Chlorite
- This discusses some chlorine compounds. Chlorite is also a type of mineral; see chlorite group.
The chlorite ion is ClO2−. A chlorite (compound) is a compound that contains this group, with chlorine in oxidation state +3. Chlorites are also known as salts of chlorous acid.
Oxidation states
Chlorine can assume oxidation states of −1, +1, +3, +5, or +7 corresponding to the anions Cl−, ClO−, ClO2−, ClO3−, or ClO4−, respectively (known as chloride, hypochlorite, chlorite, chlorate, and perchlorate.)
| oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| anion name | chloride | hypochlorite | chlorite | chlorate | perchlorate |
| formula | Cl− | ClO− | ClO2− | ClO3− | ClO4− |
| structure | The chloride ion | The hypochlorite ion | The chlorite ion | The chlorate ion | 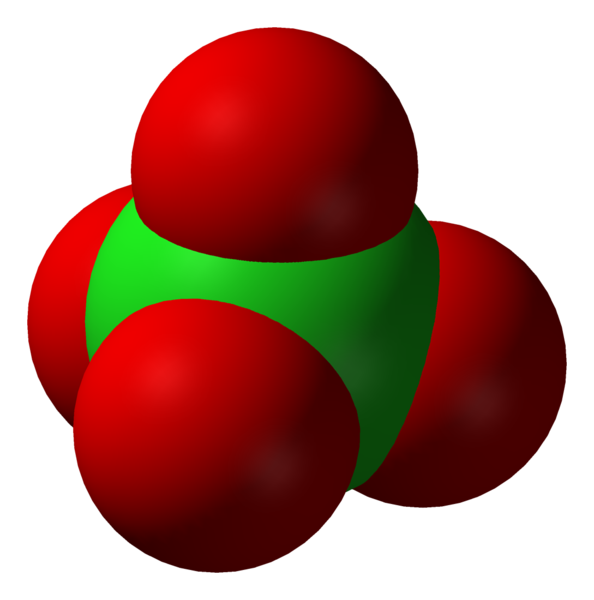
|
Some chlorite compounds
- sodium chlorite, NaClO2
- magnesium chlorite, Mg(ClO2)2
Manufacture
The free acid, chlorous acid, HClO2, is only stable at low concentrations. Since it cannot be concentrated, it is not a commercial product. However, the corresponding sodium salt, sodium chlorite, NaClO2 is stable and inexpensive enough to be commercially available. The corresponding salts of heavy metals (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and NH4+) decompose explosively with heat or shock.
Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate in a strong acid solution with a suitable reducing agent (for example, sodium chloride, sulfur dioxide, or hydrochloric acid). The chlorine dioxide is then absorbed into an alkaline solution and reduced with hydrogen peroxide, H2O2 yielding sodium chlorite.
Usage
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection in a few municipal water treatment plants after conversion to chlorine dioxide. An advantage in this application, as compared to the more commonly used chlorine, is that trihalomethanes are not produced from organic contaminants. Sodium chlorite, NaClO2 also finds application as a component of contact lens cleaning solution under the trade name purite.
Sodium chlorite, like many oxidizers, should be protected from inadvertent contamination by organic materials to avoid the formation of an explosive mixture.
References
- Chemistry of the Elements, N.N. Greenwood and A. Earnshaw, Pergamon Press, 1984.
- Kirk-Othmer Concise Encyclopedia of Chemistry, Martin Grayson, Editor, John Wiley & Sons, Inc., 1985