Ferumoxytol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS:
Only administer Feraheme when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions [SEE WARNINGS AND PRECAUTIONS (5.1)]. Observe for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following Feraheme infusion including monitoring of blood pressure and pulse during and after Feraheme administration [SEE WARNINGS AND PRECAUTIONS (5.1)]. Hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated |
Overview
Ferumoxytol is a iron replacement product that is FDA approved for the treatment of iron deficiency anemia in adult patients with chronic kidney disease. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, nausea, dizziness, hypotension, constipation, and peripheral edema.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Feraheme is indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease (CKD)
Dosage
- The recommended dose of Feraheme is an initial 510 mg dose followed by a second 510 mg dose 3 to 8 days later. Administer Feraheme as an intravenous infusion in 50-200 mL 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP over at least 15 minutes. Administer while the patient is in a reclined or semi-reclined position.
- Feraheme, when added to intravenous infusion bags containing either 0.9% Sodium Chloride Injection, USP (normal saline), or 5% Dextrose Injection, USP, at concentrations of 2-8 mg elemental iron per mL, should be used immediately but may be stored at controlled room temperature (25°C ± 2°C) for up to 4 hours.
- The dosage is expressed in terms of mg of elemental iron, with each mL of Feraheme containing 30 mg of elemental iron. Evaluate the hematologic response (hemoglobin, ferritin, iron and transferrin saturation) at least one month following the second Feraheme infusion. The recommended Feraheme dose may be readministered to patients with persistent or recurrent iron deficiency anemia.
- For patients receiving hemodialysis, administer Feraheme once the blood pressure is stable and the patient has completed at least one hour of hemodialysis. Monitor for signs and symptoms of hypotension following each Feraheme infusion.
- Allow at least 30 minutes between administration of Feraheme and administration of other medications that could potentially cause serious hypersensitivity reactions and/or hypotension, such as chemotherapeutic agents or monoclonal antibodies.
- Inspect parenteral drug products visually for the absence of particulate matter and discoloration prior to administration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ferumoxytol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ferumoxytol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ferumoxytol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ferumoxytol in pediatric patients.
Contraindications
- Known hypersensitivity to Feraheme or any of its components
History of allergic reaction to any intravenous iron product
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS:
Only administer Feraheme when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions [SEE WARNINGS AND PRECAUTIONS (5.1)]. Observe for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following Feraheme infusion including monitoring of blood pressure and pulse during and after Feraheme administration [SEE WARNINGS AND PRECAUTIONS (5.1)]. Hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated |
Serious Hypersensitivity Reactions
- Fatal and serious hypersensitivity reactions including anaphylaxis, presenting with cardiac/ cardiorespiratory arrest, clinically significant hypotension, syncope, or unresponsiveness have occurred in patients receiving Feraheme. Other adverse reactions potentially associated with hypersensitivity have occurred (pruritus, rash, urticaria, and wheezing). These reactions have occurred following the first dose or subsequent doses in patients in whom a previous Feraheme dose was tolerated.
- Patients with a history of multiple drug allergies may have a greater risk of anaphylaxis with parenteral iron products. Carefully consider the potential risks and benefits before administering Feraheme to these patients.
- Only administer Feraheme when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions. Closely observe patients for signs and symptoms of hypersensitivity including monitoring of blood pressure and pulse during and after Feraheme administration for at least 30 minutes and until clinically stable following completion of each infusion.
- In clinical studies predominantly in patients with CKD, serious hypersensitivity reactions were reported in 0.2% (3/1,726) of subjects receiving Feraheme. Other adverse reactions potentially associated with hypersensitivity (e.g., pruritus, rash, urticaria or wheezing) were reported in 3.7% (63/1,726) of these subjects. In other trials excluding patients with Stages 4 and 5 CKD, moderate to severe hypersensitivity reactions were reported in 2.6% (26/1014) of patients treated with Feraheme.
- In the post-marketing experience, fatal and serious anaphylactic type reactions presenting with cardiac/ cardiorespiratory arrest, clinically significant hypotension, syncope, and unresponsiveness have been reported. Elderly patients with multiple or serious co-morbidities who experience hypersensitivity reactions and/or hypotension following administration of Feraheme may have more severe outcomes.
Hypotension
- Severe adverse reactions of clinically significant hypotension have been reported. In clinical studies, hypotension was reported in 1.9% (33/1,726) of subjects, including three patients with serious hypotensive reactions. Hypotension has also been reported in the post-marketing experience. Monitor patients for signs and symptoms of hypotension following each Feraheme administration.
Iron Overload
- Excessive therapy with parenteral iron can lead to excess storage of iron with the possibility of iatrogenic hemosiderosis. Regularly monitor the hematologic response during parenteral iron therapy. Do not administer Feraheme to patients with iron overload.
- In the 24 hours following administration of Feraheme, laboratory assays may overestimate serum iron and transferrin bound iron by also measuring the iron in the Feraheme complex.
Magnetic Resonance (MR) Imaging
- Administration of Feraheme may transiently affect the diagnostic ability of MR imaging. Anticipated MR imaging studies should be conducted prior to the administration of Feraheme. Alteration of MR imaging studies may persist for up to 3 months following the last Feraheme dose. If MR imaging is required within 3 months after Feraheme administration, use T1- or proton density-weighted MR pulse sequences to minimize the Feraheme effects; MR imaging using T2-weighted pulse sequences should not be performed earlier than 4 weeks after the administration of Feraheme. Maximum alteration of vascular MR imaging is anticipated to be evident for 1 – 2 days following Feraheme administration.
- Feraheme will not interfere with X-ray, computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasound or nuclear medicine imaging.
Adverse Reactions
Clinical Trials Experience
- Feraheme administration may cause serious hypersensitivity reactions and hypotension.
- In clinical studies, 1,726 subjects were exposed to Feraheme; 1,562 of these had CKD and 164 did not have CKD. Of these subjects 46% were male and the median age was 63 years (range of 18 to 96 years).
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug may not reflect the rates observed in practice.
Adverse Reactions in Clinical Studies
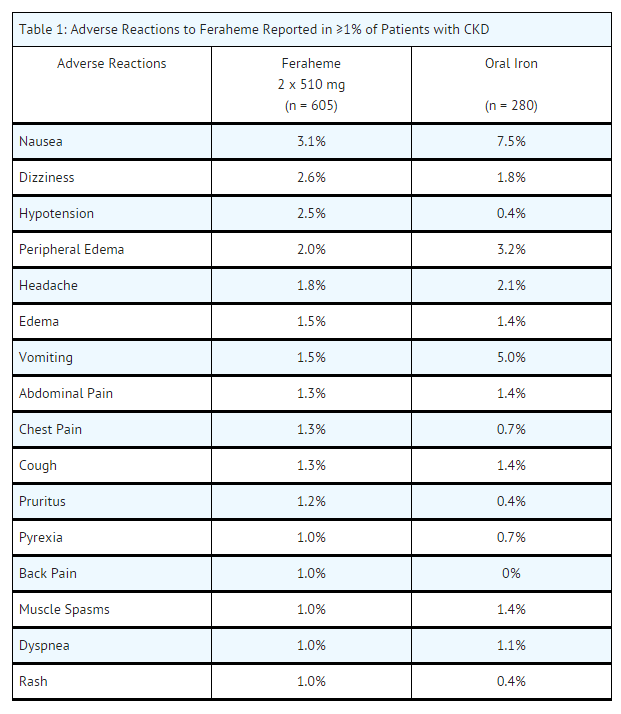
- Across the three randomized clinical trials, a total of 605 patients were exposed to two injections of 510 mg of Feraheme and a total of 280 patients were exposed to 200 mg/day of oral iron for 21 days. Most patients received their second Feraheme injection 3 to 8 days after the first injection.
- Adverse reactions related to Feraheme and reported by ≥ 1% of Feraheme-treated patients in the randomized clinical trials are listed in Table 1. Diarrhea (4.0%), constipation (2.1%) and hypertension (1.0%) have also been reported in Feraheme-treated patients.
- In clinical trials, adverse reactions leading to treatment discontinuation and occurring in ≥ 2 Feraheme-treated patients included hypotension, infusion site swelling, increased serum ferritin level, chest pain, diarrhea, dizziness, ecchymosis, pruritus, chronic renal failure, and urticaria.
- Following completion of the controlled phase of the trials, 69 patients received two additional 510 mg intravenous injections of Feraheme (for a total cumulative dose of 2.04 g). Adverse reactions following this repeat Feraheme dosing were similar in character and frequency to those observed following the first two intravenous injections.
- In a placebo-controlled, cross-over trial, 713 patients with CKD received a single 510 mg dose of Feraheme. Adverse reactions reported by these patients were similar in character and frequency to those observed in other clinical trials.
Postmarketing Experience
- Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The following serious adverse reactions have been reported from the post-marketing experience with Feraheme: fatal, life-threatening, and serious anaphylactic-type reactions, cardiac/cardiorespiratory arrest, clinically significant hypotension, syncope, unresponsiveness, loss of consciousness, tachycardia/rhythm abnormalities, angioedema, ischemic myocardial events, congestive heart failure, pulse absent, and cyanosis. These adverse reactions have usually occurred within 30 minutes after the administration of Feraheme. Reactions have occurred following the first dose or subsequent doses of Feraheme.
Drug Interactions
- Drug-drug interaction studies with Feraheme were not conducted. Feraheme may reduce the absorption of concomitantly administered oral iron preparations.
Use in Specific Populations
Pregnancy
- There are no studies of Feraheme in pregnant women. In animal studies, ferumoxytol caused fetal malformations and decreased fetal weights at maternally toxic doses of 6 times the estimated human daily dose. Use Feraheme during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Administration of ferumoxytol during organogenesis, at doses of 31.6 mg Fe/kg/day in rats and 16.5 mg Fe/kg/day in rabbits, did not result in maternal or fetal effects. These doses are approximately 2 times the estimated human daily dose based on body surface area. In rats, administration of ferumoxytol during organogenesis at a maternally toxic dose of 100 mg Fe/kg/day, approximately 6 times the estimated human daily dose based on body surface area, caused a decrease in fetal weights. In rabbits, administration of ferumoxytol during organogenesis at a maternally toxic dose of 45 mg Fe/kg/day, approximately 6 times the estimated human daily dose based on body surface area, was associated with external and/or soft tissue fetal malformations and decreased fetal weights.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ferumoxytol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ferumoxytol during labor and delivery.
Nursing Mothers
- It is not known whether Feraheme is present in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to avoid Feraheme, taking into account the importance of Feraheme to the mother and the known benefits of nursing.
Pediatric Use
- The safety and effectiveness of Feraheme in pediatric patients (less than 18 years old) have not been established.
Geriatic Use
- In controlled clinical trials, 330 patients ≥ 65 years of age were treated with Feraheme. No overall differences in safety and efficacy were observed between older and younger patients in these trials, but greater sensitivity of older individuals cannot be ruled out. In general, dose administration to an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients with multiple or serious co-morbidities who experience hypersensitivity reactions and/or hypotension following administration of Feraheme may have more severe outcomes. The potential risks and benefits of Feraheme administration should be carefully considered in these patients
Gender
There is no FDA guidance on the use of Ferumoxytol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ferumoxytol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ferumoxytol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ferumoxytol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ferumoxytol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ferumoxytol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Closely observe patients for signs and symptoms of hypersensitivity including monitoring of blood pressure and pulse during and after Feraheme administration for at least 30 minutes and until clinically stable following completion of each infusion
- Monitor patients for signs and symptoms of hypotension following each Feraheme administration
- Regularly monitor the hematologic response during parenteral iron therapy
IV Compatibility
There is limited information regarding IV Compatibility of Ferumoxytol in the drug label.
Overdosage
- Limited data are available regarding overdosage of Feraheme in humans.
- Excessive dosages of Feraheme may lead to accumulation of iron in storage sites potentially leading to hemosiderosis. Do not administer Feraheme to patients with iron overload
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox E numberTemplate:Chembox AppearanceTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox RefractIndexTemplate:Chembox Supplement| Template:Chembox header2 | Ferumoxytol | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Fe3O4 FeO.Fe2O3 | |
| Molar mass | 231.533 g/mol |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
- Feraheme consists of a superparamagnetic iron oxide that is coated with a carbohydrate shell, which helps to isolate the bioactive iron from plasma components until the iron-carbohydrate complex enters the reticuloendothelial system macrophages of the liver, spleen and bone marrow. The iron is released from the iron-carbohydrate complex within vesicles in the macrophages. Iron then either enters the intracellular storage iron pool (e.g., ferritin) or is transferred to plasma transferrin for transport to erythroid precursor cells for incorporation into hemoglobin.
Structure
- Feraheme, an iron replacement product, is a non-stoichiometric magnetite (superparamagnetic iron oxide) coated with polyglucose sorbitol carboxymethylether. The overall colloidal particle size is 17-31 nm in diameter. The chemical formula of Feraheme is Fe5874O8752-C11719H18682O9933Na414 with an apparent molecular weight of 750 kDa.
- Feraheme Injection is an aqueous colloidal product that is formulated with mannitol. It is a black to reddish brown liquid, and is provided in single use vials containing 510 mg of elemental iron. Each mL of the sterile colloidal solution of Feraheme Injection contains 30 mg of elemental iron and 44 mg of mannitol, and has low bleomycin-detectable iron. The formulation is isotonic with an osmolality of 270-330 mOsm/kg. The product contains no preservatives, and has a pH of 6 to 8.
Pharmacodynamics
Cardiac Electrophysiology
- In a randomized, positive- and placebo-controlled, parallel-group study, healthy subjects received a supratherapeutic regimen of Feraheme (1.02 g given as two 510 mg doses within 24 hours), placebo or a single dose of 400 mg moxifloxacin (positive control). Results demonstrated no effect of Feraheme on QT interval durations. No clinically meaningful effect of Feraheme on heart rate was observed.
Pharmacokinetics
- The pharmacokinetic (PK) behavior of Feraheme has been examined in healthy subjects and in patients with CKD stage 5D on hemodialysis. Feraheme exhibited dose-dependent, capacity-limited elimination from plasma with a half life of approximately 15 hours in humans. The clearance (CL) was decreased by increasing the dose of Feraheme. Volume of distribution (Vd) was consistent with plasma volume, and the mean maximum observed plasma concentration (Cmax) and terminal half-life (t1/2) values increased with dose. The estimated values of CL and Vd following two 510 mg doses of Feraheme administered intravenously within 24 hours were 69.1 mL/hr and 3.16 L, respectively. The Cmax and time of maximum concentration (tmax) were 206 mcg/mL and 0.32 hr, respectively. Rate of infusion had no influence on Feraheme PK parameters. No gender differences in Feraheme PK parameters were observed. Feraheme is not removed by hemodialysis.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Ferumoxytol was not tested for carcinogenic effects. In standard genotoxicity tests, ferumoxytol showed no evidence of mutagenic activity in an in vitro Ames test or clastogenic activity in either an in vitro chromosomal aberration assay or an in vivo micronucleus assay.
- No adverse effects on fertility or general reproductive performance were noted in animal studies. Ferumoxytol had no effect on male or female fertility or general reproductive function in rats.
Clinical Studies
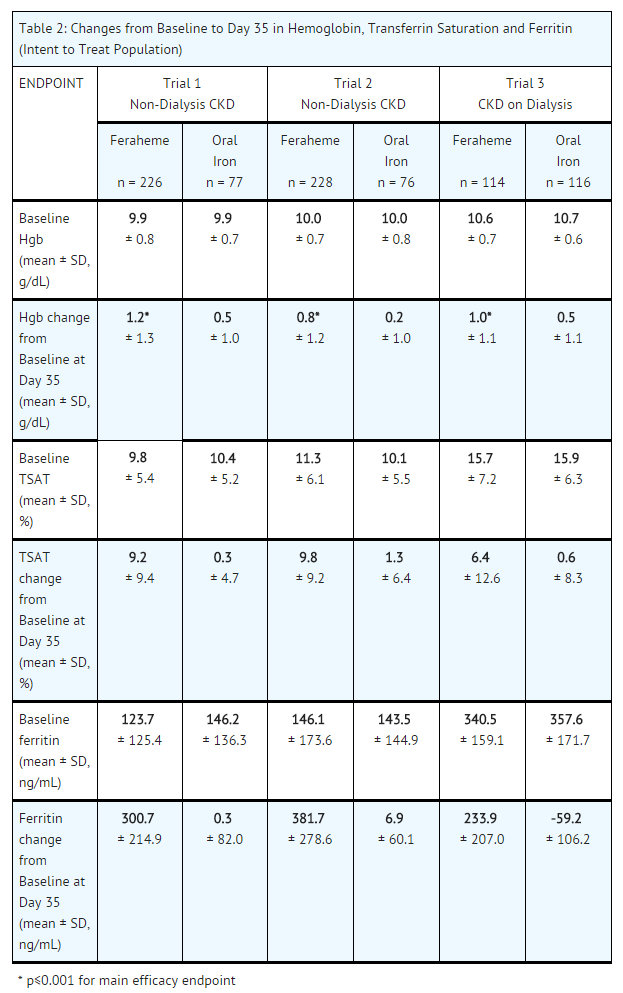
- The safety and efficacy of Feraheme for the episodic treatment of iron deficiency anemia in patients with CKD were assessed in three randomized, open-label, controlled clinical trials (Trial 1, 2 and 3). These trials also included an uncontrolled, follow-up phase in which patients with persistent iron deficiency anemia could receive two additional 510 mg intravenous injections of Feraheme. The major efficacy results from the controlled phase of each study are shown in Table 2.
- In all three trials, patients with CKD and iron deficiency anemia were randomized to treatment with Feraheme or oral iron. Feraheme was administered as two 510 mg intravenous single doses and oral iron (ferrous fumarate) was administered as a total daily dose of 200 mg elemental iron daily for 21 days. The major trial outcomes assessed the change in hemoglobin from baseline to Day 35. Trial 1 and 2 enrolled patients with non-dialysis dependent CKD and Trial 3 enrolled patients who were undergoing hemodialysis.
- In Trial 1, the mean age of patients was 66 years (range, 23 to 95); 60% were female; 65% were Caucasian, 32% were Black, and 2% were other races. In the Feraheme and oral iron groups, 42% and 44% of patients, respectively, were receiving erythropoiesis stimulating agents (ESAs) at baseline.
- In Trial 2, the mean age of patients was 65 years (range, 31 to 96); 61% were female; 58% were Caucasian, 35% were Black, and 7% were other races. In the Feraheme and oral iron groups, 36% and 43% of patients, respectively, were receiving ESAs at baseline.
- In Trial 3, the mean age of patients was 60 years (range, 24 to 87); 43% were female; 34% were Caucasian, 59% were Black, and 7% were other races. All patients were receiving ESAs.
- Table 2 shows the Baseline and mean change to Day 35 in hemoglobin (Hgb, g/dL), transferrin saturation (TSAT, %) and ferritin (ng/mL) in each treatment group for Trial 1, 2, and 3.
- Following completion of the controlled phase of each of the Phase 3 trials, patients who were iron deficient and anemic could receive two additional 510 mg intravenous injections of Feraheme for a total cumulative dose of 2.04 g. Overall, 69 patients received two additional 510 mg intravenous injections of Feraheme, and on Day 35 following these additional injections, the majority of these patients (70%) experienced an increase in hemoglobin and iron parameters (TSAT and ferritin). The mean change (±SD) in hemoglobin level from the retreatment baseline for patients with an increase in hemoglobin was 0.86 (± 0.68) g/dL and was 0.5 (± 0.8) g/dL for all patients.
How Supplied
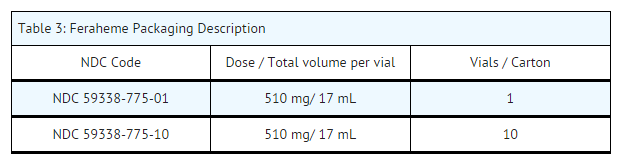
- Feraheme is available in single use vials in the following package sizes
Storage
- Store at 20° to 25°C (68° to 77°F). Excursions permitted to 15° – 30°C (59° – 86°F)
Images
Drug Images
{{#ask: Page Name::Ferumoxytol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Ingredients and Appearance
{{#ask: Label Page::Ferumoxytol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Prior to Feraheme administration:
- Question patients regarding a history of allergy to intravenous iron or any medications.
- Advise patients of the serious risks associated with Feraheme.
- Advise patients to immediately report any signs and symptoms of hypersensitivity that may develop during and following Feraheme administration, such as rash, itching, dizziness, lightheadedness, swelling and breathing problems. Advise patients to seek immediate medical attention if these occur
PATIENT INFORMATION FERAHEME (FER-UH-HEEM) (FERUMOXYTOL) INJECTION
Precautions with Alcohol
- Alcohol-Ferumoxytol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- FERAHEME®[2]
Look-Alike Drug Names
There is limited information regarding the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ "Ferumoxytol".