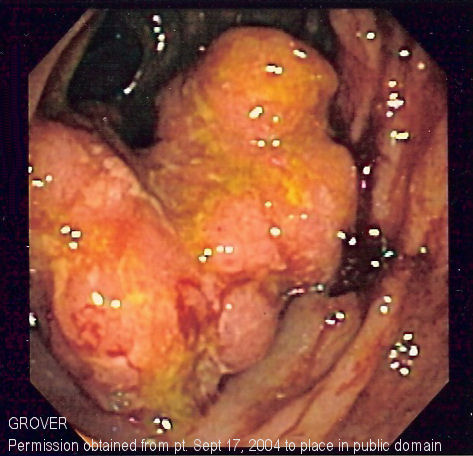
Colorectal cancer screening
|
Colorectal cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Colorectal cancer screening On the Web |
|
American Roentgen Ray Society Images of Colorectal cancer screening |
|
Risk calculators and risk factors for Colorectal cancer screening |
To view the screening of familial adenomatous polyposis (FAP), click here
To view the screening of hereditary nonpolyposis colorectal cancer (HNPCC), click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: ; Roukoz A. Karam, M.D.[2] Elliot B. Tapper, M.D.; Saarah T. Alkhairy, M.D.
Overview
Early detection of premalignant colorectal masses or early-stage colorectal cancers is essential in treating these patients and possibly preventing cancer or colorectal cancer related death. According to the USPSTF (United States Preventive Services Task Force): Screening for colorectal cancer is recommended among adults older than 50 years of age and do not have an increased risk of developing the disease (average-risk adults).
Clinical practice guidelines
According to the USPSTF (United States Preventive Services Task Force):[1]
- Screening for colorectal cancer is recommended among adults older than 50 years of age and do not have an increased risk of developing the disease (average-risk adults).
- Decision to screen for colorectal cancer among adults aged 76 to 85 years is an individual one.
- Screening would be more beneficial for healthier individuals that are able to undergo possible treatment.
- Screening for colorectal cancer among adults aged 86 years and older is not recommended.
- No preference is given to one screening modality over the other
- Decision should be shared and according to the patient's preferences when it comes to choosing an option.
- Screening options:[1]
- Colonoscopy
- FIT
- Fecal immunochemical testing for occult blood
- Flexible sigmoidoscopy
- Flexible sigmoidoscopy + FIT
- CT colonography
- FIT-DNA
- multitargeted stool DNA testing
- gFOBT
- Guaiac-based fecal occult blood testing
Screening for colorectal cancer in individuals that are at increased risk of developing the disease is different and depends on several factors:[2][1]
- Family history of colorectal cancer before age 50
- Begin screening at an earlier age
- Risk of rapid disease progression
- Perform screening more frequently
- Family history of HNPCC or FAP
- Use most sensitive screening modality: colonoscopy
Screening protocols summarized in the figures below:
Protocols have been summarized according to USPSTF guidelines.[1]



Types of Screening Methods
Fecal Occult Blood Testing
- A fecal occult blood test is a test for blood in the stool.
- There are two types of tests that can be used for detecting occult blood in stools:[1][3]
- FIT
- Fecal immunochemical testing for occult blood
- gFOBT
- Guaiac-based fecal occult blood testing
- FIT
- Use of low-sensitivity guaiac fecal tests is not recommended due to its of low sensitivity.[1]
Endoscopy
- A sigmoidoscopy is a lighted probe (sigmoidoscope) that is inserted into the rectum and lower colon to check for polyps and other abnormalities.[4]
- A colonoscopy is a lighted probe (colonoscope) that is inserted into the rectum and the entire colon to look for polyps and other abnormalities that may be caused by cancer. A colonoscopy has the advantage that if polyps are found during the procedure they can be immediately removed, and the tissue can also be taken for biopsy. The American Society for Gastrointestinal Endoscopy has released quality indicators for screening colonoscopy, which include:[4]
- Documentation of prep quality
- Photo documentation of cecal intubation
- Withdrawal time of 6 minutes or more
- Adenoma detection rate of greater than 25% in males and 15% in females greater than 50 years old
CT colonography
- Also known as Virtual Colonoscopy
- Requires special workstation software in order for the radiologist to interpret
- This technique is approaching colonoscopy in sensitivity for polyps
- Any polyps found must still be removed by standard colonoscopy[5]
Fecal DNA testing
- Multitargeted stool DNA testing
- Ability to detect mutations from DNA shed by colorectal cancer[6]
Accuracy of screening methods
Advanced adenomas are defined as being ≥10 mm, having villous histology, or having high grade dysplasia. Advanced neoplasia is defined as cancer or advanced adenoma[7].
| Method | Sensitivity | Specificity |
|---|---|---|
| Colorectal cancer | ||
| Colonoscopy | NA | NA |
| Fecal Immunochemical Test (FIT) | 74 | 94 |
| Cologuard (sDNA + FIT) | 93 | 85 |
| Advanced adenoma | ||
| Colonoscopy (for adenoma > 10 mm) | 89 to 95 | NA |
| Fecal Immunochemical Test (FIT) | 23 | 96 |
| Cologuard (sDNA + FIT) | 43 | 89 |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW; et al. (2016). "Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement". JAMA. 315 (23): 2564–2575. doi:10.1001/jama.2016.5989. PMID 27304597.
- ↑ Lieberman, David A. (2009). "Screening for Colorectal Cancer". New England Journal of Medicine. 361 (12): 1179–1187. doi:10.1056/NEJMcp0902176. ISSN 0028-4793.
- ↑ Duffy MJ, van Rossum LG, van Turenhout ST, Malminiemi O, Sturgeon C, Lamerz R; et al. (2011). "Use of faecal markers in screening for colorectal neoplasia: a European group on tumor markers position paper". Int J Cancer. 128 (1): 3–11. doi:10.1002/ijc.25654. PMID 20824704.
- ↑ 4.0 4.1 Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE; et al. (2006). "Quality indicators for colonoscopy". Am J Gastroenterol. 101 (4): 873–85. doi:10.1111/j.1572-0241.2006.00673.x. PMID 16635231.
- ↑ Morrin MM, LaMont JT (2003). "Screening virtual colonoscopy--ready for prime time?". N Engl J Med. 349 (23): 2261–4. doi:10.1056/NEJMe038181. PMID 14657435.
- ↑ Calistri D, Rengucci C, Bocchini R, Saragoni L, Zoli W, Amadori D (2003). "Fecal multiple molecular tests to detect colorectal cancer in stool". Clin Gastroenterol Hepatol. 1 (5): 377–83. PMID 15017656.
- ↑ Lin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E; et al. (2016). "Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force". U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. PMID 27441328.
- ↑ Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR (2021). "Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 325 (19): 1978–1997. doi:10.1001/jama.2021.4417. PMID 34003220 Check
|pmid=value (help).