Cephalexin microbiology
| Cephalexin |
|---|
| CEPHALEXIN®,KEFLEX® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
| Indications and Usage |
| Contraindications |
| Warnings and Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| How Supplied |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
In vitro tests demonstrate that the cephalosporins are bactericidal because of their inhibition of cell-wall synthesis. Cephalexin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobes, Gram-positive:
Staphylococcus aureus (including penicillinase-producing strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Aerobes, Gram-negative:
Moraxella (Branhamella) catarrhalis
Note—Methicillin-resistant staphylococci and most strains of enterococci (Enterococcus faecalis [formerly Streptococcus faecalis]) are resistant to cephalosporins, including cephalexin. It is not active against most strains of Enterobacter spp., Morganella morganii, and Proteus vulgaris. It has no activity against Pseudomonas spp. or Acinetobacter calcoaceticus. Penicillin-resistant Streptococcus pneumoniae is usually cross-resistant to beta-lactam antibiotics.
Susceptibility Tests
Dilution techniques Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MIC’s). These MIC’s provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC’s should be determined using a standardized procedure. Standardized procedures are based on a dilution method1-3 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of cephalothin powder. The MIC values should be interpreted according to the following:
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
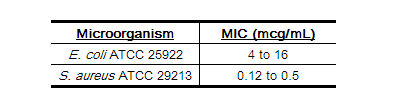
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard cephalothin powder should provide the following MIC values:
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2,3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 mcg cephalothin to test the susceptibility of microorganisms to cephalexin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 mcg cephalothin disk should be interpreted according to the following:
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cephalexin.
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30 mcg cephalothin disk should provide the following zone diameters in these laboratory test quality control strains:
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/050405s097lbl.pdf