%), and bacteria in sputum (5%).
- The incidence of adverse reactions below is based upon two double-blind, placebo-controlled, 48-week clinical trials (Trials 1 and 2) in a total of 213 patients with CF ages 6 to 53 who have a G551D mutation in the CFTR gene and who were treated with Ivacaftor 150 mg orally or placebo twice daily. Table 1 shows adverse reactions occurring in ≥8% of Ivacaftor-treated patients with CF who have a G551D mutation in the CFTR gene that also occurred at a higher rate than in the placebo-treated patients in the two double-blind, placebo-controlled trials.
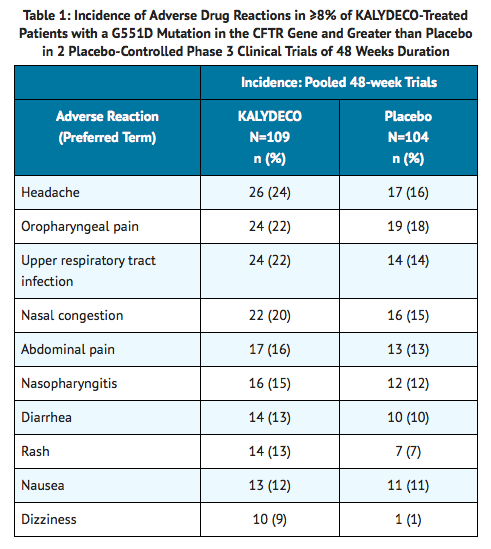
Adverse reactions in the 48-week clinical trials that occurred in the Ivacaftor group at a frequency of 4 to 7% where rates exceeded that in the placebo group include:
- Infections and infestations: rhinitis
- Investigations: aspartate aminotransferase increased, bacteria in sputum, blood glucose increased, hepatic enzyme increased
- Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal chest pain, myalgia
- Nervous system disorders: sinus headache
- Respiratory, thoracic and mediastinal disorders: pharyngeal erythema, pleuritic pain, sinus congestion, wheezing.
- Skin and subcutaneous tissue disorders: acne
- Laboratory abnormalities:
- Transaminase Elevations: During 48-week placebo-controlled clinical studies, the incidence of maximum transaminase (ALT or AST) >8, >5 or >3 × ULN was 2%, 3% and 6% in Ivacaftor-treated patients and 2%, 2% and 8% in placebo-treated patients, respectively. Two patients (2%) on placebo and 1 patient (0.5 %) on Ivacaftor permanently discontinued treatment for elevated transaminases, all >8 × ULN. Two patients treated with Ivacaftor were reported to have serious adverse reactions of elevated liver transaminases compared to none on placebo.
Postmarketing Experience
There is limited information regarding Ivacaftor Postmarketing Experience in the drug label.
Drug Interactions
Inhibitors of CYP3A
- Ivacaftor is a sensitive CYP3A substrate. Co-administration with ketoconazole, a strong CYP3A inhibitor, significantly increased ivacaftor exposure measured as area under the curve (AUC) by 8.5-fold. Based on simulations of these results, a reduction of the Ivacaftor dose to 150 mg twice a week is recommended for co-administration with strong CYP3A inhibitors, such as ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, and clarithromycin. Co-administration with fluconazole, a moderate inhibitor of CYP3A, increased ivacaftor exposure by 3-fold. Therefore, a reduction of the Ivacaftor dose to 150 mg once daily is recommended for patients taking concomitant moderate CYP3A inhibitors, such as fluconazole and erythromycin.
- Co-administration of Ivacaftor with grapefruit juice, which contains one or more components that moderately inhibit CYP3A, may increase exposure of ivacaftor. Therefore, food containing grapefruit or Seville oranges should be avoided during treatment with Ivacaftor.
Inducers of CYP3A
- Co-administration with rifampin, a strong CYP3A inducer, significantly decreased ivacaftor exposure (AUC) by approximately 9-fold. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's Wort is not recommended.
Potential for ivacaftor to affect other drugs
CYP3A and/or P-gp Substrates
- Ivacaftor and its M1 metabolite have the potential to inhibit CYP3A and P-gp. Co-administration with midazolam, a sensitive CYP3A substrate, increased midazolam exposure 1.5-fold, consistent with weak inhibition of CYP3A by ivacaftor. Co-administration with digoxin, a sensitive P-gp substrate, increased digoxin exposure by 1.3-fold, consistent with weak inhibition of P-gp by ivacaftor. Administration of Ivacaftor may increase systemic exposure of drugs that are substrates of CYP3A and/or P-gp, which may increase or prolong their therapeutic effect and adverse events. Therefore, caution and appropriate monitoring are recommended when co-administering Ivacaftor with CYP3A and/or P-gp substrates, such as digoxin, cyclosporine, and tacrolimus.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Ivacaftor in pregnant women. Ivacaftor was not teratogenic in rats at approximately 6 times the maximum recommended human dose (MRHD) (based on summed AUCs for ivacaftor and its metabolites at a maternal dose of 200 mg/kg/day). Ivacaftor was not teratogenic in rabbits at approximately 12 times the MRHD (on an ivacaftor AUC basis at a maternal dose of 100 mg/kg/day, respectively). Placental transfer of ivacaftor was observed in pregnant rats and rabbits. Because animal reproduction studies are not always predictive of human response, Ivacaftor should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ivacaftor in women who are pregnant.
Labor and Delivery
- Ivacaftor is excreted into the milk of lactating female rats. Excretion of ivacaftor into human milk is probable. There are no human studies that have investigated the effects of ivacaftor on breast-fed infants. Caution should be exercised when Ivacaftor is administered to a nursing woman.
Nursing Mothers
There is no FDA guidance on the use of Ivacaftor in women who are nursing.
Pediatric Use
- The safety and efficacy of Ivacaftor in patients 6 to 17 years of age with CF who have a G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene has been demonstrated.
- The safety and efficacy of Ivacaftor in patients with CF younger than age 6 years have not been established.
Geriatic Use
- CF is largely a disease of children and young adults. Clinical trials of Ivacaftor did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
Gender
- The effect of gender on Ivacaftor pharmacokinetics was evaluated using population pharmacokinetics of data from clinical studies of Ivacaftor No dose adjustments are necessary based on gender.
Race
There is no FDA guidance on the use of Ivacaftor with respect to specific racial populations.
Renal Impairment
- Ivacaftor has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is necessary for patients with mild to moderate renal impairment; however, caution is recommended while using Ivacaftor in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min) or end-stage renal disease.
- Ivacaftor has not been studied in patients with mild, moderate or severe renal impairment (creatinine clearance less than or equal to 30 mL/min) or in patients with end-stage renal disease. No dose adjustments are recommended for mild and moderate renal impairment patients because of minimal elimination of ivacaftor and its metabolites in urine (only 6.6% of total radioactivity was recovered in the urine in a human PK study); however, caution is recommended when administering Ivacaftor to patients with severe renal impairment or end-stage renal disease.
Hepatic Impairment
- No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A reduced dose of 150 mg once daily is recommended in patients with moderate hepatic impairment (Child-Pugh Class B). Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C) but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a dose of 150 mg once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment.
- Patients with moderately impaired hepatic function (Child-Pugh Class B, score 7 to 9) had similar ivacaftor Cmax, but an approximately two-fold increase in ivacaftor AUC0-∞ compared with healthy subjects matched for demographics. Based on simulations of these results, a reduced Ivacaftor dose of 150 mg once daily is recommended for patients with moderate hepatic impairment. The impact of mild hepatic impairment (Child-Pugh Class A) on pharmacokinetics of ivacaftor has not been studied, but the increase in ivacaftor AUC0-∞ is expected to be less than two-fold. Therefore, no dose adjustment is necessary for patients with mild hepatic impairment. The impact of severe hepatic impairment (Child-Pugh Class C, score 10-15) on pharmacokinetics of ivacaftor has not been studied. The magnitude of increase in exposure in these patients is unknown but is expected to be substantially higher than that observed in patients with moderate hepatic impairment. When benefits are expected to outweigh the risks, Ivacaftor should be used with caution in patients with severe hepatic impairment at a dose of 150 mg given once daily or less frequently.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ivacaftor in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ivacaftor in patients who are immunocompromised.
Patients with CF who are Homozygous for the F508del Mutation in the CFTR Gene
- Efficacy results from a double-blind, placebo-controlled trial in patients with CF who are homozygous for the F508del mutation in the CFTR gene showed no statistically significant difference in forced expiratory volume exhaled in one second (FEV1) over 16 weeks of Ivacaftor treatment compared to placebo. Therefore, Ivacaftor should not be used in patients homozygous for the F508del mutation in the CFTR gene.
Administration and Monitoring
Administration
There is limited information regarding Ivacaftor Administration in the drug label.
Monitoring
There is limited information regarding Ivacaftor Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ivacaftor and IV administrations.
Overdosage
- There have been no reports of overdose with Ivacaftor. The highest single dose used in a clinical study was 800 mg in a solution formulation without any treatment-related adverse events.
- The highest repeated dose was 450 mg (in a tablet formulation) every 12 hours for 4.5 days (9 doses) in a trial evaluating the effect of Ivacaftor on ECGs in healthy subjects. Adverse events reported at a higher incidence compared to placebo included dizziness and diarrhea.
- No specific antidote is available for overdose with Ivacaftor Treatment of overdose with Ivacaftor consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Pharmacology
| |
Ivacaftor
| |
| Systematic (IUPAC) name | |
| N-(2,4-Di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | R07 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 392.490 g/mol |
| SMILES | & |
| Synonyms | VX-770 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 99% |
| Metabolism | CYP3A |
| Half life | 12 hrs (single dose) |
| Excretion | 88% faeces |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Ivacaftor is a potentiator of the CFTR protein. The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. Ivacaftor facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein.
- In vitro, ivacaftor increased CFTR-mediated transepithelial current (IT) in rodent cells expressing the G551D-CFTR protein following addition of a cyclic adenosine monophosphate (cAMP) agonist with an EC50 of 100 ± 47 nM; however, ivacaftor did not increase IT in the absence of cAMP agonist. Ivacaftor also increased IT in human bronchial epithelial cells expressing G551D-CFTR protein following addition of a cAMP agonist by 10-fold with an EC50 of 236 ± 200 nM. Ivacaftor increased the open probability of G551D-CFTR protein in single channel patch clamp experiments using membrane patches from rodent cells expressing G551D-CFTR protein by 6-fold versus untreated cells after addition of PKA and ATP. In addition to G551D-CFTR, ivacaftor increased the channel-open probability of other mutant CFTR forms expressed in rodent cells, resulting in enhanced CFTR-mediated IT. These mutant CFTR forms included G178R-, S549N-, S549R-, G551S-, G970R-, G1244E-, S1251N-, S1255P-, and G1349D-CFTR. In vitro responses do not necessarily correspond to in vivo pharmacodynamic response or clinical benefit.
Structure
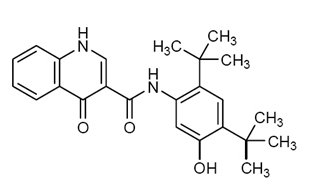
- Its molecular formula is C24H28N2O3 and its molecular weight is 392.49. Ivacaftor has the following structural formula:

Pharmacodynamics
Sweat Chloride Evaluation
- Changes in sweat chloride response to Ivacaftor were evaluated in four clinical trials. In two randomized, double-blind, placebo-controlled clinical trials in patients with a G551D mutation in the CFTR gene, one in patients 12 and older (Trial 1) and the other in patients 6-11 years of age (Trial 2), the treatment difference (between Ivacaftor and placebo) in mean change in sweat chloride from baseline through Week 24 was -48 mmol/L (95% CI -51, -45) and -54 mmol/L (95% CI -62, -47), respectively. These changes persisted through 48 weeks. In a 16-week randomized, double-blind, placebo-controlled, parallel-group clinical trial in patients with CF age 12-years and older who were homozygous for the F508del mutation in the CFTR gene (Trial 3), the treatment difference in mean change in sweat chloride from baseline through 8 weeks of treatment was -3 mmol/L (95% CI -6, -0.2). In a two-part, randomized, double-blind, placebo-controlled, crossover clinical trial in patients with CF who had a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene (Trial 4), the treatment difference in mean change in sweat chloride from baseline through 8 weeks of treatment was -49 mmol/L (95% CI -57, -41). In Trial 4, mean changes in sweat chloride for the mutations for which Ivacaftor is indicated ranged from -51 to -78, whereas the range for individual subjects with the G970R mutations was -1 to -11 mmol/L. There was no direct correlation between decrease in sweat chloride levels and improvement in lung function (FEV1).
ECG Evaluation
- The effect of multiple doses of ivacaftor 150 mg and 450 mg twice daily on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT study in 72 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on Fridericia's correction method (QTcF) was below 10 ms, the threshold for regulatory concern.
Pharmacokinetics
- The pharmacokinetics of ivacaftor is similar between healthy adult volunteers and patients with CF.
After oral administration of a single 150 mg dose to healthy volunteers in a fed state, peak plasma concentrations (Tmax) occurred at approximately 4 hours, and the mean (±SD) for AUC and Cmax were 10600 (5260) ng*hr/mL and 768 (233) ng/mL, respectively.
- After every 12-hour dosing, steady-state plasma concentrations of ivacaftor were reached by days 3 to 5, with an accumulation ratio ranging from 2.2 to 2.9.
Absorption
- The exposure of ivacaftor increased approximately 2- to 4-fold when given with food containing fat. Therefore, Ivacaftor should be administered with fat-containing food. Examples of fat-containing foods include eggs, butter, peanut butter, and cheese pizza. The median (range) Tmax is approximately 4.0 (3.0; 6.0) hours in the fed state.
Distribution
- Ivacaftor is approximately 99% bound to plasma proteins, primarily to alpha 1-acid glycoprotein and albumin. Ivacaftor does not bind to human red blood cells. The mean apparent volume of distribution (Vz/F) of ivacaftor after a single dose of 275 mg of Ivacaftor in the fed state was similar for healthy subjects and patients with CF. After oral administration of 150 mg every 12 hours for 7 days to healthy volunteers in a fed state, the mean (±SD) for apparent volume of distribution was 353 (122) L.
Metabolism
- Ivacaftor is extensively metabolized in humans. In vitro and clinical studies indicate that ivacaftor is primarily metabolized by CYP3A. M1 and M6 are the two major metabolites of ivacaftor in humans. M1 has approximately one-sixth the potency of ivacaftor and is considered pharmacologically active. M6 has less than one-fiftieth the potency of ivacaftor and is not considered pharmacologically active.
Elimination
- Following oral administration, the majority of ivacaftor (87.8%) is eliminated in the feces after metabolic conversion. The major metabolites M1 and M6 accounted for approximately 65% of the total dose eliminated with 22% as M1 and 43% as M6. There was negligible urinary excretion of ivacaftor as unchanged parent. The apparent terminal half-life was approximately 12 hours following a single dose. The mean apparent clearance (CL/F) of ivacaftor was similar for healthy subjects and patients with CF. The CL/F (SD) for the 150 mg dose was 17.3 (8.4) L/hr in healthy subjects.
Nonclinical Toxicology
There is limited information regarding Ivacaftor Nonclinical Toxicology in the drug label.
Clinical Studies
Trials in Patients with CF who have a G551D Mutation in the CFTR Gene
Dose Ranging
- Dose ranging for the clinical program consisted primarily of one double-blind, placebo-controlled, crossover trial in 39 adult (mean age 31 years) Caucasian patients with CF who had FEV1 ≥40% predicted. Twenty patients with median predicted FEV1 at baseline of 56% (range: 42% to 109%) received Ivacaftor 25, 75, 150 mg or placebo every 12 hours for 14 days and 19 patients with median predicted FEV1 at baseline of 69% (range: 40% to 122%) received Ivacaftor 150, 250 mg or placebo every 12 hours for 28 days. The selection of the 150 mg every 12 hours dose was primarily based on nominal improvements in lung function (pre-dose FEV1) and changes in pharmacodynamic parameters (sweat chloride and nasal potential difference). The twice-daily dosing regimen was primarily based on an apparent terminal plasma half-life of approximately 12 hours. Selection of the 150 mg dose of Ivacaftor for children 6 to 11 years of age was based on achievement of comparable pharmacokinetics as those observed for adult patients.
Efficacy
- The efficacy of Ivacaftor in patients with CF who have a G551D mutation in the CFTR gene was evaluated in two randomized, double-blind, placebo-controlled clinical trials in 213 clinically stable patients with CF (109 receiving Ivacaftor 150 mg twice daily). All eligible patients from these trials were rolled over into an open-label extension study.
- Trial 1 evaluated 161 patients with CF who were 12 years of age or older (mean age 26 years) with FEV1 at screening between 40-90% predicted [mean FEV1 64% predicted at baseline (range: 32% to 98%)]. Trial 2 evaluated 52 patients who were 6 to 11 years of age (mean age 9 years) with FEV1 at screening between 40-105% predicted [mean FEV1 84% predicted at baseline (range: 44% to 134%)]. Patients who had persistent Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus isolated from sputum at screening and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the upper limit of normal were excluded.
- Patients in both trials were randomized 1:1 to receive either 150 mg of Ivacaftor or placebo every 12 hours with food containing fat for 48 weeks in addition to their prescribed CF therapies (e.g., tobramycin, dornase alfa). The use of inhaled hypertonic saline was not permitted. The primary efficacy endpoint in both studies was improvement in lung function as determined by the mean absolute change from baseline in percent predicted pre-dose FEV1 through 24 weeks of treatment.
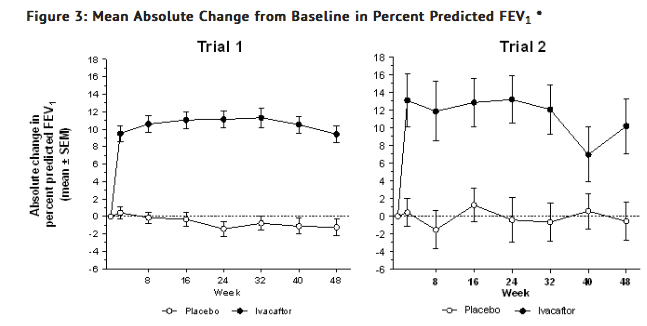
- In both studies, treatment with Ivacaftor resulted in a significant improvement in FEV1. The treatment difference between Ivacaftor and placebo for the mean absolute change in percent predicted FEV1 from baseline through Week 24 was 10.6 percentage points (P < 0.0001) in Trial 1 and 12.5 percentage points (P < 0.0001) in Trial 2 (Figure 3). These changes persisted through 48 weeks. Improvements in percent predicted FEV1 were observed regardless of age, disease severity, sex, and geographic region.
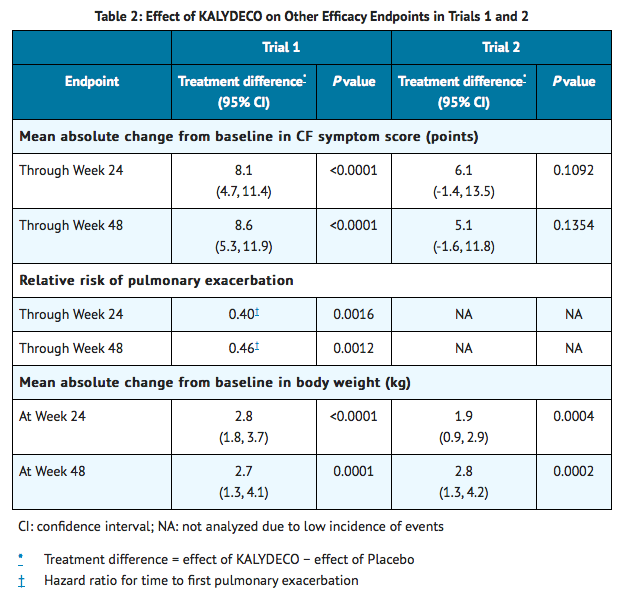
- Other efficacy variables included absolute change in sweat chloride from baseline to Week 24, time to first pulmonary exacerbation through Week 48 (Trial 1 only), absolute change in weight from baseline to Week 48, and improvement in cystic fibrosis symptoms including relevant respiratory symptoms such as cough, sputum production, and difficulty breathing. For the purpose of the study, a pulmonary exacerbation was defined as a change in antibiotic therapy (IV, inhaled, or oral) as a result of 4 or more of 12 pre-specified sino-pulmonary signs/symptoms. Patients treated with Ivacaftor demonstrated statistically significant improvements in risk of pulmonary exacerbations, CF symptoms (in Trial 1 only), and gain in body weight (Table 2). Weight data, when expressed as body mass index normalized for age and sex in patients <20 years of age, was consistent with absolute change from baseline in weight.
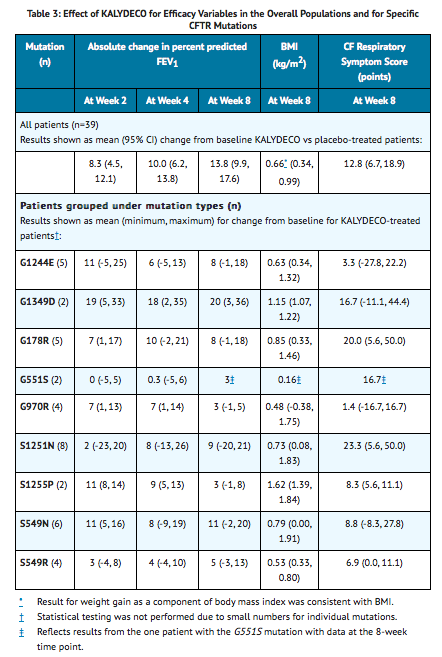
Trial in Patients with a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R Mutation in the CFTR Gene
- The efficacy and safety of Ivacaftor in patients with CF who have a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene were evaluated in a two-part, randomized, double-blind, placebo-controlled, crossover design clinical trial in 39 patients with CF (Trial 4). Patients who completed Part 1 of this trial continued into the 16-week open-label Part 2 of the study. The mutations studied were G178R, S549N, S549R, G551S, G970R, G1244E, S1251N, S1255P, and G1349D. for efficacy in patients with a G551D mutation.
- Patients were 6 years of age or older (mean age 23 years) with FEV1 ≥40% at screening [mean FEV1 at baseline 78% predicted (range: 43% to 119%)]. Patients with evidence of colonization with Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the upper limit of normal at screening were excluded.
- Patients were randomized 1:1 to receive either 150 mg of Ivacaftor or placebo every 12 hours with food containing fat for 8 weeks in addition to their prescribed CF therapies during the first treatment period and crossed over to the other treatment for the second 8 weeks. The two 8-week treatment periods were separated by a 4- to 8-week washout period. The use of inhaled hypertonic saline was not permitted.
- The primary efficacy endpoint was improvement in lung function as determined by the mean absolute change from baseline in percent predicted FEV1 through 8 weeks of treatment. Other efficacy variables included absolute change from baseline in sweat chloride through 8 weeks of treatment, absolute change from baseline in body mass index (BMI) at 8 weeks of treatment (including body weight at 8 weeks), and improvement in cystic fibrosis symptoms (including relevant respiratory symptoms such as cough, sputum production, and difficulty breathing) through 8 weeks of treatment. For the overall population of the 9 mutations studied, treatment with Ivacaftor compared to placebo resulted in significant improvement in percent predicted FEV1 [10.7 through Week 8 (P < 0.0001)], BMI [0.66 kg/m2 at Week 8 (P < 0.0001)], and cystic fibrosis respiratory symptom score [9.6 through Week 8 (P = 0.0004)]; however, there was a high degree of variability of efficacy responses among the 9 mutations (Table 3). Based on clinical and pharmacodynamic (sweat chloride) responses to ivacaftor, efficacy in patients with the G970R mutation could not be established.
Trial in Patients Homozygous for the F508del Mutation in the CFTR Gene
- Trial 3 was a 16-week randomized, double-blind, placebo-controlled, parallel-group trial in 140 patients with CF age 12 years and older who were homozygous for the F508del mutation in the CFTR gene and who had FEV1 ≥40% predicted. Patients were randomized 4:1 to receive Ivacaftor 150 mg (n=112) every twelve hours or placebo (n=28) in addition to their prescribed CF therapies. The mean age of patients enrolled was 23 years and the mean baseline FEV1 was 79% predicted (range 40% to 129%). As in Trials 1 and 2, patients who had persistent Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus isolated from sputum at screening and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the upper limit of normal were excluded. The use of inhaled hypertonic saline was not permitted.
- The primary endpoint was improvement in lung function as determined by the mean absolute change from baseline through Week 16 in percent predicted FEV1. Treatment with Ivacaftor resulted in no improvement in FEV1 relative to placebo in patients with CF homozygous for the F508del mutation in the CFTR gene [mean absolute change from baseline through Week 16 in percent predicted FEV1 was 1.5% and -0.2% for patients in the Ivacaftor and placebo-treated groups, respectively (P = 0.15)]. There were no meaningful differences between patients treated with Ivacaftor compared to placebo for secondary endpoints (change in CF symptoms, change in weight, or change in sweat chloride concentration).
How Supplied

Storage
Store at 20-25ºC (68-77ºF); excursions permitted to 15-30ºC (59-86ºF)
Images
Drug Images
{{#ask: Page Name::Ivacaftor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ivacaftor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ivacaftor Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Ivacaftor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Ivacaftor Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ivacaftor |Label Name=Ivecaftor package.png
}}