Coenzyme Q - cytochrome c reductase
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
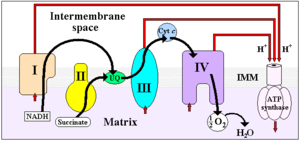
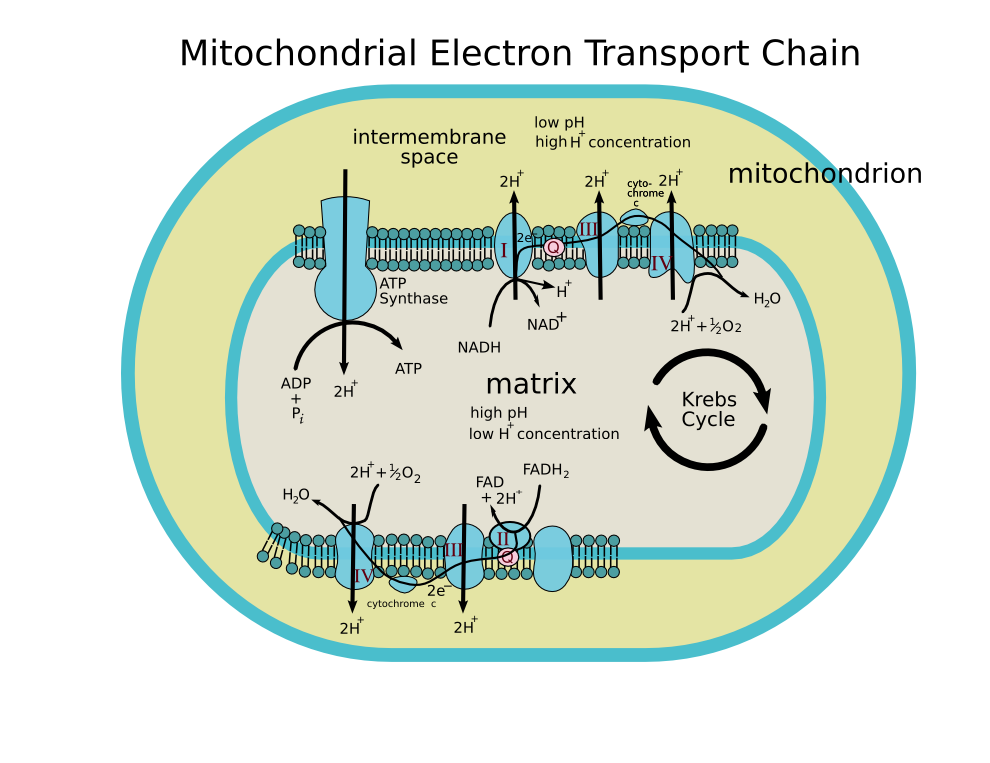
The coenzyme Q : cytochrome c — oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain (EC 1.10.2.2), playing a critical role in biochemical generation of ATP (oxidative phosphorylation). Complex III is a multisubunit transmembrane lipoprotein encoded by both the mitochondrial (cytochrome b) and the nuclear genomes (all other subunits). Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders.
Reaction
It catalyzes the reduction of cytochrome c by oxidation of coenzyme Q (CoQ) and the concomitant pumping of 4 protons from the mitochondrial matrix to the intermembrane space:
- QH2 + 2 cytochrome c (FeIII) + 2 H+in → Q + 2 cytochrome c (FeII) + 4 H+out
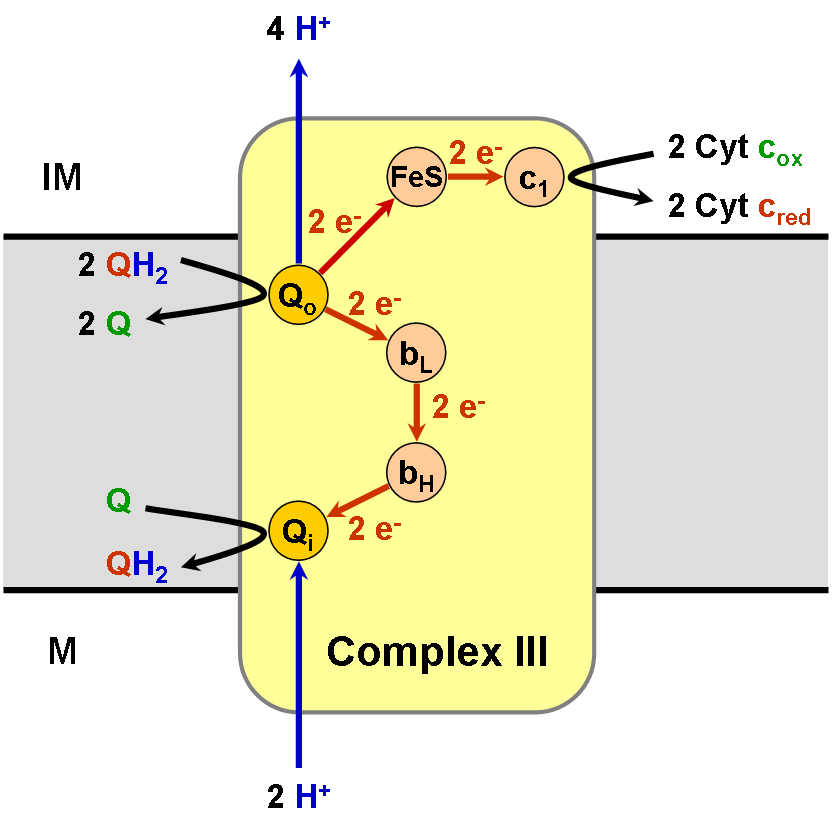
In the process called Q cycle,[1][2] two protons are consumed from the matrix (M), four protons are released into the inter membrane space (IM) and two electrons are passed to cytochrome c.
Structure
Compared to the other major proton pumping subunits of the electron transport chain, the number of subunits found can be small, as small as three polypeptide chains. This number does increase, and eleven subunits are found in higher animals [3]. Three subunits have prosthetic groups. The cytochrome b subunit has two b-type hemes (bL and bH), the cytochrome c sununit has one c-type heme (c1), and the Rieske Iron Sulfur Protein subunit (ISP) has a two iron, two sulfur iron-sulfur cluster (2Fe•2S).
Structures of complex III: PDB: 1KYO, PDB: 1L0L
Inhibitors of complex III
There are three distinct groups of Complex III inhibitors.
- Antimycin A binds to the Qi site and inhibits the transfer of electrons in Complex III from heme bH to oxidized Q (Qi site inhibitor).
- Myxothiazol and stigmatellin binds to the Qo site and inhibits the transfer of electrons from reduced QH2 to the Rieske Iron sulfur protein. Myxothiazol and stigmatellin bind to distinct pockets within the Qo site.
- Myxothiazol binds very close to cytochrome bL (hence termed a "proximal" inhibitor).
- Stigmatellin binds near the Rieske Iron sulfur protein, with which it strongly interacts.
Some have been commercialized as fungicides (the strobilurin derivates) and as anti-malaria agents (atovaquone).
Oxygen free radicals
A small fraction of electrons leave the electron transport chain before reaching complex IV. Premature electron leakage to oxygen results in the formation of superoxide. The relevance of this otherwise minor side reaction is that superoxide and other reactive oxygen species are highly toxic and are thought to play a role in several pathologies, including aging (the free radical theory of aging). Electron leakage occurs mainly at the Qo site and is stimulated by antimycin A. Antimycin A locks the b hemes in the reduced state by preventing their re-oxidation at the Qi site, which in turn causes the steady state concentrations of the Qo semiquinone to rise, the latter species reacting with oxygen to form superoxide. The effect of high membrane potential is thought to have a similar effect [4]. Superoxide produced at the Qo site can be released both into the mitochondrial matrix [5][6] and intermembrane space (from where it can reach the cytosol [7][8]). This could be explained by the fact that Complex III might produce superoxide as membrane permeable HO2 rather than as membrane impermeable O2- [9].
References
- ↑ Kramer, D. M.; Roberts, A. G.; Muller, F.; Cape, J.; Bowman, M. K. Q-cycle bypass reactions at the Qo site of the cytochrome bc1 (and related) complexes. Methods Enzymol. 382:21-45; 2004. PMID 15047094
- ↑ Crofts, A.R. (2004). The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 66, 689-733. PMID 14977419
- ↑ Iwata S., Lee J.W., Okada K., Lee J.K., Iwata M., Rasmussen B., Link T.A., Ramaswamy S., Jap B.K. (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281: 64-71
- ↑ Skulachev, V. P. (1996) Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 29, 169-202
- ↑ Muller, F. (2000) The Nature and Mechanism of Superoxide production by the Electron Transport Chain: Its relevance to aging. J. Amer. Aging Assoc. 23, 227-253
- ↑ Muller, F. L., Liu, Y. and Van Remmen, H. (2004) Complex III Releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279, 49064-49073
- ↑ Han, D., Williams, E. and Cadenas, E. (2001) Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 353, 411-416.
- ↑ Muller, F. (2000) The Nature and Mechanism of Superoxide production by the Electron Transport Chain: Its relevance to aging. J. Amer. Aging Assoc. 23, 227-253
- ↑ Muller, F. L., Liu, Y. and Van Remmen, H. (2004) Complex III Releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279, 49064-49073
See also
Additional images
-
ETC
-
ETC
Template:Diphenol family oxidoreductases
de:Cytochrom c Reduktase it:Ubiquinolo-citocromo c reduttasi