Granisetron (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Granisetron (injection) is an antiemetic that is FDA approved for the prophylaxis of prevention of nausea and/or vomiting. Common adverse reactions include asthenia, headache, somnolence, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Granisetron Injection is indicated for the following.
- The prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Prevention of Chemotherapy-Induced Nausea and Vomiting
- The recommended dosage for Granisetron Injection is 10 mcg/kg administered intravenously within 30 minutes before initiation of chemotherapy, and only on the day(s) chemotherapy is given.
Infusion Preparation
- Granisetron Injection may be administered intravenously either undiluted over 30 seconds, or diluted with 0.9% Sodium Chloride or 5% Dextrose and infused over 5 minutes.
Stability
- Intravenous infusion of Granisetron Injection should be prepared at the time of administration. However, Granisetron Injection has been shown to be stable for at least 24 hours when diluted in 0.9% Sodium Chloride or 5% Dextrose and stored at room temperature under normal lighting conditions.
- As a general precaution, Granisetron Injection should not be mixed in solution with other drugs. Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit.
- Geriatric Patients, Renal Failure Patients or Hepatically Impaired Patients - No dosage adjustment is recommended
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Granisetron in adult patients.
Non–Guideline-Supported Use
Chemotherapy-induced nausea and vomiting, Delayed Injection site pain - Propofol adverse reaction
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Pediatric Patients
- The recommended dose in pediatric patients 2 to 16 years of age is 10 mcg/kg. Pediatric patients under 2 years of age have not been studied.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Granisetron in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Granisetron in pediatric patients.
Contraindications
- Granisetron Injection is contraindicated in patients with known hypersensitivity to the drug or to any of its components.
Warnings
- Hypersensitivity reactions may occur in patients who have exhibited hypersensitivity to other selective 5-HT3 receptor antagonists
Adverse Reactions
Clinical Trials Experience
- QT prolongation has been reported with Granisetron Injection.
Chemotherapy-Induced Nausea and Vomiting
- The following have been reported during controlled clinical trials or in the routine management of patients. The percentage figures are based on clinical trial experience only. TABLE 7 gives the comparative frequencies of the five most commonly reported adverse events (≥3%) in patients receiving Granisetron Injection, in single-day chemotherapy trials.
- These patients received chemotherapy, primarily cisplatin, and intravenous fluids during the 24-hour period following Granisetron Injection administration. Events were generally recorded over seven days post-Granisetron Injection administration.
- In the absence of a placebo group, there is uncertainty as to how many of these events should be attributed to Granisetron, except for headache, which was clearly more frequent than in comparison groups.
- In over 3,000 patients receiving Granisetron Injection (2 to 160 mcg/kg) in single-day and multiple-day clinical trials with emetogenic cancer therapies, adverse events, other than those in TABLE 7, were observed; attribution of many of these events to Granisetron is uncertain.
Hepatic
- In comparative trials, mainly with cisplatin regimens, elevations of AST and ALT (>2 times the upper limit of normal) following administration of Granisetron Injection occurred in 2.8% and 3.3% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2.1%; ALT: 2.4%).
Cardiovascular
- Hypertension (2%); hypotension, arrhythmias such as sinus bradycardia, atrial fibrillation, varying degrees of A-V block, ventricular ectopy including non-sustained tachycardia, and ECG abnormalities have been observed rarely.
Central Nervous System
- Agitation, anxiety, CNS stimulation and insomnia were seen in less than 2% of patients. Extrapyramidal syndrome occurred rarely and only in the presence of other drugs associated with this syndrome.
Hypersensitivity
- Rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylaxis, shortness of breath, hypotension, urticaria) have been reported.
Other
- Fever (3%), taste disorder (2%), skin rashes (1%). In multiple-day comparative studies, fever occurred more frequently with Granisetron Injection (8.6%) than with comparative drugs (3.4%, P<0.014), which usually included dexamethasone.
Postmarketing Experience
- QT prolongation has been reported with Granisetron
Drug Interactions
There is limited information regarding Granisetron (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in pregnant rats at intravenous doses up to 9 mg/kg/day (54 mg/m2/day, 146 times the recommended human dose based on body surface area) and pregnant rabbits at intravenous doses up to 3 mg/kg/day (35.4 mg/m2/day, 96 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to granisetron.
- There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Benzyl alcohol may cross the placenta. Granisetron Injection 1 mg/mL is preserved with benzyl alcohol and should be used in pregnancy only if the benefit outweighs the potential risk.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Granisetron (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Granisetron (injection) during labor and delivery.
Nursing Mothers
- It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Granisetron Injection is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients under 2 years of age have not been established.
- Benzyl alcohol, a component of Granisetron 1 mg/mL, has been associated with serious adverse events and death, particularly in neonates.
- The “gasping syndrome,” characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and metabolites in blood and urine, has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low birth weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the “gasping syndrome,” the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity.
- Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
Geriatic Use
- During chemotherapy clinical trials, 713 patients 65 years of age or older received Granisetron Injection. Effectiveness and safety were similar in patients of various ages.
Gender
There is no FDA guidance on the use of Granisetron (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Granisetron (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Granisetron (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Granisetron (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Granisetron (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Granisetron (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
There is limited information regarding Granisetron (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Granisetron (injection) and IV administrations.
Overdosage
- There is no specific antidote for Granisetron Injection overdosage. In case of overdosage, symptomatic treatment should be given. Overdosage of up to 38.5 mg of granisetron hydrochloride injection, USP has been reported without symptoms or only the occurrence of a slight headache.
Pharmacology
Mechanism of Action
- Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-, alpha2- or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors.
- Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy-induced vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3 receptors. This evokes vagal afferent discharge and may induce vomiting. Animal studies demonstrate that, in binding to 5-HT3 receptors, granisetron blocks serotonin stimulation and subsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, a single granisetron injection prevented vomiting due to high-dose cisplatin or arrested vomiting within 5 to 30 seconds.
- In most human studies, granisetron has had little effect on blood pressure, heart rate or ECG. No evidence of an effect on plasma prolactin or aldosterone concentrations has been found in other studies.
- Granisetron Injection exhibited no effect on oro-cecal transit time in normal volunteers given a single intravenous infusion of 50 mcg/kg or 200 mcg/kg. Single and multiple oral doses slowed colonic transit in normal volunteers.
Structure
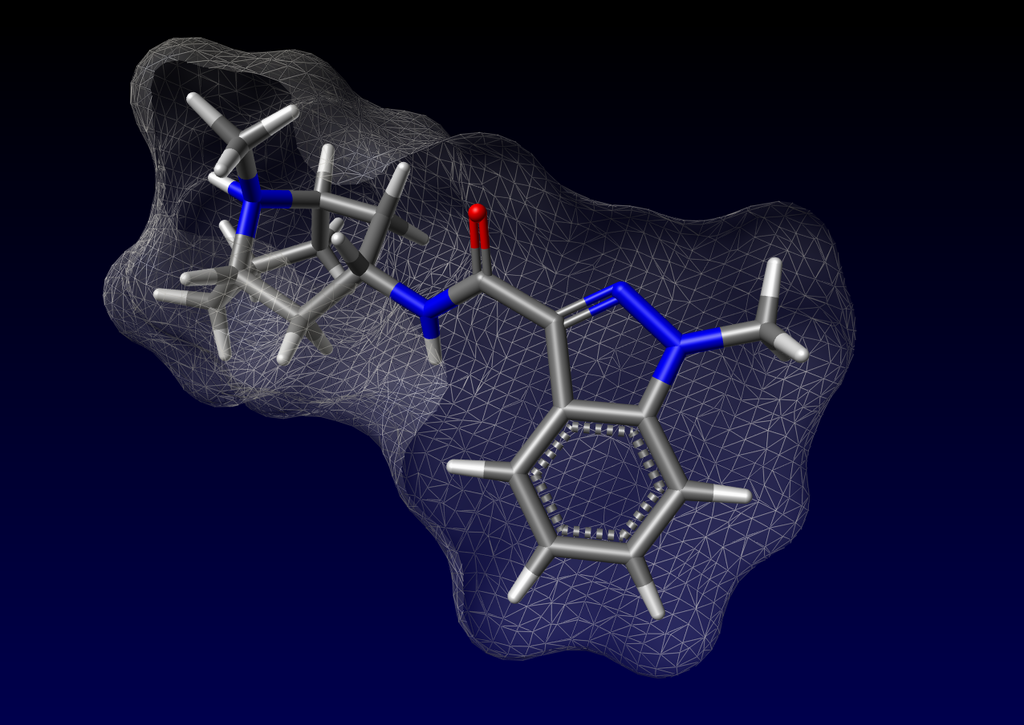
- Granisetron Hydrochloride Injection, USP (granisetron hydrochloride) is an anti-nauseant and antiemetic agent. Chemically it is endo-N-(9-methyl-9-azabi-cyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride with a molecular weight of 348.9 (312.4 free base). Its empirical formula is C18H24N4O•HCl, while its chemical structure is:
- Granisetron hydrochloride, USP is a white to off-white solid that is readily soluble in water and normal saline at 20°C. Granisetron Injection is a clear, colorless, sterile, nonpyrogenic, aqueous solution for intravenous administration.
- Granisetron Hydrochloride Injection, USP 1 mg/mL is available in 1 mL Single-use and 4 mL Multi-use vials. Granisetron Hydrochloride Injection, USP 0.1 mg/mL is available in a 1 mL Single-use vial.
- 1 mg/mL: Each mL contains:
- Active: Granisetron hydrochloride 1.12 mg, equivalent to granisetron, 1 mg;
- Preservative: Benzyl Alcohol, 10 mg;
- Inactives: Sodium Chloride, 9 mg; Citric Acid, 2 mg; Sodium Citrate, Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (approximately 4.0 to 6.0), and Water for Injection q.s. to 1 mL.
- 0.1 mg/mL: Each mL contains:
- Active: Granisetron hydrochloride 0.112 mg, equivalent to granisetron, 0.1 mg;
- Preservative: None;
- Inactives: Sodium Chloride, 9 mg; Citric Acid, 2 mg; Sodium Citrate, Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (approximately 4.0 to 6.0), and Water for Injection q.s. to 1 mL.
Pharmacodynamics
There is limited information regarding Granisetron (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
Chemotherapy-Induced Nausea and Vomiting
- In adult cancer patients undergoing chemotherapy and in volunteers, mean pharmacokinetic data obtained from an infusion of a single 40 mcg/kg dose of Granisetron Injection are shown below
Distribution
Plasma protein binding is approximately 65% and granisetron distributes freely between plasma and red blood cells.
Metabolism
- Granisetron metabolism involves N-demethylation and aromatic ring oxidation followed by conjugation. In vitro liver microsomal studies show that granisetron's major route of metabolism is inhibited by ketoconazole, suggestive of metabolism mediated by the cytochrome P-450 3A subfamily. Animal studies suggest that some of the metabolites may also have 5-HT3 receptor antagonist activity.
Elimination
- Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately 12% of the administered dose is eliminated unchanged in the urine in 48 hours. The remainder of the dose is excreted as metabolites, 49% in the urine, and 34% in the feces.
Subpopulations
Gender
- There was high inter- and intra-subject variability noted in these studies. No difference in mean AUC was found between males and females, although males had a higher Cmax generally.
Elderly
- The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years), given a single 40 mcg/kg intravenous dose of Granisetron Injection, were generally similar to those in younger healthy volunteers; mean values were lower for clearance and longer for half-life in the elderly patients.
Pediatric Patients
- A pharmacokinetic study in pediatric cancer patients (2 to 16 years of age), given a single 40 mcg/kg intravenous dose of Granisetron Injection, showed that volume of distribution and total clearance increased with age. No relationship with age was observed for peak plasma concentration or terminal phase plasma half-life. When volume of distribution and total clearance are adjusted for body weight, the pharmacokinetics of granisetron are similar in pediatric and adult cancer patients.
Renal Failure Patients
- Total clearance of granisetron was not affected in patients with severe renal failure who received a single 40 mcg/kg intravenous dose of Granisetron Injection.
Hepatically Impaired Patients
- A pharmacokinetic study in patients with hepatic impairment due to neoplastic liver involvement showed that total clearance was approximately halved compared to patients without hepatic impairment. Given the wide variability in pharmacokinetic parameters noted in patients, dosage adjustment in patients with possible hepatic functional impairment is not necessary.
Nonclinical Toxicology
There is limited information regarding Granisetron (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
- Chemotherapy-Induced Nausea and Vomiting
- Single-Day Chemotherapy
- Cisplatin-Based Chemotherapy
- In a double-blind, placebo-controlled study in 28 cancer patients, Granisetron Injection, administered as a single intravenous infusion of 40 mcg/kg, was significantly more effective than placebo in preventing nausea and vomiting induced by cisplatin chemotherapy
- Granisetron Injection was also evaluated in a randomized dose response study of cancer patients receiving cisplatin ≥75 mg/m2. Additional chemotherapeutic agents included: anthracyclines, carboplatin, cytostatic antibiotics, folic acid derivatives, methylhydrazine, nitrogen mustard analogs, podophyllotoxin derivatives, pyrimidine analogs, and vinca alkaloids.
- Granisetron Injection doses of 10 and 40 mcg/kg were superior to 2 mcg/kg in preventing cisplatin-induced nausea and vomiting, but 40 mcg/kg was not significantly superior to 10 mcg/kg
- Granisetron Injection was also evaluated in a double-blind, randomized dose response study of 353 patients stratified for high (≥80 to 120 mg/m2) or low (50 to 79 mg/m2) cisplatin dose. Response rates of patients for both cisplatin strata are given in TABLE 4.
- For both the low and high cisplatin strata, the 10, 20, and 40 mcg/kg doses were more effective than the 5 mcg/kg dose in preventing nausea and vomiting within 24 hours of chemotherapy administration. The 10 mcg/kg dose was at least as effective as the higher doses.
Moderately Emetogenic Chemotherapy
- Granisetron Injection, 40 mcg/kg, was compared with the combination of chlorpromazine (50 to 200 mg/24 hours) and dexamethasone (12 mg) in patients treated with moderately emetogenic chemotherapy, including primarily carboplatin >300 mg/m2, cisplatin 20 to 50 mg/m2 and cyclophosphamide >600 mg/m2. Granisetron Injection was superior to the chlorpromazine regimen in preventing nausea and vomiting.
- In other studies of moderately emetogenic chemotherapy, no significant difference in efficacy was found between Granisetron doses of 40 mcg/kg and 160 mcg/kg.
Repeat-Cycle Chemotherapy
- In an uncontrolled trial, 512 cancer patients received Granisetron Injection, 40 mcg/kg, prophylactically, for two cycles of chemotherapy, 224 patients received it for at least four cycles, and 108 patients received it for at least six cycles.
- Granisetron Injection efficacy remained relatively constant over the first six repeat cycles, with complete response rates (no vomiting and no moderate or severe nausea in 24 hours) of 60% to 69%. No patients were studied for more than 15 cycles.
Pediatric Studies
- A randomized double-blind study evaluated the 24-hour response of 80 pediatric cancer patients (age 2 to 16 years) to Granisetron Injection 10, 20 or 40 mcg/kg. Patients were treated with cisplatin ≥60 mg/m2, cytarabine ≥3 g/m2, cyclophosphamide ≥1 g/m2 or nitrogen mustard ≥6 mg/m2.
- A second pediatric study compared Granisetron Injection 20 mcg/kg to chlorpromazine plus dexamethasone in 88 patients treated with ifosfamide ≥3 g/m2/day for two or three days. Granisetron Injection was administered on each day of ifosfamide treatment. At 24 hours, 22% of Granisetron Injection patients achieved complete response (no vomiting and no moderate or severe nausea in 24 hours) compared with 10% on the chlorpromazine regimen. The median number of vomiting episodes with Granisetron Injection was 1.5; with chlorpromazine it was 7.0.
How Supplied
- Granisetron Hydrochloride Injection, USP 1 mg/mL (free base), is supplied in 1 mL Single-use Vials and 4 mL Multi-use Vials. CONTAINS BENZYL ALCOHOL.
- NDC 17478-546-02 (package of 1 Single-use Vial)
- NDC 17478-546-05 (package of 1 Multi-use Vial)
- Granisetron Hydrochloride Injection, USP 0.1 mg/mL (free base), is supplied in 1 mL Single-use Vials. CONTAINS NO PRESERVATIVE.
- NDC 17478-547-01 (package of 10 Single-use Vial)
Storage
- Granisetron Hydrochloride Injection, USP 1 mg/mL (free base), is supplied in 1 mL Single-use Vials and 4 mL Multi-use Vials. CONTAINS BENZYL ALCOHOL.
- NDC 17478-546-02 (package of 1 Single-use Vial)
- NDC 17478-546-05 (package of 1 Multi-use Vial)
- Granisetron Hydrochloride Injection, USP 0.1 mg/mL (free base), is supplied in 1 mL Single-use Vials. CONTAINS NO PRESERVATIVE.
- NDC 17478-547-01 (package of 10 Single-use Vial)
Images
Drug Images
{{#ask: Page Name::Granisetron (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Granisetron (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Granisetron (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Granisetron interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GRANISETRON HYDROCHLORIDE ®[1]
Look-Alike Drug Names
There is limited information regarding Granisetron (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G 01.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G02.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G03.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G04.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G05.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=G06.jpg
}}
{{#subobject:
|Label Page=Granisetron (injection) |Label Name=Ingredients.png
}}