Anti-inhibitor coagulant complex
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* Thrombotic and thromboembolic events have been reported during postmarketing surveillance following infusion of FEIBA VH or Feiba NF, particularly following the administration of high doses and/or in patients with thrombotic risk factors.
|
Overview
Anti-inhibitor coagulant complex is a human antihemophilic factor that is FDA approved for the {{{indicationType}}} of spontaneous bleeding episodes or in hemophilia A and hemophilia B patients with inhibitors. There is a Black Box Warning for this drug as shown here. Common adverse reactions include high antibody titer.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hemophilia A and B
- Feiba NF (Anti-Inhibitor Coagulant Complex) is indicated for the control of spontaneous bleeding episodes or to cover surgical interventions in hemophilia A and hemophilia B patients with inhibitors.
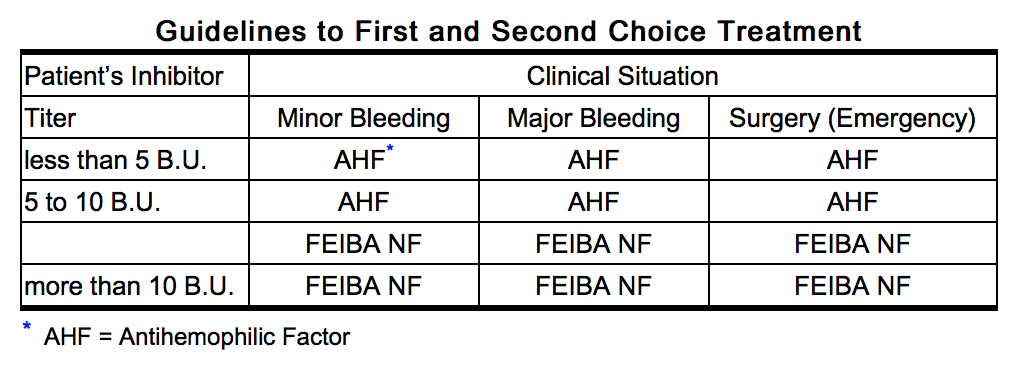
- Clinical experience suggests that patients with a Factor VIII inhibitor titer of less than 5 B.U. may be successfully treated with Antihemophilic Factor. Patients with titers ranging between 5 and 10 B.U. may either be treated with Antihemophilic Factor or Feiba NF. Cases with Factor VIII inhibitor titers greater than 10 B.U. have generally been refractory to treatment with Antihemophilic Factor.
- Inadequate response to treatment may result from an abnormal platelet count or impaired platelet function3-5 that were present before treatment with Feiba NF, nanofiltered and vapor-heated.
- Dosing Information
- Treatment should be initiated and supervised by a physician experienced in the management of hemophilia.
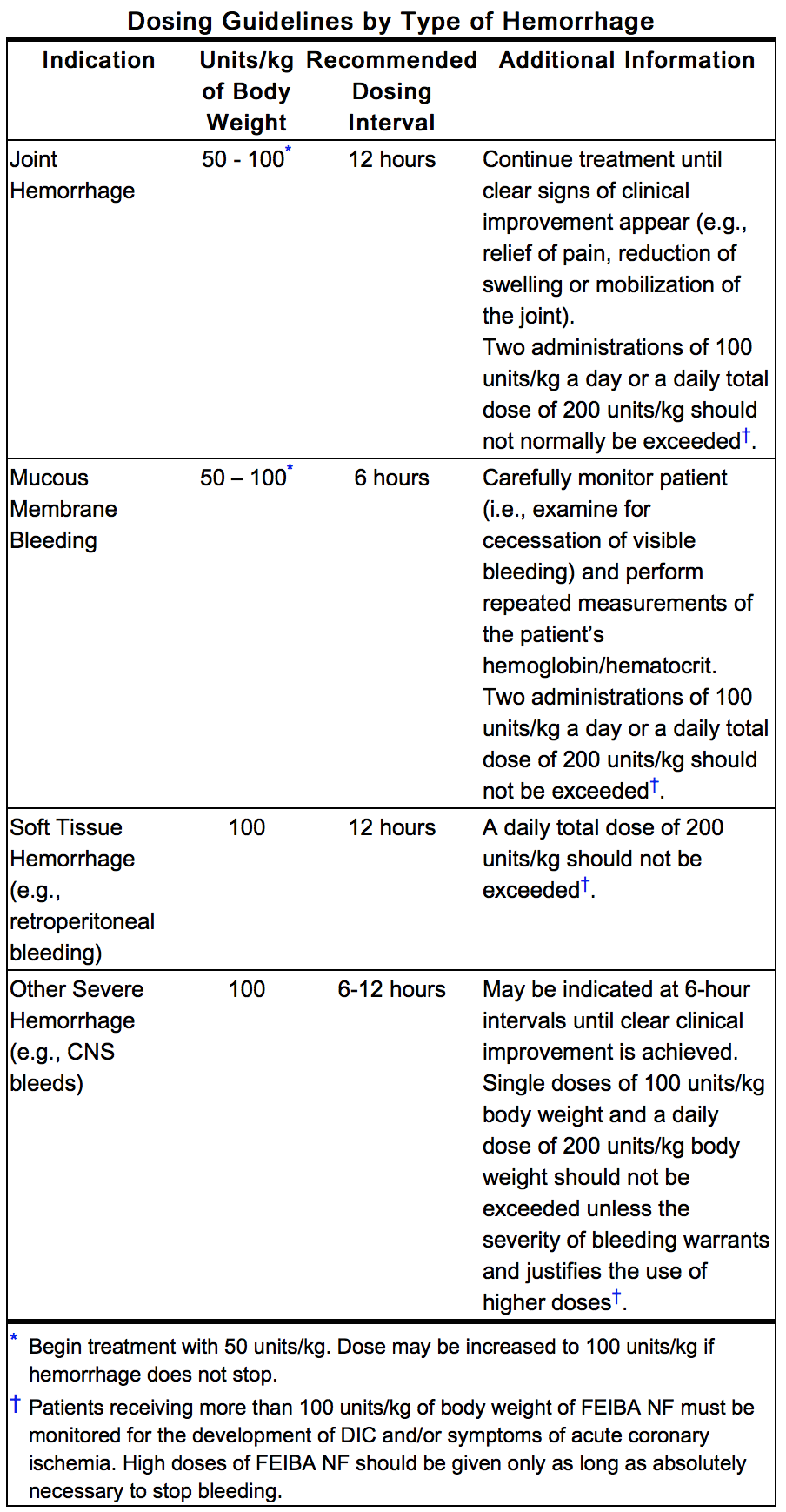
- Clinical trials demonstrated that the response to treatment with FEIBA may differ from patient to patient with no correlation to the patient’s inhibitor titer. Response may also vary between different types of hemorrhage (e.g. joint hemorrhage vs. CNS hemorrhage). As a general guideline, a dosage range of 50 to 100 Units of Feiba NF, per kg of body weight is recommended. However, care should be taken to distinguish between the following four indications:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anti-inhibitor coagulant complex in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anti-inhibitor coagulant complex in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- No data are available regarding the use of Feiba NF in newborns.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anti-inhibitor coagulant complex in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anti-inhibitor coagulant complex in pediatric patients.
Contraindications
- The use of Feiba NF is contraindicated:
- in patients who have known anaphylactic or severe hypersensitivity reactions to the product.
- in patients who are known to have a normal coagulation mechanism.
- for the treatment of bleeding episodes resulting from coagulation factor deficiencies in the absence of inhibitors to coagulation factor VIII or coagulation factor IX.
- in patients with significant signs of disseminated intravascular coagulation (DIC).
- in patients with acute thrombosis or embolism (including myocardial infarction).
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* Thrombotic and thromboembolic events have been reported during postmarketing surveillance following infusion of FEIBA VH or Feiba NF, particularly following the administration of high doses and/or in patients with thrombotic risk factors.
|
- At first sign or symptom of an infusion/hypersensitivity reaction or a thrombotic/thromboembolic event, Feiba NF administration should be stopped immediately and diagnostic and therapeutic measures initiated as appropriate.
Anaphylaxis and Hypersensitivity Reactions
- Allergic-type hypersensitivity reactions, including severe anaphylactoid reactions, have been reported following the infusion of FEIBA. The symptoms include urticaria, angioedema, gastrointestinal manifestations, bronchospasm, and hypotension; these reactions can be severe and can be systemic (e.g., anaphylaxis with urticaria and angioedema, bronchospasm, and circulatory shock). If signs and symptoms of severe allergic reactions occur, immediately discontinue administration of Feiba NF and provide appropriate supportive care. Have epinephrine immediately available to treat, if appropriate, any acute severe hypersensitivity reactions. Other infusion reactions, such as chills, pyrexia, and hypertension have also been reported.
- In patients with known or suspected hypersensitivity to Feiba NF, the expected benefit and the risk of re-exposure must be carefully weighed, taking into account the known or suspected type of the patient’s hypersensitivity (allergic or non-allergic), including potential remedial and/or preventative therapy or alternative therapeutic agents.
Thrombotic and Thromboembolic Events
- Thrombotic and thromboembolic events (including disseminated intravascular coagulation (DIC), venous thrombosis, pulmonary embolism, myocardial infarction, and stroke) have been reported following infusion of FEIBA VH or Feiba NF, particularly following the administration of high doses and/or in patients with thrombotic risk factors. The possible presence of such risk factors should always be considered in patients with congenital and acquired hemophilia. Thromboembolic events are well recognized potential complications of FEIBA infusion. Many of these events occurred with doses above 200 units/kg/day or in patients with other risk factors for thromboembolic events. A single dose of 100 units/kg body weight and a daily dose of 200 units/kg body weight should not be exceeded unless the severity of bleeding warrants and justifies the use of higher doses. Patients receiving more than 100 units/kg of body weight of Feiba NF must be monitored for the development of DIC and/or symptoms of acute coronary ischemia. High doses of Feiba NF should be given only as long as absolutely necessary to stop bleeding.
- Patients with disseminated intravascular coagulation (DIC), advanced atherosclerotic disease, crush injury, septicemia, or concomitant treatment with recombinant factor VIIa have an increased risk of developing thrombotic events due to circulating tissue factor (TF) or predisposing coagulopathy.
- FEIBA VH or Feiba NF should be used with particular caution and only if there are no therapeutic alternatives in patients at risk of DIC, arterial or venous thrombosis.
- Feiba NF should not be given to patients with significant signs of disseminated intravascular coagulation (DIC) or fibrinolysis. Infusion of Feiba NF should not exceed single dosage of 100 units per kg of body weight and daily doses of 200 units per kg body weight. Thrombotic events have been identified through post-marketing surveillance following FEIBA use for each of the approved indications. The incidence of thrombotic events cannot be determined from post-marketing data.
Transmission of Infectious Agents
- Feiba NF (Anti-Inhibitor Coagulant Complex), nanofiltered and vapor heated, is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the variant Creutzfeldt-Jakob disease (vCJD) agent. There is also the possibility that unknown infectious agents may be present in such products. The risk that such products will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, such products may still potentially transmit disease.
- Record the batch number of the product every time Feiba NF is administered to a patient, and consider appropriate vaccination (against hepatitis A and B virus) of patients who receive Feiba NF.
- ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare providers to Baxter Healthcare Corporation, at 1-800-423-2862 (in the U.S.) and/or to FDA Med Watch (1-800-FDA-1088 orwww.fda.gov/medwatch).
Precautions
- Thrombotic and Thromboembolic Complications
- Caution should be used when administering FEIBA VH or Feiba NF to patients with an increased risk of thromboembolic complications. These include, but are not limited to, patients with a history of coronary heart disease, liver disease, disseminated intravascular coagulation, post-operative immobilization, elderly patients and neonates. In each of these situations, the potential benefit of treatment with FEIBA VH or Feiba NF should be weighed against the risk of these complications.
- Patients who receive FEIBA VH or Feiba NF should be monitored for development of signs or symptoms of DIC, acute coronary ischemia, and signs and symptoms of other thrombotic and thromboembolic events. If clinical signs of intravascular coagulation occur, which include changes in blood pressure, changes in pulse rate, respiratory distress, chest pain and/or cough, the infusion should be stopped promptly and appropriate diagnostic and therapeutic measures are to be initiated.
- Laboratory indications of DIC are decreased fibrinogen, decreased platelet count, and/or presence of fibrin-fibrinogen degradation products (FDP). Other indications of DIC include significantly prolonged thrombin time, prothrombin time, or partial thromboplastin time.
- Concominant Medications
- It has been reported that Feiba NF and antifibrinolytics have been given simultaneously without complications. No adequate and well-controlled studies of the combined or sequential use of Feiba NF and recombinant Factor VIIa or antifibrinolytics have been conducted. The possibility of thrombotic events should be considered when systemic antifibrinolytics such as tranexamic acid and aminocaproic acid are used during treatment with Feiba NF. It is recommended not to use antifibrinolytics until 12 hours after the administration of Feiba NF.
- Discordant Response to Bypassing Agents
- Due to patient-specific factors the response to a bypassing agent can vary, and in a given bleeding situation patients experiencing insufficient response to one agent may respond to another agent.
- Anamnestic Responses
- Administration of Feiba NF to patients with inhibitors may result in an initial “anamnestic” rise in inhibitor levels. Anamnestic responses with rise in Factor VIII inhibitor titer have been observed in 20% of the cases. These anamnestic rises have not been observed to interfere with the efficacy of FEIBA. Upon continued administration of Feiba NF, inhibitors may decrease over time.
Adverse Reactions
Clinical Trials Experience
- The adverse reactions presented in this section have been reported from two studies with FEIBA for the treatment of bleeding episodes in pediatric and adult patients with hemophilia A or B and inhibitors to factors VIII or IX. One study also enrolled acquired hemophilia patients with factor VIII inhibitors (4 of 49 patients).
Postmarketing Experience
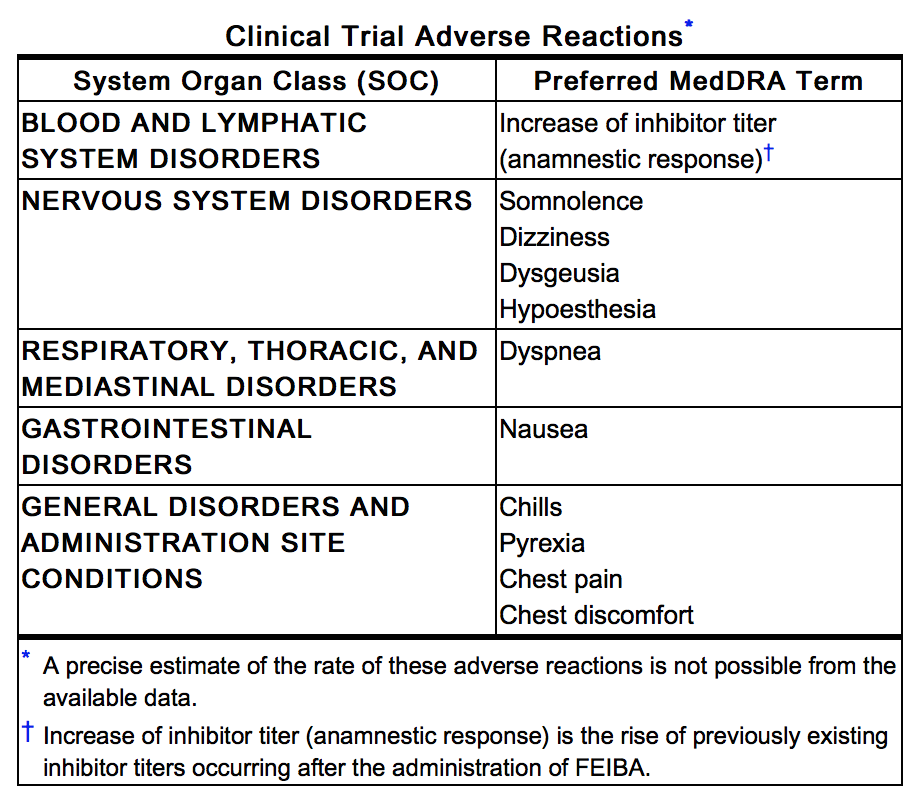
- The following adverse reactions have been reported in the post-marketing experience, listed by MedDRA System Organ Class (SOC), then by Preferred Term in order of severity, where feasible. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Vascular
Thrombosis, Venous thrombosis, Arterial thrombosis, Hypotension, Hypertension, Flushing
Cardiovascular
Myocardial infarction, Tachycardia
Digestive
Vomiting, Diarrhea, Abdominal discomfort
Hematologic and Lymphatic
Disseminated intravascular coagulation
Neurologic
Paresthesia, Thrombotic stroke, Embolic stroke, Headache
Respiratory
Pulmonary embolism, Bronchospasm, Wheezing, Cough
Skin and Hypersensitivy Reactions
Anaphylactic reaction, Hypersensitivity, Angioedema, Urticaria, Pruritus, Rash
Miscellaneous
Malaise, Feeling hot, Injection site pain.
Drug Interactions
- No compatibility studies have been performed with Feiba NF. Therefore, Feiba NF must not be mixed with other medicinal products or solvents.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- The safety of Feiba NF during pregnancy has not been established. Animal reproduction studies have not been conducted with Feiba NF. It is also not known whether Feiba NF can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing Feiba NF.Pregnancy and the postpartum period are characterized by an increased risk of thrombosis, and several complications of pregnancy are associated with an increased risk of DIC.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Anti-inhibitor coagulant complex in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Anti-inhibitor coagulant complex during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Feiba NF is administered to a nursing woman. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing Feiba NF.
Pediatric Use
- No data are available regarding the use of Feiba NF in newborns.
Geriatic Use
- Clinical studies of Feiba NF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Anti-inhibitor coagulant complex with respect to specific gender populations.
Race
There is no FDA guidance on the use of Anti-inhibitor coagulant complex with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Anti-inhibitor coagulant complex in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Anti-inhibitor coagulant complex in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Anti-inhibitor coagulant complex in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Anti-inhibitor coagulant complex in patients who are immunocompromised.
Administration and Monitoring
Administration
Reconstitution
- Feiba NF contains no preservatives. Aseptic technique should be used throughout the entire reconstitution process and the solution should then be used immediately.
- Allow the unopened vials of Feiba NF (concentrate) and Sterile Water for Injection (diluent) to reach room temperature (not above 37°C, 98°F).
- Remove caps from the concentrate and diluent vials to expose central portions of the rubber stoppers.
- Disinfect the rubber stoppers of both vials using a germicidal solution. Place the vials on an even surface and allow them to dry.
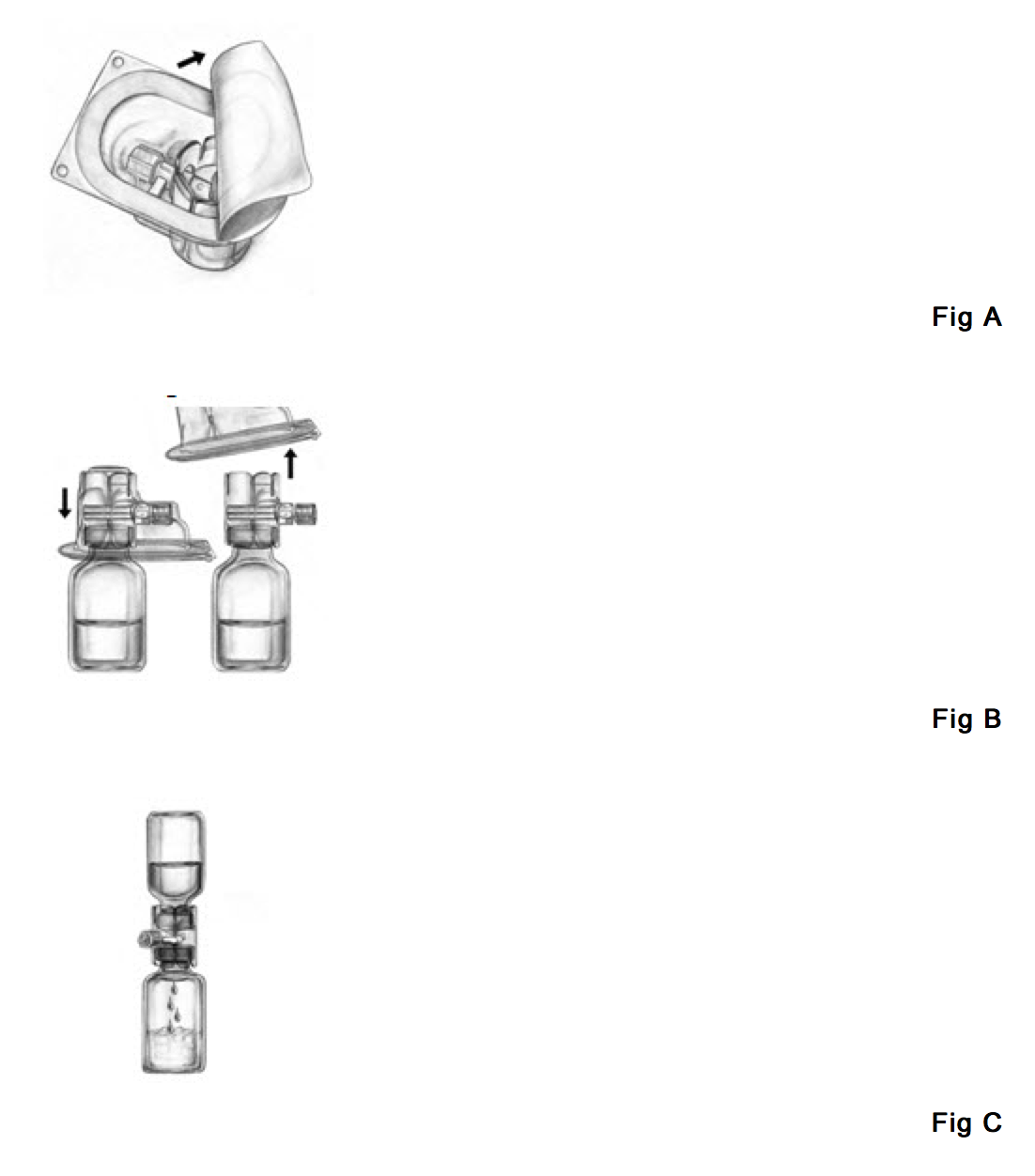
- Open the package of BAXJECT device by peeling away the lid without touching the inside (Fig. A).
- Do not remove the device from the package. Turn the package over and insert the plastic spike through diluent stopper. (Fig. B).
- Grip the package at its edge and pull the package off the device (Fig. B).
- Turn the system over, so that the vial is on top. Quickly insert the other plastic spike into the Feiba NF stopper (Fig. C). The vacuum will draw the diluent into the Feiba NF vial. Please make sure that the connection of the two vials should be done expeditiously to close the open fluid pathway created by the first insertion of the spike to the diluent vial.
- Swirl gently until Feiba NF is completely dissolved. Make sure that Feiba NF has been dissolved completely; otherwise, active material will not pass through the device filter.
- Do not refrigerate after reconstitution!
- After complete reconstitution of Feiba NF its injection or infusion should be commenced as promptly as practicable, but must be completed within three hours following reconstitution. The solution must be given by intravenous injection or intravenous drip infusion.
- Rate of Administration:
- The maximum injection or infusion rate must not exceed 2 units per kg of body weight per minute. For a patient with a body weight of 75 kg, this corresponds to an infusion rate of 2.5 - 7.5 mL per minute depending on the number of units per vial (see label on vial).
- Intravenous Injection or Infusion:
- Inspect for particulate matter and discoloration after reconstituting the concentrate as described under Reconstitution prior to administration. The appearance of the solution should be colorless to slightly yellowish and essentially free of visible particles. Do not use solutions that are cloudy or have deposits.
- Mixing of Feiba NF with other products or substances must be avoided. It is advisable to flush venous access lines with isotonic saline prior to and after infusion of Feiba NF.
- If devices other than those supplied with Feiba NF are used, ensure use of an adequate filter.
- Plastic Luer lock syringes are recommended for use with this product since protein such as Feiba NF tends to stick to the surface of all-glass syringes.
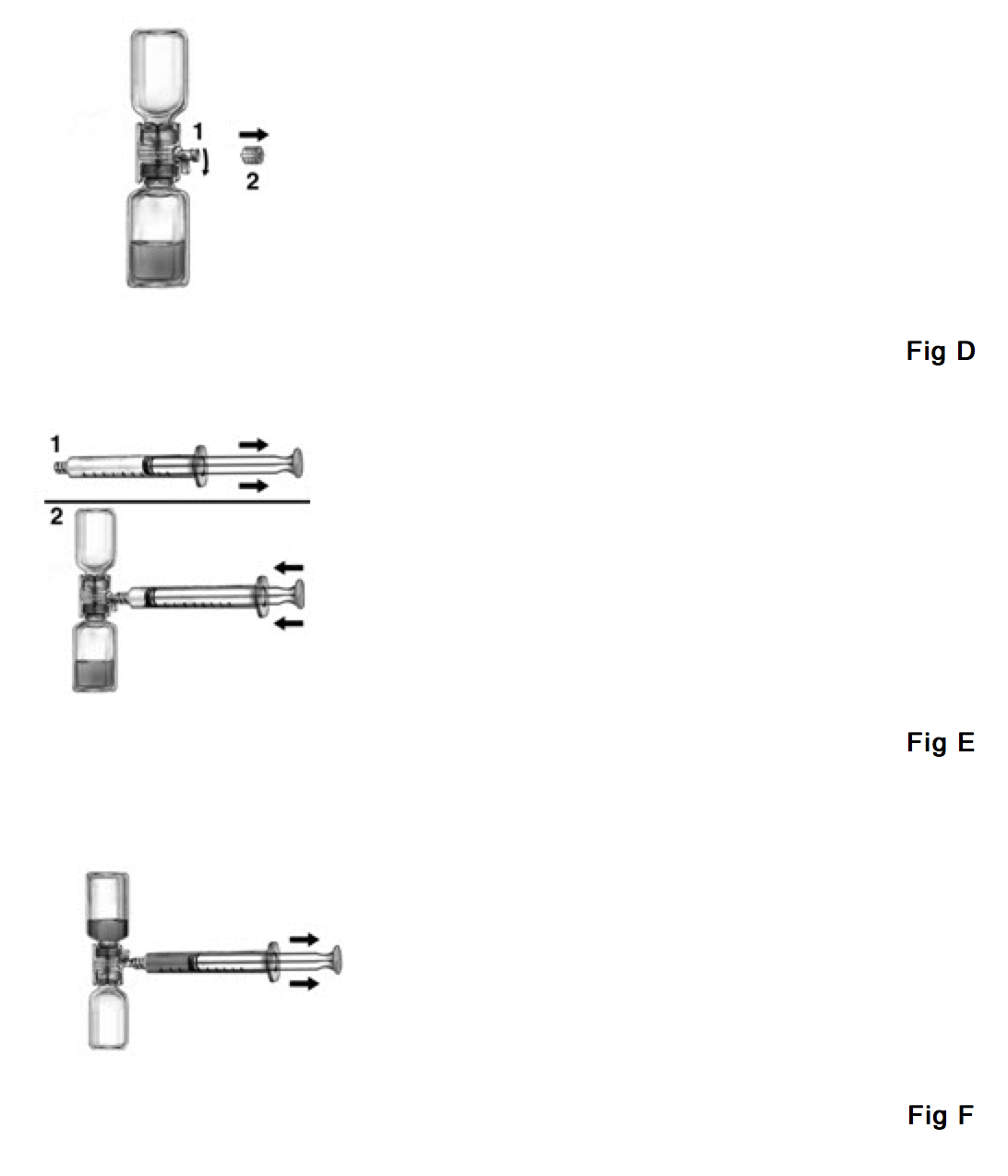
- Turn the BAXJECT device handle down towards the Feiba NF concentrate vial and remove the cap attached to the syringe connection of the BAXJECT device (Fig. D).
- Draw air into the syringe, connect the syringe to the BAXJECT device, inject air into the concentrate vial (Fig. E).
- While keeping the syringe plunger in place, turn the system upside down (concentrate vial now on top). Draw the concentrate into the syringe by pulling the plunger back slowly (Fig. F).
- Turn the BAXJECT handle to its original position (facing side way).
- Disconnect the syringe, attach a suitable needle and inject or infuse intravenously as instructed under Rate of Administration.
Monitoring
- Perform a platelet count at the time of initial use of the product and if anticipated treatment responses are not achieved. A sufficient number of functionally intact platelets is considered to be necessary for the efficacy of the product.
- No direct measure of hemostatic efficacy with bypassing agents is available. Coagulation tests such as whole blood clotting time (WBCT), aPTT and TEG may not correlate with clinical improvement. For this reason, attempts at normalizing these values by increasing the dose of Feiba NF may not be successful and are strongly discouraged because of the potential hazard of producing DIC by overdose.
IV Compatibility
There is limited information regarding IV Compatibility of Anti-inhibitor coagulant complex in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- The risk of thrombotic and thromboembolic events (including DIC, myocardial infarction, venous thrombosis, and pulmonary embolism) may be increased with high doses of Feiba NF. Some of the reported events occurred with doses above 200 U/kg or with patients with other risk factors for thromboembolic events.
Chronic Overdose
There is limited information regarding Chronic Overdose of Anti-inhibitor coagulant complex in the drug label.
Pharmacology
There is limited information regarding Anti-inhibitor coagulant complex Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Mechanism of Action of Anti-inhibitor coagulant complex in the drug label.
Structure
- Feiba NF (Anti-Inhibitor Coagulant Complex), nanofiltered and vapor heated, is a freeze-dried sterile human plasma fraction with Factor VIII inhibitor bypassing activity. In vitro, Feiba NF shortens the activated partial thromboplastin time (APTT) of plasma containing Factor VIII inhibitor. Factor VIII inhibitor bypassing activity is expressed in arbitrary units. One unit of activity is defined as that amount of Feiba NF that shortens the APTT of a high titer Factor VIII inhibitor reference plasma to 50% of the blank value.
- Feiba NF contains Factors II, IX, and X, mainly non-activated, and Factor VII mainly in the activated form. The product contains approximately equal unitages of Factor VIII inhibitor bypassing activity and Prothrombin Complex Factors. In addition, 1–6 units of Factor VIII coagulant antigen (FVIII C:Ag) per mL are present. The preparation contains only traces of factors of the kinin generating system. It contains no heparin.
- Reconstituted Feiba NF contains 4 mg of trisodium citrate and 8 mg of sodium chloride per mL.
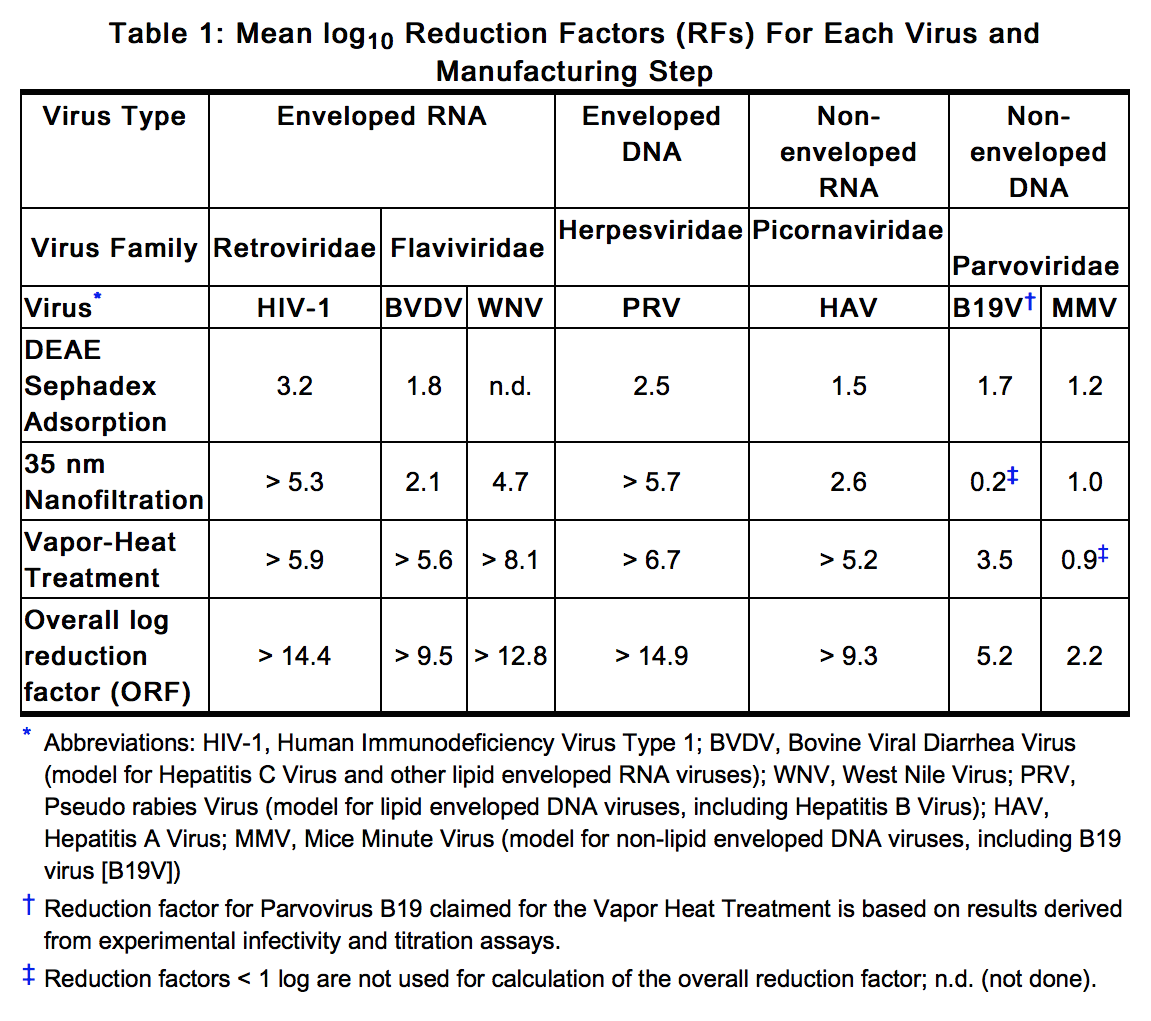
- Feiba NF is manufactured from large plasma pools of human plasma. Screening against potentially infectious agents begins with the donor selection process and continues throughout plasma collection and plasma preparation. Each individual plasma donation used in the manufacture of Feiba NF is collected only at FDA approved blood establishments and is tested by FDA licensed serological tests for Hepatitis B Surface Antigen (HBsAg), and for antibodies to Human Immunodeficiency Virus (HIV-1/HIV-2) and Hepatitis C Virus (HCV) in accordance with the U.S. regulatory requirements. As an additional safety measure, mini-pools of the plasma are tested for the presence of HIV-1 and HCV by FDA licensed Nucleic Acid Testing (NAT) and found negative. In addition, two dedicated and independent virus removal/inactivation steps have been integrated into the manufacturing process, namely 35 nm nanofiltration and a vapor heat treatment process. In addition, the DEAE-Sephadex adsorption contributes to the virus safety profile of Feiba NF. Despite these measures, such products can still potentially transmit disease.
- In vitro spiking studies have been used to validate the capability of the manufacturing process to remove and inactivate viruses. To establish the minimum applicable virus clearance capacity of the manufacturing process, these virus clearance studies were performed under extreme conditions (e.g. at minimum incubation times and temperatures below specifications for vapor-heat treatment). Virus clearance studies for Feiba NF performed in accordance with good laboratory practices have demonstrated, that the manufacturing process of Feiba NF ensures a high margin of safety with respect to adventitious viruses (Table 1).
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Anti-inhibitor coagulant complex in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Anti-inhibitor coagulant complex in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Anti-inhibitor coagulant complex in the drug label.
Clinical Studies
- Feiba NF is identical in formulation to FEIBA VH. Biochemical and preclinical studies have confirmed the comparability of Feiba NF and FEIBA VH.
- The safety and efficacy of FEIBA has been demonstrated by two prospective clinical trials1,2. The first, conducted by Sixma and collaborators, was a randomized double-blind study comparing the effect of FEIBA and PROTHROMPLEX IMMUNO (a non-activated prothrombin complex concentrate) in 15 patients with hemophilia A and inhibitors to Factor VIII. A total of 150 bleeding episodes (primarily joint and musculoskeletal plus a few mucocutaneous) were treated. A single dose of 88 Units per kg of body weight was used uniformly for treatments with FEIBA . The study showed that, based on subjective patient evaluation, FEIBA was fully effective in 41.0% and partly effective in 24.6% of episodes (i.e. combined effectiveness of 65.6%), while PROTHROMPLEX IMMUNO was rated fully effective in 25.0% and partly effective in 21.4% of episodes (i.e. combined effectiveness of 46.4%).
- The second study with FEIBA was a multicenter study conducted by Hilgartner et al2. This study was conducted in 44 hemophilia A subjects with inhibitors, 3 hemophilia B subjects with inhibitors and 2 acquired FVIII inhibitor subjects. It was designed to evaluate the efficacy of FEIBA in the treatment of joint, mucous membrane, musculocutaneous and emergency bleeding episodes such as central nervous system hemorrhages and surgical bleedings. In 49 patients with inhibitor titers of greater than 5 Bethesda Units (from nine co-operating hemophilia centers), 489 single doses were given for the treatment of 165 bleeding episodes. The usual dosage was 50 Units per kg of body weight, repeated at 12-hour intervals (6-hour intervals in mucous membrane bleedings), if necessary. Bleeding was controlled in 153 episodes (93%). In 130 (78%) of the episodes, hemostasis was achieved with one or more infusions within 36 hours. Of these, 36% were controlled with one infusion within 12 hours. An additional 14% of episodes responded after more than 36 hours.
- Of the 489 single doses, 18 (3.7%) caused minor transient reactions in recipients. Out of 49 patients, 10 (20%) showed a rise in their inhibitor titers. In 5 of these patients (10%), the rise was tenfold or more. However, of these 10 patients, 3 had received Factor VIII or Factor IX concentrates within 2 weeks prior to treatment with FEIBA. These anamnestic rises have not been observed to interfere with the efficacy of FEIBA.
How Supplied
- Feiba NF is available in single-dose vials in the following nominal dosage strengths:
- Blue - 500 Units per vial (NDC 64193-223-02)
- Green - 1000 Units per vial (NDC 64193-224-02)
- Purple - 2500 Units per vial (NDC 64193-225-02)
- The number of Units of Factor VIII inhibitor bypassing activity is stated on the label of each vial.
- Feiba NF is packaged with a suitable volume (20 mL or 50 mL) of Sterile Water for Injection, U.S.P., one BAXJECT Needleless Transfer Device, and one Package Insert.
- The 50 mL SWFI stoppers are not latex-free and may contain Dry Natural Rubber Latex.
- Storage
- Store at room temperature, not to exceed 25°C (77°F).
- Avoid freezing, which may damage the diluent vial.
- Store in the original package in order to protect from light.
Storage
There is limited information regarding Anti-inhibitor coagulant complex Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Anti-inhibitor coagulant complex |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Anti-inhibitor coagulant complex |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients receiving Feiba NF of the benefits and risks associated with treatment. Advise patients of the early signs of hypersensitivity reactions. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment.
- Some viruses, such as parvovirus B19 or hepatitis A, are particularly difficult to remove or inactivate at this time. Pregnant women and immune-compromised individuals should be informed of the symptoms of parvovirus B19 (i.e. fever, drowsiness, chills, and runny nose followed about two weeks later by a rash, and joint pain). Inform all patients of the symptoms of hepatitis A infection (i.e. several days to weeks of poor appetite, tiredness, and low-grade fever followed by nausea, vomiting, and upper abdominal pain). Dark urine and a yellowed complexion are also common symptoms of Hepatitis A infection.
- Advise patients/caregivers to record the batch number of the product every time Feiba NF is administered outside a healthcare setting.
- Advise patients to report any adverse reactions or problems following Feiba NF administration to their physician or healthcare provider.
Non-Hemophilic Patients
- Non-hemophilic patients with acquired inhibitors against Factors VIII, IX or XII may have both a bleeding tendency and an increased risk of thrombosis at the same time.
Precautions with Alcohol
- Alcohol-Anti-inhibitor coagulant complex interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Feiba NF®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Feiba NF (anti-inhibitor coagulant complex) kit".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Anti-inhibitor coagulant complex
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Anti-inhibitor coagulant complex |Label Name=Anti-inhibitor coagulant complex07.png
}}
{{#subobject:
|Label Page=Anti-inhibitor coagulant complex |Label Name=Anti-inhibitor coagulant complex08.png
}}
{{#subobject:
|Label Page=Anti-inhibitor coagulant complex |Label Name=Anti-inhibitor coagulant complex09.png
}}
{{#subobject:
|Label Page=Anti-inhibitor coagulant complex |Label Name=Anti-inhibitor coagulant complex10.png
}}