Aztreonam (inhalation)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aztreonam (inhalation) is an antibiotic that is FDA approved for the treatment of respiratory symptoms in cystic fibrosis (CF) patients with pseudomonas aeruginosa. Common adverse reactions include chest discomfort, abdominal pain, cough, nasal congestion, wheezing, and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Aztreonam is indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric.
- Aztreonam is indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric patients below the age of 7 years, patients with FEV1 <25% or >75% predicted, or patients colonized with Burkholderia cepacia.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of aztreonam and other antibacterial drugs, aztreonam should be used only to treat patients with CF known to have Pseudomonas aeruginosa in the lungs.
- Dosing Information - The recommended dose of aztreonam for both adults and pediatric patients 7 years of age and older is one single-use vial (75 mg of aztreonam.
Dosing Information
- The recommended dose of aztreonam for both adults and pediatric patients 7 years of age and older is one single-use vial (75 mg of aztreonam) reconstituted with 1 mL of sterile diluent administered 3 times a day for a 28-day course (followed by 28 days off aztreonam therapy). Dosage is not based on weight or adjusted for age. Doses should be taken at least 4 hours apart.
- Aztreonam is administered by inhalation using an Altera® Nebulizer System. Patients should use a bronchodilator before administration of aztreonam.
Instructions for Aztreonam Reconstitution
- Aztreonam should be administered immediately after reconstitution. Do not reconstitute aztreonam until ready to administer a dose.
- Take one amber glass vial containing aztreonam and one diluent ampule from the carton. To open the glass vial, carefully remove the metal ring by lifting or pulling the tab and remove the gray rubber stopper. Twist the tip off the diluent ampule and squeeze the liquid into the glass vial. Replace the rubber stopper, then gently swirl the vial until contents have completely dissolved.
- The empty vial, stopper, and diluent ampule should be disposed of properly upon completion of dosing.
Instructions for Aztreonam Administration
- Aztreonam is administered by inhalation using an Altera Nebulizer System. Aztreonam should not be administered with any other nebulizer. Aztreonam should not be mixed with any other drugs in the Altera Nebulizer Handset.
- Aztreonam is not for intravenous or intramuscular administration.
- Patients should use a bronchodilator before administration of aztreonam. Short-acting bronchodilators can be taken between 15 minutes and 4 hours prior to each dose of aztreonam. Alternatively, long-acting bronchodilators can be taken between 30 minutes and 12 hours prior to administration of aztreonam. For patients taking multiple inhaled therapies, the recommended order of administration is as follows: bronchodilator, mucolytics, and lastly, aztreonam.
- To administer aztreonam, pour the reconstituted solution into the handset of the nebulizer system. Turn the unit on. Place the mouthpiece of the handset in your mouth and breathe normally only through your mouth. Administration typically takes between 2 and 3 minutes. Further patient instructions on how to administer aztreonam are provided in the FDA-APPROVED PATIENT LABELING. Instructions on testing nebulizer functionality and cleaning the handset are provided in the Instructions for Use included with the nebulizer system.
- A dose of aztreonam consists of a single-use vial of sterile, lyophilized aztreonam (75 mg) reconstituted with a 1 mL ampule of sterile diluent (0.17% sodium chloride).
- A dose of aztreonam consists of a single-use vial of sterile, lyophilized aztreonam (75 mg) reconstituted with a 1 mL ampule of sterile diluent (0.17% sodium chloride). Reconstituted aztreonam is administered by inhalation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam (inhalation) in adult patients.
Non–Guideline-Supported Use
- Bacterial musculoskeletal infection[1]
- Febrile neutropenia, Empiric therapy[2]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Aztreonam (inhalation) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam (inhalation) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam (inhalation) in pediatric patients.
Contraindications
- Aztreonam is contraindicated in patients with a known allergy to aztreonam.
Warnings
Allergic Reactions
- Severe allergic reactions have been reported following administration of aztreonam for injection to patients with no known history of exposure to aztreonam. In addition, allergic reaction with facial rash, facial swelling, and throat tightness was reported with aztreonam in clinical trials. If an allergic reaction to aztreonam occurs, stop administration of aztreonam and initiate treatment as appropriate.
- Caution is advised when administering aztreonam to patients if they have a history of beta-lactam allergy, although patients with a known beta-lactam allergy have received aztreonam in clinical trials and no severe allergic reactions were reported. A history of allergy to beta-lactam antibiotics, such as penicillins, cephalosporins, and/or carbapenems, may be a risk factor, since cross-reactivity may occur.
Bronchospasm
- Bronchospasm is a complication associated with nebulized therapies, including aztreonam. Reduction of 15% or more in forced expiratory volume in 1 second (FEV1) immediately following administration of study medication after pretreatment with a bronchodilator was observed in 3% of patients treated with aztreonam.
Decreases in FEV1 After 28-Day Treatment Cycle
- In clinical trials, patients with increases in FEV1 during a 28-day course of aztreonam were sometimes treated for pulmonary exacerbations when FEV1 declined after the treatment period. Healthcare providers should consider a patient's baseline FEV1 measured prior to aztreonam therapy and the presence of other symptoms when evaluating whether post-treatment changes in FEV1 are caused by a pulmonary exacerbation.
Development of Drug-Resistant Bacteria
- Prescribing aztreonam in the absence of known Pseudomonas aeruginosa infection in patients with CF is unlikely to provide benefit and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of drugs cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of aztreonam was evaluated in 344 patients from two placebo-controlled trials and one open-label follow-on trial. In controlled trials, 146 patients with CF received 75 mg aztreonam 3 times a day for 28 days.
- TABLE 1 displays adverse reactions reported in more than 5% of patients treated with aztreonam 3 times a day in placebo-controlled trials. The listed adverse reactions occurred more frequently in aztreonam-treated patients than in placebo-treated patients.
Postmarketing Experience
- In addition to adverse reactions reported from clinical trials, the following possible adverse reactions have been identified during post-approval use of aztreonam.
- Because these events have been reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS
Drug Interactions
- No formal clinical studies of drug interactions with aztreonam have been conducted.
Use in Specific Populations
Pregnancy
- No reproductive toxicology studies have been conducted with aztreonam. However, studies were conducted with aztreonam for injection. Aztreonam has been shown to cross the placenta and enter fetal circulation. No evidence of embryo or fetotoxicity or teratogenicity has been shown in studies with pregnant rats and rabbits. In rats receiving aztreonam for injection during late gestation and lactation, no drug induced changes in maternal, fetal or neonatal parameters were observed. These animal reproduction and developmental toxicity studies used parenteral routes of administration that would provide systemic exposures far in excess of the average peak plasma levels measured in humans following aztreonam therapy.
- No adequate and well-controlled studies of aztreonam for injection or aztreonam in pregnant women have been conducted. Because animal reproduction studies are not always predictive of human response, aztreonam should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aztreonam (inhalation) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aztreonam (inhalation) during labor and delivery.
Nursing Mothers
- Following administration of aztreonam for injection, aztreonam is excreted in human milk at concentrations that are less than one percent of those determined in simultaneously obtained maternal serum. Peak plasma concentrations of aztreonam following administration of aztreonam (75 mg) are approximately 1% of peak concentrations observed following IV aztreonam (500 mg). Therefore, use of aztreonam during breastfeeding is unlikely to pose a risk to infants.
Pediatric Use
- Patients 7 years and older were included in clinical trials with aztreonam. Fifty-five patients under 18 years of age received aztreonam in placebo-controlled trials. No dose adjustments were made for pediatric patients. Pyrexia was more commonly reported in pediatric patients than in adult patients. Safety and effectiveness in pediatric patients below the age of 7 years have not been established.
Geriatic Use
Clinical trials of aztreonam did not include aztreonam-treated patients aged 65 years of age and older to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Aztreonam (inhalation) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aztreonam (inhalation) with respect to specific racial populations.
Renal Impairment
- Aztreonam is known to be excreted by the kidney. Placebo-controlled clinical trials with aztreonam excluded patients with abnormal baseline renal function (defined as serum creatinine greater than 2 times the upper limit of normal range). Given the low systemic exposure of aztreonam following administration of aztreonam, clinically relevant accumulation of aztreonam is unlikely to occur in patients with renal impairment. Therefore, aztreonam may be administered to patients with mild, moderate and severe renal impairment with no dosage adjustment.
Hepatic Impairment
There is no FDA guidance on the use of Aztreonam (inhalation) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aztreonam (inhalation) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aztreonam (inhalation) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Aztreonam (inhalation) Monitoring in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Aztreonam (inhalation) in the drug label.
Overdosage
- No overdoses have been reported with aztreonam in clinical trials to date. In clinical trials, 225 mg doses of aztreonam via inhalation were associated with higher rates of drug-related respiratory adverse reactions, particularly cough. Since the peak plasma concentration of aztreonam following administration of aztreonam (75 mg) is approximately 0.6 mcg/mL, compared to a serum concentration of 54 mcg/mL following administration of aztreonam for injection (500 mg), no systemic safety issues associated with aztreonam overdose are anticipated.
Pharmacology
Mechanism of Action
- Aztreonam is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Aztreonam has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
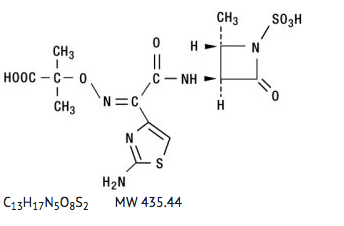
Structure
- Aztreonam is designated chemically as (Z)-2-[(2-amino-4-thiazolyl)(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. Structural formula:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Aztreonam (inhalation) in the drug label.
Pharmacokinetics
- Single 30-minute intravenous infusions of 500 mg, 1 g, and 2 g doses of Aztreonam in healthy subjects produced aztreonam peak serum levels of 54 mcg/mL, 90 mcg/mL, and 204 mcg/mL, respectively, immediately after administration; at 8 hours, serum levels were 1 mcg/mL, 3 mcg/mL, and 6 mcg/mL, respectively (Figure 1). Single 3-minute intravenous injections of the same doses resulted in serum levels of 58 mcg/mL, 125 mcg/mL, and 242 mcg/mL at 5 minutes following completion of injection.
Serum concentrations of aztreonam following completion of single intravenous infusions of 500 mg, 1 g, and 2 g doses are depicted in Figure 1.

- The serum levels of aztreonam following single 500 mg, 1 g, or 2 g intravenous doses of Aztreonam exceed the MIC90 for Neisseria sp., Haemophilus influenzae, and most genera of the Enterobacteriaceae for 8 hours (for Enterobacter sp., the 8-hour serum levels exceed the MIC for 80% of strains). For Pseudomonas aeruginosa, a single 2 g intravenous dose produces serum levels that exceed the MIC90 for approximately 4 to 6 hours. All of the above doses of Aztreonam result in average urine levels of aztreonam that exceed the MIC90 for the same pathogens for up to 12 hours.
- When aztreonam pharmacokinetics were assessed for adult and pediatric patients, they were found to be comparable (down to 9 months old). The serum half-life of aztreonam averaged 1.7 hours (1.5-2.0) in subjects with normal renal function, independent of the dose. In healthy subjects, based on a 70 kg person, the serum clearance was 91 mL/min and renal clearance was 56 mL/min; the apparent mean volume of distribution at steady-state averaged 12.6 liters, approximately equivalent to extracellular fluid volume.
- In elderly patients, the mean serum half-life of aztreonam increased and the renal clearance decreased, consistent with the age-related decrease in creatinine clearance.1-4 The dosage of Aztreonam should be adjusted accordingly. In patients with impaired renal function, the serum half-life of aztreonam is prolonged. The serum half-life of aztreonam is only slightly prolonged in patients with hepatic impairment since the liver is a minor pathway of excretion.
- Average urine concentrations of aztreonam were approximately 1100 mcg/mL, 3500 mcg/mL, and 6600 mcg/mL within the first 2 hours following single 500 mg, 1 g, and 2 g intravenous doses of Aztreonam (30-minute infusions), respectively. The range of average concentrations for aztreonam in the 8- to 12-hour urine specimens in these studies was 25 to 120 mcg/mL. In healthy subjects, aztreonam is excreted in the urine about equally by active tubular secretion and glomerular filtration.
- Approximately 60% to 70% of an intravenous dose was recovered in the urine by 8 hours. Urinary excretion of a single intravenous dose was essentially complete by 12 hours after injection. About 12% of a single intravenous radiolabeled dose was recovered in the feces. Unchanged aztreonam and the inactive beta-lactam ring hydrolysis product of aztreonam were present in feces and urine.
- Intravenous administration of a single 500 mg or 1 g dose of Aztreonam every 8 hours for 7 days to healthy subjects produced no apparent accumulation of aztreonam or modification of its disposition characteristics; serum protein binding averaged 56% and was independent of dose.
- Renal function was monitored in healthy subjects given aztreonam; standard tests (serum creatinine, creatinine clearance, BUN, urinalysis, and total urinary protein excretion) as well as special tests (excretion of N-acetyl-β-glucosaminidase, alanine aminopeptidase, and β2-microglobulin) were used. No abnormal results were obtained.
Aztreonam achieves measurable concentrations in the following body fluids and tissues: none|400px
- The concentration of aztreonam in saliva at 30 minutes after a single 1 g intravenous dose (9 patients) was 0.2 mcg/mL; in human milk at 2 hours after a single 1 g intravenous dose (6 patients), 0.2 mcg/mL; in amniotic fluid at 6 to 8 hours after a single 1 g intravenous dose (5 patients), 2 mcg/mL. The concentration of aztreonam in peritoneal fluid obtained 1 to 6 hours after multiple 2 g intravenous doses ranged between 12 mcg/mL and 90 mcg/mL in 7 of 8 patients studied.
- Aztreonam given intravenously rapidly reaches therapeutic concentrations in peritoneal dialysis fluid; conversely, aztreonam given intraperitoneally in dialysis fluid rapidly produces therapeutic serum levels.
- Concomitant administration of probenecid or furosemide and aztreonam causes clinically insignificant increases in the serum levels of aztreonam. Single-dose intravenous pharmacokinetic studies have not shown any significant interaction between aztreonam and concomitantly administered gentamicin, nafcillin sodium, cephradine, clindamycin, or metronidazole. No reports of disulfiram-like reactions with alcohol ingestion have been noted; this is not unexpected since aztreonam does not contain a methyl-tetrazole side chain.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Aztreonam (inhalation) in the drug label.
Clinical Studies
- Aztreonam was evaluated over a period of 28 days of treatment in a randomized, double-blind, placebo-controlled, multicenter trial that enrolled patients with CF and P. aeruginosa. This trial was designed to evaluate improvement in respiratory symptoms. Patients 7 years of age and older and with FEV1 of 25% to 75% predicted were enrolled. All patients received aztreonam or placebo on an outpatient basis administered with the Altera Nebulizer System. All patients were required to take a dose of an inhaled bronchodilator (beta-agonist) prior to taking a dose of aztreonam or placebo. Patients were receiving standard care for CF, including drugs for obstructive airway diseases.
- The trial enrolled 164 patients with CF and P. aeruginosa. The mean age was 30 years, and the mean baseline FEV1 % predicted was 55%; 43% were females and 96% were Caucasian. These patients were randomized in a 1:1 ratio to receive either aztreonam (75 mg) or volume-matched placebo administered by inhalation 3 times a day for 28 days. Patients were required to have been off antibiotics for at least 28 days before treatment with study drug. The primary efficacy endpoint was improvement in respiratory symptoms on the last day of treatment with aztreonam or placebo. Respiratory symptoms were also assessed two weeks after the completion of treatment with aztreonam or placebo. Changes in respiratory symptoms were assessed using a questionnaire that asks patients to report on symptoms like cough, wheezing, and sputum production.
- Improvement in respiratory symptoms was noted for aztreonam-treated patients relative to placebo-treated patients on the last day of drug treatment. Statistically significant improvements were seen in both adult and pediatric patients, but were substantially smaller in adult patients. Two weeks after completion of treatment, a difference in respiratory symptoms between treatment groups was still present, though the difference was smaller.
- Pulmonary function, as measured by FEV1 (L), increased from baseline in patients treated with aztreonam (see FIGURE 1). The treatment difference at Day 28 between aztreonam-treated and placebo-treated patients for percent change in FEV1 (L) was statistically significant at 10% (95% CI: 6%, 14%). Improvements in FEV1 were comparable between adult and pediatric patients. Two weeks after completion of drug treatment, the difference in FEV1 between aztreonam and placebo groups had decreased to 6% (95% CI: 2%, 9%).

How Supplied
- Each kit for a 28-day course of aztreonam contains 84 sterile vials of aztreonam and 88 ampules of sterile diluent packed in 2 cartons, each carton containing a 14-day supply. The four additional diluent ampules are provided in case of spillage.

Storage
- Aztreonam vials and diluent ampules should be stored in the refrigerator at 2 °C to 8 °C (36 °F to 46 °F) until needed. Once removed from the refrigerator, aztreonam and diluent may be stored at room temperature (up to 25 °C / 77 °F) for up to 28 days. Do not separate the aztreonam vials from the diluent ampules. aztreonam should be protected from light.
- Do not use aztreonam if it has been stored at room temperature for more than 28 days. Do not use aztreonam beyond the expiration date stamped on the vial. Do not use diluent beyond the expiration date embossed on the ampule.
- Aztreonam should be used immediately upon reconstitution. Do not reconstitute more than one dose at a time.
- Do not use diluent or reconstituted aztreonam if it is cloudy or if there are particles in the solution.
Images
Drug Images
{{#ask: Page Name::Aztreonam (inhalation) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Aztreonam (inhalation) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Aztreonam (inhalation) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CAYSTON®[3]
Look-Alike Drug Names
There is limited information regarding Aztreonam (inhalation) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Conrad DA, Williams RR, Couchman TL, Lentnek AL (1991). "Efficacy of aztreonam in the treatment of skeletal infections due to Pseudomonas aeruginosa". Rev Infect Dis. 13 Suppl 7: S634–9. PMID 2068473 PMID: 2068473 Check
|pmid=value (help). - ↑ Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA; et al. (2011). "Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america". Clin Infect Dis. 52 (4): e56–93. doi:10.1093/cid/cir073. PMID 21258094 PMID: 21258094 Check
|pmid=value (help). - ↑ "CAYSTON- aztreonam".
