Ceftaroline labels and packages
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Teflaro®(ceftaroline fosamil) for injection
Distributed by: Forest Pharmaceuticals, Inc. Subsidiary of Forest Laboratories, Inc. St. Louis, MO 63045, USA
Manufactured by: Facta Farmaceutici S.p.A. Nucleo Industriale S. Atto–S. Nicolò a Tordino 64020 Teramo, Italy
Teflaro® is a registered trademark of Forest Laboratories, Inc.
IF95USCFR08 ©2010-2013 Forest Laboratories, Inc. All rights reserved.
Principal Display Panel – 400 mg Vial Label
Rx only NDC 0456-0400-01
Teflaro® ceftaroline fosamil for injection 400 mg/vial
For Intravenous Infusion Only.
See package insert for dosage information.
Single use vial – Discard after use
 |
Principal Display Panel – 400 mg Carton Label
NDC 0456-0400-10 Rx only 10 Single-Use Vials
Teflaro® (ceftaroline fosamil) for injection
400 mg/vial
FOR INTRAVENOUS INFUSION ONLY CONSTITUTED SOLUTION MUST BE FURTHER DILUTED FOR INTRAVENOUS INFUSION
 |
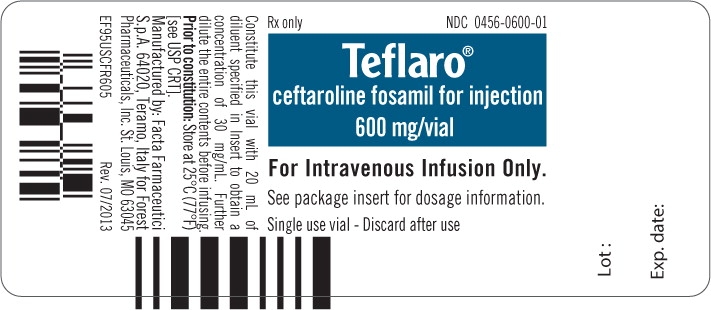
Principal Display Panel – 600 mg Vial Label
Rx only NDC 0456-0600-01
Teflaro® ceftaroline fosamil for injection 600 mg/vial
For Intravenous Infusion Only.
See package insert for dosage information.
Single use vial – Discard after use
 |
Principal Display Panel – 600 mg Carton Label
NDC 0456-0600-10 Rx only 10 Single-Use Vials
Teflaro® (ceftaroline fosamil) for injection
600 mg/vial
FOR INTRAVENOUS INFUSION ONLY CONSTITUTED SOLUTION MUST BE FURTHER DILUTED FOR INTRAVENOUS INFUSION
 |
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/200327s001lbl.pdf