Cefoxitin description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
DESCRIPTION
The drug chamber is filled with cefoxitin sodium USP, a semi-synthetic, broad-spectrum cepha antibiotic sealed under nitrogen for intravenous administration. It is derived from cephamycin C, which is produced by Streptomyces lactamdurans. Its chemical name is sodium (6R,7S)-3-(hydroxymethyl)-7-methoxy-8-oxo-7-[2-(2-thienyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate carbamate (ester). The empirical formula is C16H16N3NaO7S2, and the molecular weight is 449.44. The structural formula is:
Cefoxitin sodium contains approximately 53.8 mg (2.3 mEq) of sodium per gram of cefoxitin activity.
Cefoxitin for Injection and Dextrose Injection is supplied as a sterile, nonpyrogenic, single use packaged combination of cefoxitin (filled using Cefoxitin Sodium USP) and Dextrose Injection (diluent). After reconstitution, each 50 mL contains cefoxitin sodium equivalent to either 1 gram or 2 grams cefoxitin. The diluent chamber contains Dextrose Injection. The concentration of Dextrose Hydrous USP in Water for Injection USP has been adjusted to render the reconstituted drug product iso-osmotic. Dextrose Hydrous USP has been added to adjust the osmolality to approximately 290 mOsmol/kg (approximately 2 g (4% w/v) and 1.1 g (2.2% w/v) to the 1 g and 2 g doses, respectively). Dextrose Injection is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
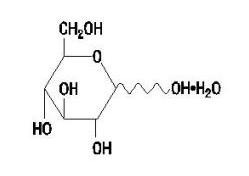
Dextrose Hydrous USP has the following structural (molecular) formula:
The molecular weight of Dextrose Hydrous USP is 198.17.
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use. When reconstituted according to instructions in the product labeling, the approximate osmolality of the reconstituted solution of Cefoxitin for Injection and Dextrose Injection is approximately 290 mOsmol/kg. After reconstitution, the pH is approximately 6.5. Solutions of Cefoxitin for Injection and Dextrose Injection range from colorless to light amber.
Not made with natural rubber latex, PVC or Di(2-ethylhexyl)phthalate (DEHP).
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/065214s013lbl.pdf