Transcatheter aortic valve replacement: Difference between revisions
No edit summary |
|||
| (215 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{| class="infobox" style="float:right;" | |||
|- | |||
| [[File:Siren.gif|30px|link=Aortic stenosis resident survival guide]]|| <br> || <br> | |||
| [[Aortic stenosis resident survival guide|'''Resident'''<br>'''Survival'''<br>'''Guide''']] | |||
|} | |||
{{SI}} | {{SI}} | ||
''' | '''Editor(s)-In-Chief:''' Roger Laham, M.D., [[C. Michael Gibson|C. Michael Gibson, M.S., M.D.]]; {{AE}} {{Sara.Zand}} Saleh El Dassouki, MD [mailto:seldassouki@hotmail.com]; {{MehdiP}}; {{TarekNafee}}; {{AKK}} | ||
'' | {{SK}} TAVI; Edward's valve; Edward's SAPIEN transcatheter heart valve; CoreValve; percutaneous aortic valve replacement; PAVR; SAVR; Surgical aortic valve replacement | ||
{{ | ==Overview== | ||
{{Main|TAVR_vs_SAVR}} | |||
[[TAVI]] is a safe and effective procedure for the treatment of severe symptomatic [[AS]] in all [[adults]] regardless of estimated [[surgical]] risk. | |||
In [[patients]] considering a [[bioprosthetic]] [[AVR]], the next step is the choice between [[SAVR]] and [[TAVI]]. When the [[surgery]] is high risk or prohibitive, [[TAVI]] or [[palliative care]] would be focused. If both [[SAVR]] and [[TAVI]] are options, [[TAVI]] durability and [[life expectancy]] should be considered. Data about the durability of [[SAVR]] is for more than 50 years, but it limited to 5 years for [[TAVI]]. [[SAVR]] valve deteriorates typically after >10 years, so longer-term is needed for evaluation of [[TAVI]] durability. Earlier [[RCTs]] comparing [[SAVR]] and [[TAVI]] in [[patients]] with a higher surgical risk included only [[older]] [[patients]], with a mean age in the mid-80s. However, more recent [[RCTs]] that included [[patients]] at low to intermediate surgical risk had a [[mean age]] in the mid-70s. There is no data about the use of [[TAVI]] in [[patients]] younger than 65 years of [[age]]. Some younger [[patients]] with [[comorbid]] [[conditions]] have a limited [[life expectancy]], whereas some [[older]] [[patients]] have a longer-than-average [[life expectancy]]. [[Comorbid]] [[cardiac]] and noncardiac [[conditions]], [[frailty]], [[dementia]], and other factors that affect [[longevity]] or [[quality of life]] should be individualized for making decision the type of approach. [[Mortality rate ]] in [[TAVI]] is lower than [[SAVR]] and is associated with a shorter hospital [[length of stay]], more rapid return to normal [[activities]], lower risk of transient or permanent [[atrial fibrillation]], lower risk of [[stroke]], less [[bleeding]], and less [[pain]] than [[SAVR]]. On the other hand, [[SAVR]] is associated with a lower risk of [[paravalvular leak]], less need for [[valve intervention]], and less need for a [[permanent pacemaker]]. | |||
==Comparing [[transcatheter aortic valve replacement]] ([[TAVR]]) and [[surgical aortic valve replacement]] ([[SAVR]])== | |||
{| style="border: 2px solid #4479BA; align="left" | |||
! style="width: 200px; background: #4479BA;" | {{fontcolor|#FFF|Characteristics}} | |||
! style="width: 300px; background: #4479BA;" | {{fontcolor|#FFF|Favors SAVR}} | |||
! style="width: 400px; background: #4479BA;" | {{fontcolor|#FFF|Favors TAVI}} | |||
! style="width: 400px; background: #4479BA;" | {{fontcolor|#FFF|Favors palliation}} | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Age]]/[[life expectancy]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Younger]] [[age]]/longer [[life expectancy]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Older]] age/fewer expected remaining years of [[life]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Limited [[life expectancy]] | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Valve]] [[anatomy]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[BAV]] | |||
*Subaortic ([[LV outflow tract]]) calcification | |||
*[[Rheumatic valve disease]] | |||
*Small or large [[aortic]] annulus | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" |Calcific [[AS]] of a [[trileaflet valve]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Prosthetic valve]] preference | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Mechanical or surgical [[bioprosthetic]] valve preferred | |||
*Concern for [[patient–prosthesis mismatch]] ([[annular]] enlargement might be considered) | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Bioprosthetic]] valve preferred | |||
*Favorable ratio of [[life expectancy]] to valve durability | |||
* In [[TAVI]] [[valve area]] is larger than same size [[SAVR]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Concurrent [[cardiac]] [[conditions]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Aortic]] dilation | |||
*Severe [[primary MR]] | |||
*Severe [[CAD]] requiring [[bypass grafting]] | |||
*[[Septal hypertrophy]] requiring [[myectomy]] | |||
*[[AF]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe [[calcification]] of the ascending [[aorta]] ([[porcelain aorta]]) | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Irreversible severe [[LV systolic dysfunction]] | |||
*Severe [[MR]] attributable to annular [[calcification]] | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Noncardiac [[conditions]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Severe [[lung]], [[liver]], or [[rena]]l disease | |||
*[[Mobility]] issues (high procedural risk with [[sternotomy]]) | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Symptoms]] likely attributable to noncardiac [[conditions]] | |||
*Severe [[dementia]] | |||
*Moderate to severe involvement of ≥2 other [[organ]] systems | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Frailty]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Not frail or few [[frailty]] measures | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" |[[Frailty]] likely to improve after [[TAVI]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe frailty unlikely to improve after [[TAVI]] | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Estimated procedural or surgical risk of [[SAVR]] or [[TAVI]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[SAVR]] risk low | |||
*[[TAVI]] risk high | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[TAVI]] risk low to medium | |||
*[[SAVR]] risk high to prohibitive | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Prohibitive [[SAVR]] risk (>15%) or post-[[TAVI]] [[life expectancy]] <1 y | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Procedural]] specific impediments | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Valve]] anatomy, annular size, or low [[coronary]] ostial height precludes [[TAVI]] | |||
*Vascular access does not allow [[transfemoral]] [[TAVI]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Previous [[cardiac surgery]] with at-risk [[coronary grafts]] | |||
*Previous [[chest irradiation]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Valve]] anatomy, annular size, or [[coronary]] ostial height precludes [[TAVI]] | |||
*[[Vascular access]] does not allow transfemoral [[TAVI]] | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Goals of Care and [[patient]] preferences and [[values]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Less uncertainty about valve durability | |||
*Avoid repeat intervention | |||
*Lower risk of [[permanent pacer]] | |||
*[[Life]] prolongation | |||
*[[Symptom]] relief | |||
*Improved long-term [[exercise capacity]] and [[quality of life]] | |||
*Avoid [[vascular]] complications | |||
* Longer [[hospital stay]], [[pain]] in [[recovery period]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*Uncertainty about valve durability and possible repeat [[intervention]] | |||
*Higher risk of [[permanent pacer]] | |||
*Life prolongation | |||
*[[Symptom]] relief | |||
*Improved [[exercise capacity]] and [[quality of life]] | |||
* Shorter [[hospital stay]], less postprocedural [[pain]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | | |||
*[[Life prolongation]] not an important goal | |||
*Avoid [[futile]] or unnecessary diagnostic or therapeutic procedures | |||
*Avoid procedural [[stroke]] risk | |||
*Avoid possibility of [[cardiac pace maker implantation]] | |||
|- | |||
|} | |||
<span style="font-size:85%">'''Abbreviations:''' | |||
'''MR:''' [[Mitral regurgitation]]; | |||
'''AVA:''' [[Aortic valve area]]; | |||
'''LVOT:''' [[Left ventricular outflow tract]] ; | |||
'''SAVR:''' [[Surgical aortic valve replacement]]; | |||
'''TAVI:''' [[Transcatheter aortic valve implantation]]; | |||
'''AF:''' [[Atrial fibrillation]]; | |||
'''CAD:''' [[Coronary artery disease]]; | |||
'''AS:''' [[Aortic stenosis]] | |||
</span> | |||
<br> | |||
{| | |||
! colspan="2" style="background: PapayaWhip;" align="center" + |The above table adopted from 2020 AHA Guideline<ref name="pmid33332149">{{cite journal |vauthors=Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C |title=2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines |journal=Circulation |volume=143 |issue=5 |pages=e35–e71 |date=February 2021 |pmid=33332149 |doi=10.1161/CIR.0000000000000932 |url=}}</ref> | |||
|- | |||
|} | |||
{| style="border: 2px solid #4479BA; align="left" | |||
! style="width: 200px; background: #4479BA;" | {{fontcolor|#FFF|Clinical characteristics}} | |||
! style="width: 300px; background: #4479BA;" | {{fontcolor|#FFF|Favours TAVI}} | |||
! style="width: 400px; background: #4479BA;" | {{fontcolor|#FFF|Favours SAVR}} | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Lower [[surgical]] risk | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Higher [[surgical]] risk | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Younger [[age]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Older [[age]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Previous [[cardiac]] [[surgery]] ([[CABG]]) | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe [[frailty]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Endocarditis]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
! style="width: 200px; background: #4479BA;" | {{fontcolor|#FFF|Anatomical and procedural factors}} | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[TAVI]] feasible via [[transfemoral]] approach | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Inaccessable Transfemoral approach or [[SAVR]] feasible | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Sequelae of [[chest]] [[radiation]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Porcelain [[aorta]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | High likelihood of severe [[patient]]-[[prosthesis]] mismatch ([[AVA]] <0.65 cm2/m2 [[BSA]]) | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe [[chest]] deformity or [[scoliosis]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Unsuitable [[aortic]] annular dimensions for [[TAVI]] device | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Bisuspid aortic valve]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Unfavourable [[valve]] morphology for [[TAVI]] (high risk of [[coronary]] obstruction due to low [[coronary]] ostia or heavy [[leaflet]]/[[LVOT]] [[calcification]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | [[Thrombus]] in [[aorta]] or [[left ventricle]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
! style="width: 200px; background: #4479BA;" | {{fontcolor|#FFF|Concomitant cardiac conditions requiring interventio}} | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Significant multi-vessel [[CAD]] requiring [[surgical]] [[revascularization]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe primary [[mitral valve]] [[disease]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Severe [[tricuspid valve]] [[disease]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Significant dilatation/[[aneurysm]] of the [[aortic]] root and/or [[ascending aorta]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|- | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | Septal [[hypertrophy]] requiring [[myomectomy]] | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | _ | |||
| style="padding: 0 5px; background: #F5F5F5; text-align: left;" | + | |||
|} | |||
<span style="font-size:85%">'''Abbreviations:''' | |||
'''AV:''' [[Aortic valve]]; | |||
'''AVA:''' [[Aortic valve area]]; | |||
'''LVOT:''' [[Left ventricular outflow tract]] ; | |||
'''SAVR:''' [[Surgical aortic valve replacement]]; | |||
'''TAVI:''' [[Transcatheter aortic valve implantation]]; | |||
'''BSA:''' [[Body surface area]]; | |||
'''CAD:''' [[Coronary artery disease]] | |||
</span> | |||
<br> | |||
{| | |||
! colspan="2" style="background: PapayaWhip;" align="center" + |The above table adopted from 2021 ESC Guideline<ref name="pmid34453165">{{cite journal |vauthors=Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W |title=2021 ESC/EACTS Guidelines for the management of valvular heart disease |journal=Eur Heart J |volume=43 |issue=7 |pages=561–632 |date=February 2022 |pmid=34453165 |doi=10.1093/eurheartj/ehab395 |url=}}</ref> | |||
|- | |||
|} | |||
<br> | |||
{| style="cellpadding=0; cellspacing= 0; width: 800px;" | |||
|- | |||
| style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF;" align=center |'''Recommendations for choice of SAVR versus TAVI for whom a bioprosthetic AVR is approperiate''' | |||
''''' | |- | ||
|style="font-size: 100; padding: 0 5px; background: #B8B8B8" align=left | ''' ([[AHA guidelines classification scheme|Class I, Level of Evidence A]]):''' | |||
|- | |||
|style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width: 70%" align=left| | |||
❑ [[SAVR]] is recommended for symptomatic and asymptomatic severe [[AS]], and any indication for [[AVR]], who are < 65 years and life expectancy >20 year<br> | |||
❑Either [[SAVR]] or transfemoral [[TAVI]] is recommended in [[symptomatic]] severe [[AS]] who are 65-80 years after evaluation about [[life expectancy]] and [[valve]] durability<br> | |||
❑[[TAVI]] is recommended in symptomatic [[severe]] [[AS]] who are >80 years or younger [[patients]] with [[life expectancy]] <10 years and no anatomic contraindication for transfemoral [[TAVI]]<br> | |||
❑ [[TAVI]] is recommended in symptomatic [[patients]] with severe [[AS]] in any age and high surgical risk or prohibitive for [[surgery]] when predicted [[survival]] is > 12 months after [[TAVI]] with acceptable [[quality of life]]<br> | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #B8B8B8" align=left | ''' ([[ AHA guidelines classification scheme|Class I, Level of Evidence B]]):''' | |||
|- | |||
|style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width: 70%" align=left| | |||
❑[[SAVR]] is recommended in preference to [[TAVI]] in asymptomatic severe [[AS]] and abnormal [[exercise stress test]], very severe [[AS]], rapid progression, and [[elevated]] [[BNP]]<br> | |||
❑ In [[asymptomatic]] [[severe]] [[AS]] in age ≤ 80 years of age and [[LVEF]] < 50 and no anatomic contraindications for transfemoral [[TAVI]], making decision between [[TAVI]] and [[SAVR]] is similar to [[symptomatic]] [[patients]]<br> | |||
== | |- | ||
|style="font-size: 100; padding: 0 5px; background: #B8B8B8" align=left | ''' ([[ AHA guidelines classification scheme|Class I, Level of Evidence C]]):''' | |||
|- | |||
|style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width: 70%" align=left| | |||
❑For symptomatic severe [[AS]] when predictive [[survival]] is <12 months after [[TAVI]] or [[SAVR]] and minimal improvement in [[quality of life]] is expected, [[palliative care]] is recommended<br> | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #B8B8B8" align=left | ''' ([[ AHA guidelines classification scheme|Class IIb, Level of Evidence C]]):''' | |||
|- | |||
|style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width: 70%" align=left| | |||
❑For critically ill [[patients]] with severe [[AS]], percutaneous [[aortic ballon dilation]] is a bridge to [[TAVI]] or [[SAVR]]<br> | |||
| | |||
|} | |||
{| | |||
! colspan="2" style="background: PapayaWhip;" align="center" + |The above table adopted from 2020 AHA Guideline<ref name="pmid33332149">{{cite journal |vauthors=Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C |title=2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines |journal=Circulation |volume=143 |issue=5 |pages=e35–e71 |date=February 2021 |pmid=33332149 |doi=10.1161/CIR.0000000000000932 |url=}}</ref> | |||
|- | |||
|} | |||
<span style="font-size:85%">'''Abbreviations:''' | |||
'''SAVR:''' [[ Surgical aortic valve replacement]]; | |||
'''TAVI:''' [[Transcutaneous aortic valve implantation]]; | |||
'''AS:''' [[Aortic stenosis]]; | |||
'''LVEF:'''[[Left ventricular ejection fraction]] ; | |||
</span> | |||
<br> | |||
==Notes== | |||
* [[Outcomes]] and durability after [[SAVR]] were excellent for both [[mechanical]] and [[bioprosthetic valves]].<ref name="pmid31433469">{{cite journal |vauthors=Burke CR, Kirkpatrick JN, Otto CM |title=Goals of care in patients with severe aortic stenosis |journal=Eur Heart J |volume=41 |issue=8 |pages=929–932 |date=February 2020 |pmid=31433469 |doi=10.1093/eurheartj/ehz567 |url=}}</ref><ref name="pmid30312995">{{cite journal |vauthors=Kumar A, Sato K, Banerjee K, Narayanswami J, Betancor J, Menon V, Mohananey D, Anumandla AK, Sawant AC, Krishnaswamy A, Tuzcu EM, Jaber W, Mick S, Svensson LG, Popović ZB, Blackstone EH, Kapadia SR |title=Hemodynamic durability of transcatheter aortic valves using the updated Valve Academic Research Consortium-2 criteria |journal=Catheter Cardiovasc Interv |volume=93 |issue=4 |pages=729–738 |date=March 2019 |pmid=30312995 |doi=10.1002/ccd.27927 |url=}}</ref> | |||
* Earlier [[RCTs]] compared [[SAVR]] and [[TAVI]] in higher surgical risk [[patients]] including only [[older]] [[patients]], with a [[mean age]] in the mid-80s. However, more recent [[RCTs]] included [[patients]] at low to intermediate [[surgical risk]] with a [[mean age]] in the mid-70s, but there were very few [[patients ]]<65 years of [[age]], so the [[evidence base]] cannot be extrapolated to these [[patients]].<ref name="pmid27683246">{{cite journal |vauthors=Siemieniuk RA, Agoritsas T, Manja V, Devji T, Chang Y, Bala MM, Thabane L, Guyatt GH |title=Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis |journal=BMJ |volume=354 |issue= |pages=i5130 |date=September 2016 |pmid=27683246 |pmc=5040923 |doi=10.1136/bmj.i5130 |url=}}</ref> | |||
* [[Valve]] durability should be considered in younger [[patients]] with longer [[life expectancy]] and lower [[surgical]] risk. <ref name="pmid28684441">{{cite journal |vauthors=Foroutan F, Guyatt GH, Otto CM, Siemieniuk RA, Schandelmaier S, Agoritsas T, Vandvik PO, Bhagra S, Bagur R |title=Structural valve deterioration after transcatheter aortic valve implantation |journal=Heart |volume=103 |issue=23 |pages=1899–1905 |date=December 2017 |pmid=28684441 |doi=10.1136/heartjnl-2017-311329 |url=}}</ref> | |||
* [[SAVR]] is recommended for adults <65 years of age unless [[life expectancy]] is limited by comorbid [[cardiac]] or noncardiac [[conditions]].<ref name="pmid31329852">{{cite journal |vauthors=Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, Pilgrim T, Petrinic T, Nikolakopoulou A, Salanti G, Søndergaard L, Verma S, Jüni P, Windecker S |title=Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis |journal=Eur Heart J |volume=40 |issue=38 |pages=3143–3153 |date=October 2019 |pmid=31329852 |doi=10.1093/eurheartj/ehz275 |url=}}</ref> | |||
* [[Patient]]’s values and preferences and risks associated with valve intervention should be considered for choosing the approach. | |||
*There are no data for the use of [[TAVI]] in [[patients]] <65 years of age. | |||
*Both [[SAVR]] and [[TAVI]] are effective approaches to [[AVR]] in adults 65 to 80 years of age. | |||
* Selected [[patients]] in [[TAVI]] compared [[SAVR]] had high-velocity severe [[AS]] (Stage D1). | |||
* [[TAVI]] is not encouraged for symptomatic [[patients]] with low-flow, low-gradient severe [[AS]] (Stages D2 and D3).<ref name="pmid29566812">{{cite journal |vauthors=Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Muñoz-García AJ, Garcia Del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto WY, Clavel MA, de Agustin A, Serra V, Schindler JT, Dahou A, Puri R, Pelletier-Beaumont E, Côté M, Pibarot P, Rodés-Cabau J |title=Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The TOPAS-TAVI Registry |journal=J Am Coll Cardiol |volume=71 |issue=12 |pages=1297–1308 |date=March 2018 |pmid=29566812 |doi=10.1016/j.jacc.2018.01.054 |url=}}</ref> | |||
* Risk of [[mortality]] is slightly lower in [[TAVI]] and is associated with a shorter hospital [[length of stay]], more rapid return to normal activities, lower risk of transient or permanent [[atrial fibrillation]], less [[bleeding]], and less [[pain]] than [[SAVR]].<ref name="pmid27683072">{{cite journal |vauthors=Foroutan F, Guyatt GH, O'Brien K, Bain E, Stein M, Bhagra S, Sit D, Kamran R, Chang Y, Devji T, Mir H, Manja V, Schofield T, Siemieniuk RA, Agoritsas T, Bagur R, Otto CM, Vandvik PO |title=Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies |journal=BMJ |volume=354 |issue= |pages=i5065 |date=September 2016 |pmid=27683072 |pmc=5040922 |doi=10.1136/bmj.i5065 |url=}}</ref> | |||
* [[SAVR]] is associated with a lower risk of [[paravalvular leak]], less need for [[valve intervention]], and less need for a [[permanent pacemaker]]. | |||
* Factors related to approach to [[TAVI]] or [[SAVR]] include [[vascular access]], comorbid [[cardiac]], and noncardiac [[conditions]], [[functional status]] and [[survival]] after [[AVR]], and [[patient]] values and preferences. | |||
*[[TAVI]] is a safe and effective procedure for the treatment of severe symptomatic [[AS]] in all [[adults]] regardless of estimated [[surgical]] risk. | |||
* The [[mortality rate]] for transfemoral [[TAVI]] is lower than that for [[SAVR]] | |||
*[[TAVI]] also is associated with a lower risk of [[stroke]], major [[bleeding]], and [[AF]], shorter [[hospital length of stay]], less [[pain]], and more rapid return to normal [[activities]]. | |||
* In comparison with [[SAVR]], [[TAVI]] results in higher rates of [[vascular]] complications, [[paravalvular regurgitation]], [[permanent pacemaker implantation]], and [[valve]] intervention. | |||
* Durability of [[TAVI]] valves is at least 5 years. | |||
* [[Valve-in-valve]] [[TAVI]] is a second [[TAVI]] within the first prosthesis when significant [[valve]] deterioration does occur. | |||
* When a [[transfemoral]] approach is not possible, other factors, such as alternative [[vascular]] access, comorbid [[cardiac]] and noncardiac [[conditions]], expected [[functional status]] and [[survival]] after [[AVR]], and [[patient]] values and preferences, must be considered. | |||
* Choosing a balloon-expandable valve or self-expanding valve depends on [[patient]] [[anatomy]] and other considerations.<ref name="pmid26271061">{{cite journal |vauthors=Abdel-Wahab M, Neumann FJ, Mehilli J, Frerker C, Richardt D, Landt M, Jose J, Toelg R, Kuck KH, Massberg S, Robinson DR, El-Mawardy M, Richardt G |title=1-Year Outcomes After Transcatheter Aortic Valve Replacement With Balloon-Expandable Versus Self-Expandable Valves: Results From the CHOICE Randomized Clinical Trial |journal=J Am Coll Cardiol |volume=66 |issue=7 |pages=791–800 |date=August 2015 |pmid=26271061 |doi=10.1016/j.jacc.2015.06.026 |url=}}</ref> | |||
* In [[patients]] with [[LVEF]] <50%, the choice of [[TAVI]] versus [[SAVR]] is likely in [[patients]] with symptomatic severe [[AS]].<ref name="pmid31525069">{{cite journal |vauthors=Furer A, Chen S, Redfors B, Elmariah S, Pibarot P, Herrmann HC, Hahn RT, Kodali S, Thourani VH, Douglas PS, Alu MC, Fearon WF, Passeri J, Malaisrie SC, Crowley A, McAndrew T, Genereux P, Ben-Yehuda O, Leon MB, Burkhoff D |title=Effect of Baseline Left Ventricular Ejection Fraction on 2-Year Outcomes After Transcatheter Aortic Valve Replacement: Analysis of the PARTNER 2 Trials |journal=Circ Heart Fail |volume=12 |issue=8 |pages=e005809 |date=August 2019 |pmid=31525069 |doi=10.1161/CIRCHEARTFAILURE.118.005809 |url=}}</ref><ref name="pmid29217004">{{cite journal |vauthors=Elmariah S, Fearon WF, Inglessis I, Vlahakes GJ, Lindman BR, Alu MC, Crowley A, Kodali S, Leon MB, Svensson L, Pibarot P, Hahn RT, Thourani VH, Palacios IF, Miller DC, Douglas PS, Passeri JJ |title=Transapical Transcatheter Aortic Valve Replacement Is Associated With Increased Cardiac Mortality in Patients With Left Ventricular Dysfunction: Insights From the PARTNER I Trial |journal=JACC Cardiovasc Interv |volume=10 |issue=23 |pages=2414–2422 |date=December 2017 |pmid=29217004 |doi=10.1016/j.jcin.2017.09.023 |url=}}</ref> | |||
* [[SAVR]] is recommended in preference to [[TAVI]] in asymptomatic [[patients]] with abnormal exercise [[blood pressure]] response, an elevated serum [[BNP]] level, rapid [[hemodynamic progression]], or very severe [[AS]] with a [[velocity]] of ≥5 m/s. | |||
* When transfemoral [[TAVI]] is not feasible, [[SAVR]] or [[palliative care]] are options that should be included in the shared decision-making discussion. | |||
* [[TAVI]] was compared with standard [[medical therapy]] in severe symptomatic inoperable [[AS ]].<ref name="pmid25788231">{{cite journal |vauthors=Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR |title=5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial |journal=Lancet |volume=385 |issue=9986 |pages=2485–91 |date=June 2015 |pmid=25788231 |doi=10.1016/S0140-6736(15)60290-2 |url=}}</ref><ref name="pmid20961243">{{cite journal |vauthors=Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S |title=Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery |journal=N Engl J Med |volume=363 |issue=17 |pages=1597–607 |date=October 2010 |pmid=20961243 |doi=10.1056/NEJMoa1008232 |url=}}</ref> | |||
* In comparison with [[medical therapy]], [[TAVI]] [[patients]] demonstrated lower rate of [[all-cause death]] and [[hospitalization]] and improvement in [[NYHA]] class. However, the rate of major [[stroke]] and major [[vascular]] complications was higher with [[TAVI]] than with standard therapy.<ref name="pmid20961243">{{cite journal |vauthors=Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S |title=Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery |journal=N Engl J Med |volume=363 |issue=17 |pages=1597–607 |date=October 2010 |pmid=20961243 |doi=10.1056/NEJMoa1008232 |url=}}</ref><ref name="pmid20961243">{{cite journal |vauthors=Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S |title=Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery |journal=N Engl J Med |volume=363 |issue=17 |pages=1597–607 |date=October 2010 |pmid=20961243 |doi=10.1056/NEJMoa1008232 |url=}}</ref> | |||
*Factors associated with a poor outcome after [[TAVI]] include advanced [[age]], [[frailty]], [[smoking]] or [[chronic obstructive pulmonary disease]], [[pulmonary hypertension]], [[liver]] disease, prior [[stroke]], [[anemia]], and other systemic [[conditions]]. | |||
* [[Patients]] with a mechanical impediment to [[SAVR]], such as a [[porcelain aorta]] or prior [[chest radiation]] damage, may have better outcomes after [[TAVI]] than do [[frail]] [[patients]] or those with moderate to severe [[disease]] in more than one other [[organ]] system. | |||
* [[TAVI]] is not recommended in [[patients]] with 1) a [[life expectancy]] of <1 year even with a successful [[procedure]] or 2) those with a chance of [[survival]] with benefit of <25% at 2 years. | |||
*[[Percutaneous aortic balloon dilation]] has a limited role in treating [[older]] [[patients]]. However, the role in treating [[children]], [[adolescents]], and young [[adults]] with [[AS]] has been shown. | |||
* The mechanism of [[balloon dilation]] is a modest reduction in the severity of [[stenosis]] in [[older]] [[patients]] and fracture of [[calcific]] deposits within the [[valve]] [[leaflets]] and, to a minor degree, stretching of the [[annulus]] and separation of the [[calcified]] or fused [[commissures]]. | |||
* Immediate [[hemodynamic]] results include a moderate reduction in the [[transvalvular]] [[pressure gradient]], but the post-dilation valve area rarely exceeds 1.0 cm2. | |||
* An early [[symptomatic]] improvement usually occurs, despite the modest change in [[valve area]]. However, serious acute complications, including acute severe [[AR]], [[restenosis]], and [[clinical deterioration]], occur within 6 to 12 months in most [[patients]]. | |||
* Therefore, in [[patients]] with [[AS]], [[percutaneous aortic balloon dilation]] is not a substitute for [[AVR]] and can be planned as a bridge to [[TAVI]] or [[SAVR]] in symptomatic severe [[AS]] and refractory [[pulmonary edema]] or [[cardiogenic shock]].<ref name="pmid29929641">{{cite journal |vauthors=Kolte D, Khera S, Vemulapalli S, Dai D, Heo S, Goldsweig AM, Aronow HD, Elmariah S, Inglessis I, Palacios IF, Thourani VH, Sharaf BL, Gordon PC, Abbott JD |title=Outcomes Following Urgent/Emergent Transcatheter Aortic Valve Replacement: Insights From the STS/ACC TVT Registry |journal=JACC Cardiovasc Interv |volume=11 |issue=12 |pages=1175–1185 |date=June 2018 |pmid=29929641 |doi=10.1016/j.jcin.2018.03.002 |url=}}</ref> | |||
==Valve Types== | |||
* There are currently 8 prosthetic transcatheter [[valve]] types which are available. Two of these are [[FDA]] approved in the United States.<ref name="pmid27012409">{{cite journal |vauthors=Vahl TP, Kodali SK, Leon MB |title=Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure |journal=J. Am. Coll. Cardiol. |volume=67 |issue=12 |pages=1472–87 |year=2016 |pmid=27012409 |doi=10.1016/j.jacc.2015.12.059 |url=}}</ref> | |||
* The CHOICE trial is a small study which compared the most prominent two valves (CORE and SAPIEN) and no major significant differences were found in primary clinical endpoints between both valves. Nevertheless, there are situations, anatomical variability, and operator preferences that continue to make one valve more preferable to the other, depending on the situation. There are currently three ongoing major trials comparing newer generation valves to the aforementioned devices. | |||
===Synopsis=== | |||
{| class="wikitable" | |||
!Valve | |||
!Type of Expansion | |||
!Device Placement | |||
!Year of Introduction | |||
!FDA approval | |||
!Available Sizes (in mm) | |||
|- | |||
|Core valve | |||
|Self expanding | |||
|TF | |||
|2005 | |||
|Yes | |||
|23, 26, 29 | |||
|- | |||
|Edwards Sapien [[valves]] | |||
|Balloon expandable | |||
|TF | |||
|2007 | |||
|Yes | |||
|20, 23, 26, 29 | |||
|- | |||
|Acurate ''neo'' [[valve]]<ref name="pmid26198875">{{cite journal |vauthors=Schäfer U, Conradi L, Diemert P, Deuschl F, Schofer N, Seiffert M, Lubos E, Schirmer J, Reichenspurner H, Blankenberg S, Treede H |title=Symetis ACURATE TAVI: review of the technology, developments and current data with this self-expanding transcatheter heart valve |journal=Minerva Cardioangiol |volume=63 |issue=5 |pages=359–69 |year=2015 |pmid=26198875 |doi= |url=}}</ref> | |||
|Self expanding | |||
|TF, TA | |||
|2011 | |||
|No | |||
|S, M, L † | |||
|- | |||
|Jena [[Valve]] | |||
|Self expanding | |||
|TF, TA | |||
|2011 | |||
|No | |||
|23, 25, 27 | |||
|- | |||
|St. Jude Medical Portico | |||
|Self expanding | |||
|TF | |||
|2014 | |||
|No | |||
|23, 25, 27, 29 | |||
|- | |||
|Direct Flow Medical [[Valve]] | |||
|Self expanding | |||
|TF | |||
|2013 | |||
|No | |||
|23, 25, 27, 29 | |||
|- | |||
|Medtronic Engager [[Valve]] | |||
|Self expanding | |||
|TF | |||
|2013 | |||
|No | |||
|23, 26 | |||
|- | |||
|Boston Scientific Lotus [[Valve]] | |||
|Mechanically Expanded | |||
|TF | |||
|2013 | |||
|No | |||
|23,25,27 | |||
|} | |||
<sup>TF: Trasfemoral, TA: Transapical</sup> | |||
† <sub>They claimed that can cover aortic annulus diameters from 21 to 27 mm.</sub> | |||
=== Description of Valves === | |||
====Core Valve==== | |||
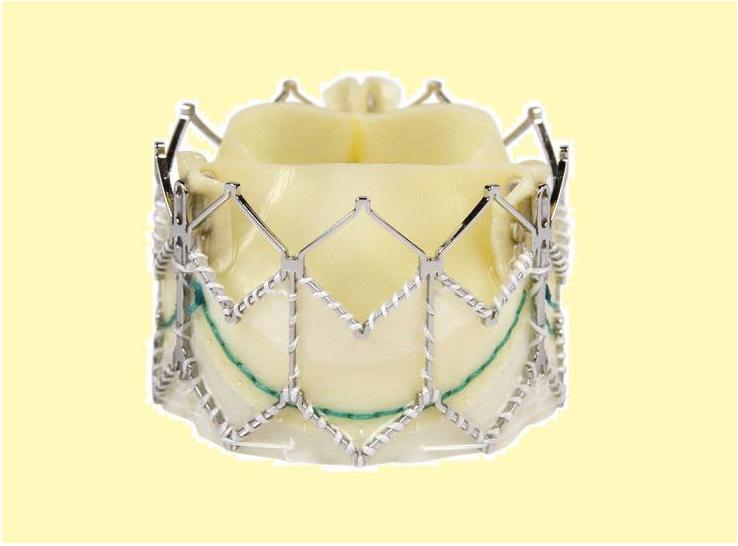
The Core-Valve device was first inserted in 2005.<ref name="pmid17015786">{{cite journal | author = Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW | title = Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study | journal = [[Circulation]] | volume = 114 | issue = 15 | pages = 1616–24 | year = 2006 | month = October | pmid = 17015786 | doi = 10.1161/CIRCULATIONAHA.106.639450 | url = http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=17015786 | issn = | accessdate = 2011-02-23}}</ref><ref> Heart Mirror J 2010; 157-159.</ref> It consists of three leaflets of bioprosthetic pericardial valve tissue mounted on a self-expendable nitinol stent, which expands from the [[left ventricular outflow tract]] ([[LVOT]]) to the ascending [[aorta]]. The Core Valve frame is currently available in 3 sizes (23 mm, 26 mm and 29 mm). The multilevel nitinol frame was designed for optimal functionality,stability,and durability. The inflow portion of the frame exerts high radial expansive force to provide proper support of the frame within the annular location.<ref>CARDIAC INTERVENTIONS TODAY,TAVI using the CoreValve revalving system,July.August 2010</ref> The design of this portion of the frame with its radial strength prevents annular recoil, allowing the frame to partially conform to the non circular shape of the aortic annulus. The center portion of the frame has very high hoop strength that resists size and shape deformation which is a very important part of the device since it contains the valve leaflets, which are supra-annular. This center portion of the frame is concave to allow normal flow of blood through the [[coronary arteries]] and coronary cannulation after implantation. The largest part of the frame is the outflow portion that exerts low radial forces and allow optimal flow of blood through the valve. For the tissue in the valves, porcine (pig) pericardium was selected due to its lower profile (compared with bovine (cow) pericardium) and its durability. The trileaflet valve is made of six individual pieces of porcine pericardium, with three pieces used to make a skirt at the inflow section of the valve thus preventing [[aortic regurgitation]] and three leaflet elements that are constructed with long commissures to distribute the aortic pressure load to the valve leaflets and the commissural posts. | |||
[[Image:CoreValve-Prosthesis-.jpg|left|200px|Core valve prosthesis]] | |||
<br><br><br><br><br><br><br><br> | |||
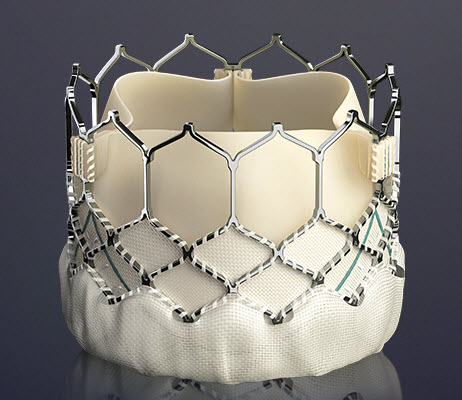
====The Edwards SAPIEN Valves==== | |||
===== SAPIEN XT ===== | |||
This prosthesis is considered the second generation of the Cribier-Edwards valve.<ref> Heart Mirror J 2010; 157-159.</ref> It is a balloon-expendable valve made of a stainless steel frame covered by a Dacron skirt where three leaflets of pericardium are sutured. The device is placed in a subcoronary position during rapid ventricular pacing, via anterograde, transapical or a retrograde transfemoral approach. It is available in two sizes (23mm and 26 mm). In the first generation the leaflets were made of equine (horse) pericardium; in the second generation they are made of bovine (cow) pericardium with improvements made in the frame suture and an increase in the skirt length to decrease the risk of [[aortic regurgitation]]. | |||
===== SAPIEN 3 ===== | |||
The Edwards SAPIEN 3 Transcatheter Heart Valve is comprised of a balloon-expandable, radiopaque, cobalt-chromium frame, trileaflet bovine pericardial tissue valve, and polyethylene terephthalate (PET) fabric skirt. The leaflets are treated according to the Carpentier-Edwards ThermaFix process. It is available in 4 sizes (20mm, 23mm, 26mm and 29mm). | |||
<gallery> | |||
Image:The Edwars SAPIEN valve.jpg|200px|The Edwards SAPIEN XT | |||
Image:Sapien3.jpg|200px|The Edwards SAPIEN 3 | |||
</gallery> | |||
====St. Jude Portico valve==== | |||
The valve stent is made from nitinol, a nickel-titanium alloy that has self-expanding properties and is radiopaque. The valve cuff is made from porcine pericardium that is sutured to the stent frame. The cuff provides the sealing area for implantation. The valve orifice is made by suturing three valve leaflets, each made from a single layer of bovine pericardium, into a tri-leaflet configuration on the stent frame. It is available in 4 sizes (23mm, 25mm, 27mm and 29mm). | |||
[[Image:Portico.jpg|left|200px|St. Jude Portico valve prosthesis]]<br><br><br><br><br><br><br><br><br><br> | |||
====Direct Flow Medical valve ==== | |||
It features a metal-free frame. The Direct Flow Medical System incorporates a polymer frame, which is expanded using pressurized saline and contrast for placement, assessment and repositioning. The saline/contrast solution is exchanged for a quick-curing polymer that solidifies and secures the valve in place once optimal positioning is reached. It is available in 4 sizes(23 mm, 25 mm, 27 mm, 29 mm). | |||
[[Image:direct flow.jpg|left|200px|Direct Flow valve prosthesis]]<br><br><br><br><br><br><br><br> | |||
====Symetis Acurate neo valve==== | |||
Acurate neo is composed of a porcine pericardial tissue valve sutured within a self-expanding nitinol stent covered by a pericardial skirt on the outer and inner surface of the stent body.It is a nitinol-based valve that incorporate features that facilitate positioning and anatomic orientation in relation to the native valve commissures and coronaries. The valve is currently implanted only transapically. | |||
It is available in three sizes (S, M, L) and its delivery system boasts an 18F outer diameter and it cover the aortic annulus diameters from 21 to 27 mm. | |||
[[Image:Acurate neo.jpg|left|200px|Acurate neo valve]]<br><br><br><br><br><br><br><br><br><br> | |||
====Medtronic Engager valve==== | |||
It has a self expanding nitinol frame and polyester skirt and bovine pericardial tissue. It is available in 2 sizes(23 mm, 26 mm) | |||
[[Image:engager.jpg|left|200px|Medtronic Engager valve prosthesis]]<br><br><br><br><br><br><br><br><br><br> | |||
====Lotus edge valve==== | |||
Lotus valve consisting of a pre-attached, stent-mounted tissue valve prosthesis and catheter delivery system for guidance and percutaneous placement of the valve. It is the first device of its kind that offers controlled mechanical expansion, which allows the valve to be fully deployed, assessed and then released, providing unparalleled control during the procedure. It is available in 3 sizes (23 mm, 25 mm and 27 mm). | |||
[[Image:lotus.jpg|left|200px|Lotus edge valve prosthesis]]<br><br><br><br><br><br><br><br><br><br> | |||
====Jena valve==== | |||
The transapical JenaValve prosthesis consists of a natural aortic porcine root bioprosthesis fitted with an outer porcine pericardial patch, a so-called skirt. The JenaValve is available in 3 sizes, 23mm, 25mm and 27mm. It is not commercially available now and is waiting for approval. | |||
[[Image:Jenavalave.jpg|left|200px|Jenavalve prosthesis]]<br><br><br><br><br><br><br><br><br><br> | |||
== Valve Sizing and Positioning == | |||
Valve sizing and positioning is of utmost importance in the success of the TAVR procedure and the risk of paraprocedural compications. The utilization of 2D echocardiography was initially used to estimate the annulus size. Currently, the gold standard for estimating the size of the aortic outlet is CT angiography focussing on the area of the valve or the perimeter of the annulus to estimate the required device size. Furthermore, the positioning of the valve must be estimated by an experienced operator as every valve type presents advantages and disadvantages according to the variability in the anatomy. Examining the left ventricular outflow tract, the origin of the coronary arteries, and the sinotubular junction constitute some of the considerations the operator must entertain. Additionally, the positioning of the aorta and the degree of calcification of the valve and the proximal aorta must be assessed in planning a successful procedure.<ref name="pmid27012409">{{cite journal| author=Vahl TP, Kodali SK, Leon MB| title=Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure. | journal=J Am Coll Cardiol | year= 2016 | volume= 67 | issue= 12 | pages= 1472-87 | pmid=27012409 | doi=10.1016/j.jacc.2015.12.059 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27012409 }} </ref> | |||
==Contraindications== | |||
General contraindications for transcatheter aortic valve implant ([[TAVI]]) through every approach include: | |||
* Aortic annulus size <18 mm or >25 mm for balloon expandable devices and <20 mm or >27 mm for self expandable devices. | |||
* Severe [[mitral regurgitation]]. | |||
* Presence of apical [[left ventricle]] [[thrombus]]. | |||
* Aortic root dimension > 45 mm at the sino-tubular junction for self-expandable prostheses. | |||
* Low position of the coronary ostia (<8 mm from the aortic annulus) | |||
* Asymmetric heavy valvular calcification which may compress the [[coronary arteries]] during the procedure of TAVI. | |||
* [[Bicuspid aortic valves]] - because of the risk of incomplete placement of the prosthesis | |||
* Intracardiac mass, [[thrombosis]], [[vegetation]] | |||
* Severe [[aortic regurgitation]] | |||
* Serum [[creatinine]] >3.0 mg/dL or patient on [[dialysis]] | |||
* [[Left ventricular]] [[ejection fraction]] <20% | |||
* Untreated [[coronary heart disease]] requiring [[revascularization]] | |||
* Hemodynamic instability | |||
* [[Upper gastrointestinal bleed]] within the past 3 months | |||
* [[Cerebrovascular accident]] ([[CVA]]) or [[transient ischemic attack]] ([[TIA]]) within the last 6 months | |||
* [[Aortic aneurysm]] or severe ilio-femoral insufficiency disease | |||
* [[Hypertrophic cardiomyopathy]] | |||
* [[Bleeding disorders]] | |||
* Active [[infective endocarditis]] | |||
* [[Life expectancy]] < 1 year | |||
* [[Comorbidities]] suggesting no improvement of [[quality of life]] after [[procedure]] | |||
The transfemoral approach has a few specific contraindications which include: | |||
* Severe tortuosity, calcification and narrowing of the iliac arteries | |||
* Previous aorta-femoral bypass | |||
* [[Abdominal aortic aneurysm]] | |||
* Severe angulation of the aorta | |||
* Severe [[atherosclerosis]] of the ascending aorta and arch of the aorta<ref name="pmid22625183">{{cite journal| author=Bapat VN, Attia RQ, Thomas M| title=Distribution of calcium in the ascending aorta in patients undergoing transcatheter aortic valve implantation and its relevance to the transaortic approach. | journal=JACC Cardiovasc Interv | year= 2012 | volume= 5 | issue= 5 | pages= 470-6 | pmid=22625183 | doi=10.1016/j.jcin.2012.03.006 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22625183 }} </ref>. | |||
[[ | Contraindications for transapical approach include: | ||
* Previous surgery to the heart | |||
* Severe [[respiratory insufficiency]] | |||
* Chest deformity | |||
==Procedure== | ==Procedure== | ||
The diseased valve is moved aside by | The diseased valve is first moved aside by aortic balloon [[valvuloplasty]]. The Corevalve prosthesis, which is loaded on a specialized delivery catheter, is advanced to the stenosed aortic valve. Once correctly positioned, the external part of the delivery system (the sheath) is progressively retracted, deploying the Corevalve Prosthesis. The delivery catheter is then closed and retrieved. | ||
{{#ev:youtube|7EhoUbWHW2A}} | |||
===Techniques=== | ===Techniques=== | ||
Two major catheter based techniques for replacing the aortic valve have been investigated <ref name="pmid19442881">{{cite journal |author=Zajarias A, Cribier AG |title=Outcomes and safety of percutaneous aortic valve replacement |journal=[[Journal of the American College of Cardiology]] |volume=53 |issue=20 |pages=1829–36 |year=2009 |month=May |pmid=19442881 |doi=10.1016/j.jacc.2008.11.059 |url=http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(09)00702-5 |accessdate=2011-03-18}}</ref> | Two major catheter based techniques for replacing the aortic valve have been investigated:<ref name="pmid19442881">{{cite journal |author=Zajarias A, Cribier AG |title=Outcomes and safety of percutaneous aortic valve replacement |journal=[[Journal of the American College of Cardiology]] |volume=53 |issue=20 |pages=1829–36 |year=2009 |month=May |pmid=19442881 |doi=10.1016/j.jacc.2008.11.059 |url=http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(09)00702-5 |accessdate=2011-03-18}}</ref> | ||
retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted. | retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted. | ||
====Retrograde Approach<ref name="pmid16461813">{{cite journal |author=Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S |title=Percutaneous aortic valve implantation retrograde from the femoral artery |journal=[[Circulation]] |volume=113 |issue=6 |pages=842–50 |year=2006 |month=February |pmid=16461813 |doi=10.1161/CIRCULATIONAHA.105.582882 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=16461813 |accessdate=2011-03-18}}</ref>==== | |||
After a routine [[aortic balloon valvuloplasty]], a 22F or 24F sheath is advanced from the femoral artery to the aorta. The manipulation of the prosthesis around the aortic arch and through the stenotic valve is facilitated by a steerable, deflectable catheter. Rapid ventricular pacing is used to decrease cardiac output while the delivery balloon is inflated to deploy the prosthesis within the annulus. | |||
====Transapical Antegrade Approach<ref name="pmid20566953">{{cite journal |author=Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O |title=Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve |journal=[[Circulation]] |volume=122 |issue=1 |pages=62–9 |year=2010 |month=July |pmid=20566953 |doi=10.1161/CIRCULATIONAHA.109.907402 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=20566953 |accessdate=2011-03-18}}</ref>==== | |||
An alternate catheter based method consists of a direct left ventricular apical puncture and antegrade aortic valve implantation via a small anterolateral thoracotomy without the need of cardiopulmonary bypass or sternotomy. This technique is used in patients with severe peripheral arterial disease and heavily calcified ascending aorta and arch (porcelain aorta) who have an increased risk of stroke and other embolic events using other approaches. | |||
====Alternative Vascular Access==== | |||
In some patients, the peripheral vascular anatomy is unsuitable for a transfemoral approach; for such reason a number of other vascular access have been suggested. The Subclavian (ie,axillary) or Transaortic access may be useful in solving such problems.<ref name="pmid19778770">{{cite journal |author=Fraccaro C, Napodano M, Tarantini G, Gasparetto V, Gerosa G, Bianco R, Bonato R, Pittarello D, Isabella G, Iliceto S, Ramondo A |title=Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system |journal=[[JACC. Cardiovascular Interventions]] |volume=2 |issue=9 |pages=828–33 |year=2009 |month=September |pmid=19778770 |doi=10.1016/j.jcin.2009.06.016 |url=http://linkinghub.elsevier.com/retrieve/pii/S1936-8798(09)00477-4 |accessdate=2011-03-23}}</ref> In a series of 54 cases treated via the Subclavian approach in the Italian National Registry, procedural success was achieved in 100% of cases.<ref name="pmid18948247">{{cite journal |author=Ruge H, Lange R, Bleiziffer S, Hutter A, Mazzitelli D, Will A, Schreiber C, Laborde JC, Bauernschmitt R |title=First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: a case report |journal=[[The Heart Surgery Forum]] |volume=11 |issue=5 |pages=E323–4 |year=2008 |pmid=18948247 |doi=10.1532/HSF98.20081021 |url=http://cardenjennings.metapress.com/openurl.asp?genre=article&id=doi:10.1532/HSF98.20081021 |accessdate=2011-03-23}}</ref> No specific complications such as vessel rupture or vertebral or internal mammary ischemia associated with Subclavian access were found. No deaths at 30 days in this series, and the 6-month mortality rate was 9.4% and was no different from those who underwent a transfemoral approach. | |||
==Complications== | ==Complications== | ||
*[[QRS]] duration: In one observational study of 270 patients, the QRS duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01). | ===Mortality=== | ||
The 30-day all-cause mortality has been estimated at 2.2%<ref name="pmid33541537">{{cite journal| author=Anwaruddin S, Desai ND, Vemulapalli S, Marquis-Gravel G, Li Z, Kosinski A | display-authors=etal| title=Evaluating Out-of-Hospital 30-Day Mortality After Transfemoral Transcatheter Aortic Valve Replacement: An STS/ACC TVT Analysis. | journal=JACC Cardiovasc Interv | year= 2021 | volume= 14 | issue= 3 | pages= 261-274 | pmid=33541537 | doi=10.1016/j.jcin.2020.10.027 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=33541537 }} </ref>. | |||
===Strokes and Transient Ischemic Attacks=== | |||
The etiology of cerebrovascular events after TAVI is thought to be related to the embolization of atherothrombotic material during advancement of the device to and across the aortic valve.<ref name="pmid20177005">{{cite journal |author=Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H |title=Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study |journal=[[Circulation]] |volume=121 |issue=7 |pages=870–8 |year=2010 |month=February |pmid=20177005 |doi=10.1161/CIRCULATIONAHA.109.855866 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=20177005 |accessdate=2011-03-22}}</ref> Magnetic resonance imaging have shown that microembolization is common with both balloon-expandable and self-expanding percutaneous valves, as well with surgical aortic valve repair (SAVR), but the presence of clinical [[stroke]]s are infrequent (2.9%-5.1%) | |||
Silent strokes detected by [[MRI]] has been estimated at 76%<ref name="pmid33517376">{{cite journal| author=Woldendorp K, Indja B, Bannon PG, Fanning JP, Plunkett BT, Grieve SM| title=Silent brain infarcts and early cognitive outcomes after transcatheter aortic valve implantation: a systematic review and meta-analysis. | journal=Eur Heart J | year= 2021 | volume= | issue= | pages= | pmid=33517376 | doi=10.1093/eurheartj/ehab002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=33517376 }} </ref>. | |||
===Aortic Regurgitation=== | |||
Significant aortic regurgitation caused by paravalvular leak after CoreValve percutaneous implantation is usually an uncommon complication that relates more frequently to low positioning of the CoreValve frame, incomplete expansion of the frame into the eccentrically shaped annulus, rigidity of the underlying aortic annulus due to calcium, or undersizing of the valve relative to the aortic annular size.<ref name="pmid19376312">{{cite journal |author=Jilaihawi H, Chin D, Vasa-Nicotera M, Jeilan M, Spyt T, Ng GA, Bence J, Logtens E, Kovac J |title=Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis |journal=[[American Heart Journal]] |volume=157 |issue=5 |pages=860–6 |year=2009 |month=May |pmid=19376312 |doi=10.1016/j.ahj.2009.02.016 |url=http://linkinghub.elsevier.com/retrieve/pii/S0002-8703(09)00148-3 |accessdate=2011-03-22}}</ref> | |||
===Vascular Access Complications=== | |||
The relatively large-caliber sheath (18F) required for placement of the percutaneous valve may be the cause of various vascular complications. One of the most common vascular events encountered are incomplete arteriotomy closure.<ref name="pmid20177005">{{cite journal |author=Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H |title=Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study |journal=[[Circulation]] |volume=121 |issue=7 |pages=870–8 |year=2010 |month=February |pmid=20177005 |doi=10.1161/CIRCULATIONAHA.109.855866 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=20177005 |accessdate=2011-03-22}}</ref> Avoiding such complications is possible; preprocedural screening using computed tomographic angiography, vascular ultrasound guidance for arterial access, and alternative (eg, subclavian) access have allowed better selection to avoid those vascular complications. | |||
===Coronary Artery Occlusion=== | |||
Coronary occlusion after TAVI is usually rare but may occur in some cases due to expansion of the native aortic valve across the orifice of the coronary ostium. This complication can be prevented with careful preprocedural screening to ensure adequate sinus of valsava width (30 mm) and height (15 mm).<ref>Gerckens U, Latsios G, Mueller R, et al. Left main PCI after trans-subclavian CoreValve implantation. Successful outcome of a combined procedure for management of a rare complication.Clin Res Cardiol. 2009;98:687-690.</ref> | |||
===Conduction Abnormalities=== | |||
Worsening or new conduction abnormalities are frequently observed with TAVI; more often when self-expandable CoreValve device is used<ref name="pmid19463319">{{cite journal| author=Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH et al.| title=Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. | journal=JACC Cardiovasc Interv | year= 2008 | volume= 1 | issue= 3 | pages= 310-6 | pmid=19463319 | doi=10.1016/j.jcin.2008.04.007 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19463319 }} </ref><ref name="pmid19110790">{{cite journal| author=Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O et al.| title=Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. | journal=EuroIntervention | year= 2008 | volume= 4 | issue= 2 | pages= 242-9 | pmid=19110790 | doi= | pmc= | url= }} </ref>. Conduction abnormalities may be due to compression of superficially running left bundle branch (in the uppermost part of ventricular septum) by the lower one third of prosthesis which exerts radial forces for secure anchoring of the stent against the native annulus and outflow septum. Hence, deeper the implantation of the prosthesis into the left ventricular outflow tract, greater is the risk of development of severe conduction defect requiring pacemaker implantation. | |||
A study in Italy reported that 77% of the patients post TAVI developed new onset or worsening of per-existing conduction abnormalities. 44% of the patients developed [[left bundle branch block]] ([[LBBB]]) and subsequently 39% of the patients underwent implantation of pacemaker. After TAVI, 6 (75%) of 8 patients with right bundle branch block ([[RBBB]]) at baseline required pacemaker implantation versus 19 (34%) of 56 patients, who had not had [[RBBB]] before TAVI. It was concluded that the RBBB was the only baseline conduction abnormality that significantly affected the occurrence of pacemaker implantation after TAVI because if patients already have a right bundle branch block, then a procedure-induced left bundle branch block will result in a complete [[atrioventricular block]] requiring a [[pacemaker]].<ref name="pmid21247519">{{cite journal| author=Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R et al.| title=Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation. | journal=Am J Cardiol | year= 2011 | volume= 107 | issue= 5 | pages= 747-54 | pmid=21247519 | doi=10.1016/j.amjcard.2010.10.054 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21247519 }} </ref><ref>http://www.medpagetoday.com/MeetingCoverage/EUROPACE/27358 accessed on July 1, 2011</ref> | |||
Other CoreValve implantation complications are:<ref name="pmid21339482">{{cite journal | author = Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, Trivedi U, Hutchinson N, De Belder AJ, Moat N, Blackman DJ, Levy RD, Manoharan G, Roberts D, Khogali SS, Crean P, Brecker SJ, Baumbach A, Mullen M, Laborde JC, Hildick-Smith D | title = Permanent Pacemaker Insertion After CoreValve Transcatheter Aortic Valve Implantation: Incidence and Contributing Factors (the UK CoreValve Collaborative) | journal = [[Circulation]] | volume = | issue = | pages = | year = 2011 | month = February | pmid = 21339482 | doi = 10.1161/CIRCULATIONAHA.109.927152 | url = http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=21339482 | issn = | accessdate = 2011-02-23}}</ref> | |||
*[[QRS]] duration: In one observational study of 270 patients, the [[QRS]] duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01). | |||
*[[Left Bundle Branch Block]] ([[LBBB]]): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001). | *[[Left Bundle Branch Block]] ([[LBBB]]): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001). | ||
*Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days). | *Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days). | ||
| Line 49: | Line 577: | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Cardiology]] | [[Category:Cardiology]] | ||
Latest revision as of 13:19, 11 July 2023
| Resident Survival Guide |
Editor(s)-In-Chief: Roger Laham, M.D., C. Michael Gibson, M.S., M.D.; Associate Editor(s)-in-Chief: Sara Zand, M.D.[1] Saleh El Dassouki, MD [2]; Seyedmahdi Pahlavani, M.D. [3]; Tarek Nafee, M.D. [4]; Arzu Kalayci, M.D. [5]
Synonyms and keywords: TAVI; Edward's valve; Edward's SAPIEN transcatheter heart valve; CoreValve; percutaneous aortic valve replacement; PAVR; SAVR; Surgical aortic valve replacement
Overview
TAVI is a safe and effective procedure for the treatment of severe symptomatic AS in all adults regardless of estimated surgical risk. In patients considering a bioprosthetic AVR, the next step is the choice between SAVR and TAVI. When the surgery is high risk or prohibitive, TAVI or palliative care would be focused. If both SAVR and TAVI are options, TAVI durability and life expectancy should be considered. Data about the durability of SAVR is for more than 50 years, but it limited to 5 years for TAVI. SAVR valve deteriorates typically after >10 years, so longer-term is needed for evaluation of TAVI durability. Earlier RCTs comparing SAVR and TAVI in patients with a higher surgical risk included only older patients, with a mean age in the mid-80s. However, more recent RCTs that included patients at low to intermediate surgical risk had a mean age in the mid-70s. There is no data about the use of TAVI in patients younger than 65 years of age. Some younger patients with comorbid conditions have a limited life expectancy, whereas some older patients have a longer-than-average life expectancy. Comorbid cardiac and noncardiac conditions, frailty, dementia, and other factors that affect longevity or quality of life should be individualized for making decision the type of approach. Mortality rate in TAVI is lower than SAVR and is associated with a shorter hospital length of stay, more rapid return to normal activities, lower risk of transient or permanent atrial fibrillation, lower risk of stroke, less bleeding, and less pain than SAVR. On the other hand, SAVR is associated with a lower risk of paravalvular leak, less need for valve intervention, and less need for a permanent pacemaker.
Comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)
| Characteristics | Favors SAVR | Favors TAVI | Favors palliation |
|---|---|---|---|
| Age/life expectancy | Younger age/longer life expectancy | Older age/fewer expected remaining years of life | Limited life expectancy |
| Valve anatomy |
|
Calcific AS of a trileaflet valve | |
| Prosthetic valve preference |
|
|
|
| Concurrent cardiac conditions |
|
Severe calcification of the ascending aorta (porcelain aorta) |
|
| Noncardiac conditions |
|
| |
| Frailty | Not frail or few frailty measures | Frailty likely to improve after TAVI | Severe frailty unlikely to improve after TAVI |
| Estimated procedural or surgical risk of SAVR or TAVI | Prohibitive SAVR risk (>15%) or post-TAVI life expectancy <1 y | ||
| Procedural specific impediments |
|
|
|
| Goals of Care and patient preferences and values |
|
|
|
Abbreviations:
MR: Mitral regurgitation;
AVA: Aortic valve area;
LVOT: Left ventricular outflow tract ;
SAVR: Surgical aortic valve replacement;
TAVI: Transcatheter aortic valve implantation;
AF: Atrial fibrillation;
CAD: Coronary artery disease;
AS: Aortic stenosis
| The above table adopted from 2020 AHA Guideline[1] |
|---|
| Clinical characteristics | Favours TAVI | Favours SAVR |
|---|---|---|
| Lower surgical risk | _ | + |
| Higher surgical risk | + | _ |
| Younger age | _ | + |
| Older age | + | _ |
| Previous cardiac surgery (CABG) | + | _ |
| Severe frailty | + | _ |
| Endocarditis | _ | + |
| Anatomical and procedural factors | ||
| TAVI feasible via transfemoral approach | + | _ |
| Inaccessable Transfemoral approach or SAVR feasible | _ | + |
| Sequelae of chest radiation | + | _ |
| Porcelain aorta | + | _ |
| High likelihood of severe patient-prosthesis mismatch (AVA <0.65 cm2/m2 BSA) | + | _ |
| Severe chest deformity or scoliosis | + | _ |
| Unsuitable aortic annular dimensions for TAVI device | _ | + |
| Bisuspid aortic valve | _ | + |
| Unfavourable valve morphology for TAVI (high risk of coronary obstruction due to low coronary ostia or heavy leaflet/LVOT calcification | _ | + |
| Thrombus in aorta or left ventricle | _ | + |
| Concomitant cardiac conditions requiring interventio | ||
| Significant multi-vessel CAD requiring surgical revascularization | _ | + |
| Severe primary mitral valve disease | _ | + |
| Severe tricuspid valve disease | _ | + |
| Significant dilatation/aneurysm of the aortic root and/or ascending aorta | _ | + |
| Septal hypertrophy requiring myomectomy | _ | + |
Abbreviations:
AV: Aortic valve;
AVA: Aortic valve area;
LVOT: Left ventricular outflow tract ;
SAVR: Surgical aortic valve replacement;
TAVI: Transcatheter aortic valve implantation;
BSA: Body surface area;
CAD: Coronary artery disease
| The above table adopted from 2021 ESC Guideline[2] |
|---|
| Recommendations for choice of SAVR versus TAVI for whom a bioprosthetic AVR is approperiate | |
| (Class I, Level of Evidence A): | |
|
❑ SAVR is recommended for symptomatic and asymptomatic severe AS, and any indication for AVR, who are < 65 years and life expectancy >20 year | |
| (Class I, Level of Evidence B): | |
|
❑SAVR is recommended in preference to TAVI in asymptomatic severe AS and abnormal exercise stress test, very severe AS, rapid progression, and elevated BNP | |
| (Class I, Level of Evidence C): | |
|
❑For symptomatic severe AS when predictive survival is <12 months after TAVI or SAVR and minimal improvement in quality of life is expected, palliative care is recommended | |
| (Class IIb, Level of Evidence C): | |
|
❑For critically ill patients with severe AS, percutaneous aortic ballon dilation is a bridge to TAVI or SAVR |
| The above table adopted from 2020 AHA Guideline[1] |
|---|
Abbreviations:
SAVR: Surgical aortic valve replacement;
TAVI: Transcutaneous aortic valve implantation;
AS: Aortic stenosis;
LVEF:Left ventricular ejection fraction ;
Notes
- Outcomes and durability after SAVR were excellent for both mechanical and bioprosthetic valves.[3][4]
- Earlier RCTs compared SAVR and TAVI in higher surgical risk patients including only older patients, with a mean age in the mid-80s. However, more recent RCTs included patients at low to intermediate surgical risk with a mean age in the mid-70s, but there were very few patients <65 years of age, so the evidence base cannot be extrapolated to these patients.[5]
- Valve durability should be considered in younger patients with longer life expectancy and lower surgical risk. [6]
- SAVR is recommended for adults <65 years of age unless life expectancy is limited by comorbid cardiac or noncardiac conditions.[7]
- Patient’s values and preferences and risks associated with valve intervention should be considered for choosing the approach.
- There are no data for the use of TAVI in patients <65 years of age.
- Both SAVR and TAVI are effective approaches to AVR in adults 65 to 80 years of age.
- Selected patients in TAVI compared SAVR had high-velocity severe AS (Stage D1).
- TAVI is not encouraged for symptomatic patients with low-flow, low-gradient severe AS (Stages D2 and D3).[8]
- Risk of mortality is slightly lower in TAVI and is associated with a shorter hospital length of stay, more rapid return to normal activities, lower risk of transient or permanent atrial fibrillation, less bleeding, and less pain than SAVR.[9]
- SAVR is associated with a lower risk of paravalvular leak, less need for valve intervention, and less need for a permanent pacemaker.
- Factors related to approach to TAVI or SAVR include vascular access, comorbid cardiac, and noncardiac conditions, functional status and survival after AVR, and patient values and preferences.
- TAVI is a safe and effective procedure for the treatment of severe symptomatic AS in all adults regardless of estimated surgical risk.
- The mortality rate for transfemoral TAVI is lower than that for SAVR
- TAVI also is associated with a lower risk of stroke, major bleeding, and AF, shorter hospital length of stay, less pain, and more rapid return to normal activities.
- In comparison with SAVR, TAVI results in higher rates of vascular complications, paravalvular regurgitation, permanent pacemaker implantation, and valve intervention.
- Durability of TAVI valves is at least 5 years.
- Valve-in-valve TAVI is a second TAVI within the first prosthesis when significant valve deterioration does occur.
- When a transfemoral approach is not possible, other factors, such as alternative vascular access, comorbid cardiac and noncardiac conditions, expected functional status and survival after AVR, and patient values and preferences, must be considered.
- Choosing a balloon-expandable valve or self-expanding valve depends on patient anatomy and other considerations.[10]
- In patients with LVEF <50%, the choice of TAVI versus SAVR is likely in patients with symptomatic severe AS.[11][12]
- SAVR is recommended in preference to TAVI in asymptomatic patients with abnormal exercise blood pressure response, an elevated serum BNP level, rapid hemodynamic progression, or very severe AS with a velocity of ≥5 m/s.
- When transfemoral TAVI is not feasible, SAVR or palliative care are options that should be included in the shared decision-making discussion.
- TAVI was compared with standard medical therapy in severe symptomatic inoperable AS .[13][14]
- In comparison with medical therapy, TAVI patients demonstrated lower rate of all-cause death and hospitalization and improvement in NYHA class. However, the rate of major stroke and major vascular complications was higher with TAVI than with standard therapy.[14][14]
- Factors associated with a poor outcome after TAVI include advanced age, frailty, smoking or chronic obstructive pulmonary disease, pulmonary hypertension, liver disease, prior stroke, anemia, and other systemic conditions.
- Patients with a mechanical impediment to SAVR, such as a porcelain aorta or prior chest radiation damage, may have better outcomes after TAVI than do frail patients or those with moderate to severe disease in more than one other organ system.
- TAVI is not recommended in patients with 1) a life expectancy of <1 year even with a successful procedure or 2) those with a chance of survival with benefit of <25% at 2 years.
- Percutaneous aortic balloon dilation has a limited role in treating older patients. However, the role in treating children, adolescents, and young adults with AS has been shown.
- The mechanism of balloon dilation is a modest reduction in the severity of stenosis in older patients and fracture of calcific deposits within the valve leaflets and, to a minor degree, stretching of the annulus and separation of the calcified or fused commissures.
- Immediate hemodynamic results include a moderate reduction in the transvalvular pressure gradient, but the post-dilation valve area rarely exceeds 1.0 cm2.
- An early symptomatic improvement usually occurs, despite the modest change in valve area. However, serious acute complications, including acute severe AR, restenosis, and clinical deterioration, occur within 6 to 12 months in most patients.
- Therefore, in patients with AS, percutaneous aortic balloon dilation is not a substitute for AVR and can be planned as a bridge to TAVI or SAVR in symptomatic severe AS and refractory pulmonary edema or cardiogenic shock.[15]
Valve Types
- There are currently 8 prosthetic transcatheter valve types which are available. Two of these are FDA approved in the United States.[16]
- The CHOICE trial is a small study which compared the most prominent two valves (CORE and SAPIEN) and no major significant differences were found in primary clinical endpoints between both valves. Nevertheless, there are situations, anatomical variability, and operator preferences that continue to make one valve more preferable to the other, depending on the situation. There are currently three ongoing major trials comparing newer generation valves to the aforementioned devices.
Synopsis
| Valve | Type of Expansion | Device Placement | Year of Introduction | FDA approval | Available Sizes (in mm) |
|---|---|---|---|---|---|
| Core valve | Self expanding | TF | 2005 | Yes | 23, 26, 29 |
| Edwards Sapien valves | Balloon expandable | TF | 2007 | Yes | 20, 23, 26, 29 |
| Acurate neo valve[17] | Self expanding | TF, TA | 2011 | No | S, M, L † |
| Jena Valve | Self expanding | TF, TA | 2011 | No | 23, 25, 27 |
| St. Jude Medical Portico | Self expanding | TF | 2014 | No | 23, 25, 27, 29 |
| Direct Flow Medical Valve | Self expanding | TF | 2013 | No | 23, 25, 27, 29 |
| Medtronic Engager Valve | Self expanding | TF | 2013 | No | 23, 26 |
| Boston Scientific Lotus Valve | Mechanically Expanded | TF | 2013 | No | 23,25,27 |
TF: Trasfemoral, TA: Transapical
† They claimed that can cover aortic annulus diameters from 21 to 27 mm.
Description of Valves
Core Valve
The Core-Valve device was first inserted in 2005.[18][19] It consists of three leaflets of bioprosthetic pericardial valve tissue mounted on a self-expendable nitinol stent, which expands from the left ventricular outflow tract (LVOT) to the ascending aorta. The Core Valve frame is currently available in 3 sizes (23 mm, 26 mm and 29 mm). The multilevel nitinol frame was designed for optimal functionality,stability,and durability. The inflow portion of the frame exerts high radial expansive force to provide proper support of the frame within the annular location.[20] The design of this portion of the frame with its radial strength prevents annular recoil, allowing the frame to partially conform to the non circular shape of the aortic annulus. The center portion of the frame has very high hoop strength that resists size and shape deformation which is a very important part of the device since it contains the valve leaflets, which are supra-annular. This center portion of the frame is concave to allow normal flow of blood through the coronary arteries and coronary cannulation after implantation. The largest part of the frame is the outflow portion that exerts low radial forces and allow optimal flow of blood through the valve. For the tissue in the valves, porcine (pig) pericardium was selected due to its lower profile (compared with bovine (cow) pericardium) and its durability. The trileaflet valve is made of six individual pieces of porcine pericardium, with three pieces used to make a skirt at the inflow section of the valve thus preventing aortic regurgitation and three leaflet elements that are constructed with long commissures to distribute the aortic pressure load to the valve leaflets and the commissural posts.
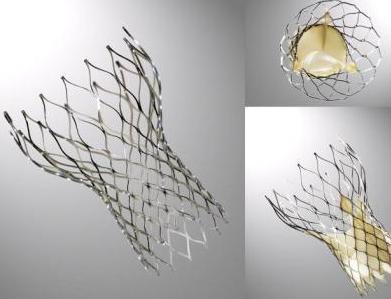
The Edwards SAPIEN Valves
SAPIEN XT
This prosthesis is considered the second generation of the Cribier-Edwards valve.[21] It is a balloon-expendable valve made of a stainless steel frame covered by a Dacron skirt where three leaflets of pericardium are sutured. The device is placed in a subcoronary position during rapid ventricular pacing, via anterograde, transapical or a retrograde transfemoral approach. It is available in two sizes (23mm and 26 mm). In the first generation the leaflets were made of equine (horse) pericardium; in the second generation they are made of bovine (cow) pericardium with improvements made in the frame suture and an increase in the skirt length to decrease the risk of aortic regurgitation.
SAPIEN 3
The Edwards SAPIEN 3 Transcatheter Heart Valve is comprised of a balloon-expandable, radiopaque, cobalt-chromium frame, trileaflet bovine pericardial tissue valve, and polyethylene terephthalate (PET) fabric skirt. The leaflets are treated according to the Carpentier-Edwards ThermaFix process. It is available in 4 sizes (20mm, 23mm, 26mm and 29mm).
-
The Edwards SAPIEN XT
-
The Edwards SAPIEN 3
St. Jude Portico valve
The valve stent is made from nitinol, a nickel-titanium alloy that has self-expanding properties and is radiopaque. The valve cuff is made from porcine pericardium that is sutured to the stent frame. The cuff provides the sealing area for implantation. The valve orifice is made by suturing three valve leaflets, each made from a single layer of bovine pericardium, into a tri-leaflet configuration on the stent frame. It is available in 4 sizes (23mm, 25mm, 27mm and 29mm).
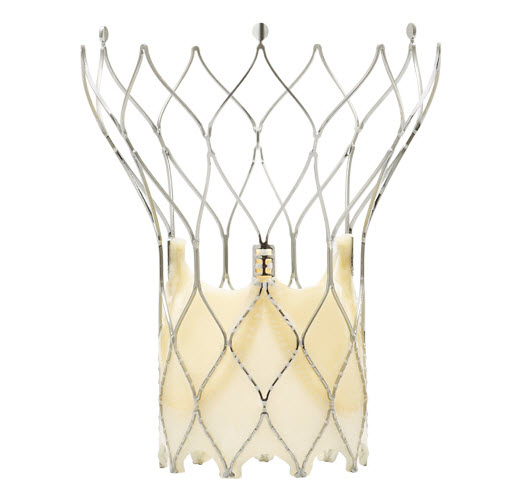
Direct Flow Medical valve
It features a metal-free frame. The Direct Flow Medical System incorporates a polymer frame, which is expanded using pressurized saline and contrast for placement, assessment and repositioning. The saline/contrast solution is exchanged for a quick-curing polymer that solidifies and secures the valve in place once optimal positioning is reached. It is available in 4 sizes(23 mm, 25 mm, 27 mm, 29 mm).

Symetis Acurate neo valve
Acurate neo is composed of a porcine pericardial tissue valve sutured within a self-expanding nitinol stent covered by a pericardial skirt on the outer and inner surface of the stent body.It is a nitinol-based valve that incorporate features that facilitate positioning and anatomic orientation in relation to the native valve commissures and coronaries. The valve is currently implanted only transapically. It is available in three sizes (S, M, L) and its delivery system boasts an 18F outer diameter and it cover the aortic annulus diameters from 21 to 27 mm.
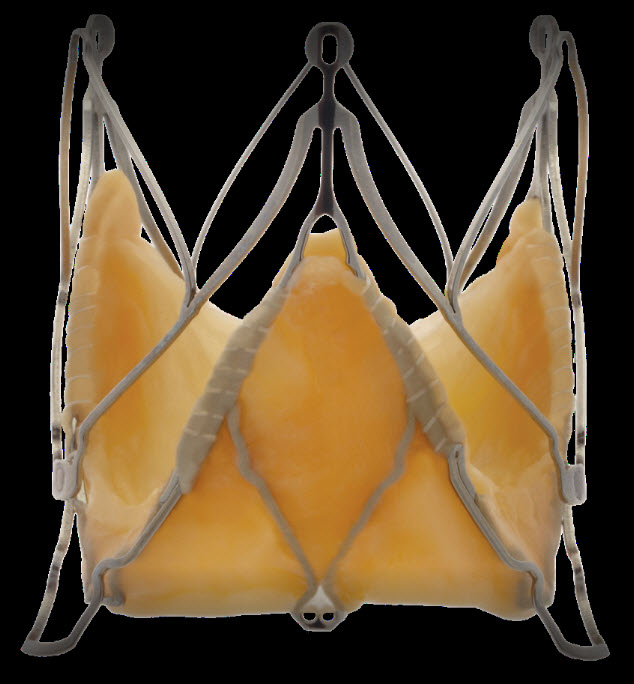
Medtronic Engager valve
It has a self expanding nitinol frame and polyester skirt and bovine pericardial tissue. It is available in 2 sizes(23 mm, 26 mm)
Lotus edge valve
Lotus valve consisting of a pre-attached, stent-mounted tissue valve prosthesis and catheter delivery system for guidance and percutaneous placement of the valve. It is the first device of its kind that offers controlled mechanical expansion, which allows the valve to be fully deployed, assessed and then released, providing unparalleled control during the procedure. It is available in 3 sizes (23 mm, 25 mm and 27 mm).

Jena valve
The transapical JenaValve prosthesis consists of a natural aortic porcine root bioprosthesis fitted with an outer porcine pericardial patch, a so-called skirt. The JenaValve is available in 3 sizes, 23mm, 25mm and 27mm. It is not commercially available now and is waiting for approval.

Valve Sizing and Positioning
Valve sizing and positioning is of utmost importance in the success of the TAVR procedure and the risk of paraprocedural compications. The utilization of 2D echocardiography was initially used to estimate the annulus size. Currently, the gold standard for estimating the size of the aortic outlet is CT angiography focussing on the area of the valve or the perimeter of the annulus to estimate the required device size. Furthermore, the positioning of the valve must be estimated by an experienced operator as every valve type presents advantages and disadvantages according to the variability in the anatomy. Examining the left ventricular outflow tract, the origin of the coronary arteries, and the sinotubular junction constitute some of the considerations the operator must entertain. Additionally, the positioning of the aorta and the degree of calcification of the valve and the proximal aorta must be assessed in planning a successful procedure.[16]
Contraindications
General contraindications for transcatheter aortic valve implant (TAVI) through every approach include:
- Aortic annulus size <18 mm or >25 mm for balloon expandable devices and <20 mm or >27 mm for self expandable devices.
- Severe mitral regurgitation.
- Presence of apical left ventricle thrombus.
- Aortic root dimension > 45 mm at the sino-tubular junction for self-expandable prostheses.
- Low position of the coronary ostia (<8 mm from the aortic annulus)
- Asymmetric heavy valvular calcification which may compress the coronary arteries during the procedure of TAVI.
- Bicuspid aortic valves - because of the risk of incomplete placement of the prosthesis
- Intracardiac mass, thrombosis, vegetation
- Severe aortic regurgitation
- Serum creatinine >3.0 mg/dL or patient on dialysis
- Left ventricular ejection fraction <20%
- Untreated coronary heart disease requiring revascularization
- Hemodynamic instability
- Upper gastrointestinal bleed within the past 3 months
- Cerebrovascular accident (CVA) or transient ischemic attack (TIA) within the last 6 months
- Aortic aneurysm or severe ilio-femoral insufficiency disease
- Hypertrophic cardiomyopathy
- Bleeding disorders
- Active infective endocarditis
- Life expectancy < 1 year
- Comorbidities suggesting no improvement of quality of life after procedure
The transfemoral approach has a few specific contraindications which include:
- Severe tortuosity, calcification and narrowing of the iliac arteries
- Previous aorta-femoral bypass
- Abdominal aortic aneurysm
- Severe angulation of the aorta
- Severe atherosclerosis of the ascending aorta and arch of the aorta[22].
Contraindications for transapical approach include:
- Previous surgery to the heart
- Severe respiratory insufficiency
- Chest deformity
Procedure
The diseased valve is first moved aside by aortic balloon valvuloplasty. The Corevalve prosthesis, which is loaded on a specialized delivery catheter, is advanced to the stenosed aortic valve. Once correctly positioned, the external part of the delivery system (the sheath) is progressively retracted, deploying the Corevalve Prosthesis. The delivery catheter is then closed and retrieved. {{#ev:youtube|7EhoUbWHW2A}}
Techniques
Two major catheter based techniques for replacing the aortic valve have been investigated:[23] retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted.
Retrograde Approach[24]
After a routine aortic balloon valvuloplasty, a 22F or 24F sheath is advanced from the femoral artery to the aorta. The manipulation of the prosthesis around the aortic arch and through the stenotic valve is facilitated by a steerable, deflectable catheter. Rapid ventricular pacing is used to decrease cardiac output while the delivery balloon is inflated to deploy the prosthesis within the annulus.
Transapical Antegrade Approach[25]
An alternate catheter based method consists of a direct left ventricular apical puncture and antegrade aortic valve implantation via a small anterolateral thoracotomy without the need of cardiopulmonary bypass or sternotomy. This technique is used in patients with severe peripheral arterial disease and heavily calcified ascending aorta and arch (porcelain aorta) who have an increased risk of stroke and other embolic events using other approaches.
Alternative Vascular Access
In some patients, the peripheral vascular anatomy is unsuitable for a transfemoral approach; for such reason a number of other vascular access have been suggested. The Subclavian (ie,axillary) or Transaortic access may be useful in solving such problems.[26] In a series of 54 cases treated via the Subclavian approach in the Italian National Registry, procedural success was achieved in 100% of cases.[27] No specific complications such as vessel rupture or vertebral or internal mammary ischemia associated with Subclavian access were found. No deaths at 30 days in this series, and the 6-month mortality rate was 9.4% and was no different from those who underwent a transfemoral approach.
Complications
Mortality
The 30-day all-cause mortality has been estimated at 2.2%[28].
Strokes and Transient Ischemic Attacks
The etiology of cerebrovascular events after TAVI is thought to be related to the embolization of atherothrombotic material during advancement of the device to and across the aortic valve.[29] Magnetic resonance imaging have shown that microembolization is common with both balloon-expandable and self-expanding percutaneous valves, as well with surgical aortic valve repair (SAVR), but the presence of clinical strokes are infrequent (2.9%-5.1%)
Silent strokes detected by MRI has been estimated at 76%[30].
Aortic Regurgitation
Significant aortic regurgitation caused by paravalvular leak after CoreValve percutaneous implantation is usually an uncommon complication that relates more frequently to low positioning of the CoreValve frame, incomplete expansion of the frame into the eccentrically shaped annulus, rigidity of the underlying aortic annulus due to calcium, or undersizing of the valve relative to the aortic annular size.[31]
Vascular Access Complications
The relatively large-caliber sheath (18F) required for placement of the percutaneous valve may be the cause of various vascular complications. One of the most common vascular events encountered are incomplete arteriotomy closure.[29] Avoiding such complications is possible; preprocedural screening using computed tomographic angiography, vascular ultrasound guidance for arterial access, and alternative (eg, subclavian) access have allowed better selection to avoid those vascular complications.
Coronary Artery Occlusion
Coronary occlusion after TAVI is usually rare but may occur in some cases due to expansion of the native aortic valve across the orifice of the coronary ostium. This complication can be prevented with careful preprocedural screening to ensure adequate sinus of valsava width (30 mm) and height (15 mm).[32]
Conduction Abnormalities
Worsening or new conduction abnormalities are frequently observed with TAVI; more often when self-expandable CoreValve device is used[33][34]. Conduction abnormalities may be due to compression of superficially running left bundle branch (in the uppermost part of ventricular septum) by the lower one third of prosthesis which exerts radial forces for secure anchoring of the stent against the native annulus and outflow septum. Hence, deeper the implantation of the prosthesis into the left ventricular outflow tract, greater is the risk of development of severe conduction defect requiring pacemaker implantation.
A study in Italy reported that 77% of the patients post TAVI developed new onset or worsening of per-existing conduction abnormalities. 44% of the patients developed left bundle branch block (LBBB) and subsequently 39% of the patients underwent implantation of pacemaker. After TAVI, 6 (75%) of 8 patients with right bundle branch block (RBBB) at baseline required pacemaker implantation versus 19 (34%) of 56 patients, who had not had RBBB before TAVI. It was concluded that the RBBB was the only baseline conduction abnormality that significantly affected the occurrence of pacemaker implantation after TAVI because if patients already have a right bundle branch block, then a procedure-induced left bundle branch block will result in a complete atrioventricular block requiring a pacemaker.[35][36]
Other CoreValve implantation complications are:[37]
- QRS duration: In one observational study of 270 patients, the QRS duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01).
- Left Bundle Branch Block (LBBB): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001).
- Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days).
- Multivariate predictors of permanent pacemaker implantation included:
- Periprocedural atrioventricular block (odds ratio, 6.29; 95% confidence interval, 3.55 to 11.15)
- Balloon pre-dilatation (odds ratio, 2.68; 95% confidence interval, 2.00 to 3.47)
- Use of a larger 29 mm CoreValve prosthesis (odds ratio, 2.50; 95% confidence interval, 1.22 to 5.11)
- The interventricular septum diameter (odds ratio, 1.18; 95% confidence interval, 1.10 to 3.06)
- A prolonged QRS duration (odds ratio, 3.45; 95% confidence interval, 1.61 to 7.40)
References
- ↑ 1.0 1.1 Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C (February 2021). "2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation. 143 (5): e35–e71. doi:10.1161/CIR.0000000000000932. PMID 33332149 Check
|pmid=value (help). - ↑ Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W (February 2022). "2021 ESC/EACTS Guidelines for the management of valvular heart disease". Eur Heart J. 43 (7): 561–632. doi:10.1093/eurheartj/ehab395. PMID 34453165 Check
|pmid=value (help). - ↑ Burke CR, Kirkpatrick JN, Otto CM (February 2020). "Goals of care in patients with severe aortic stenosis". Eur Heart J. 41 (8): 929–932. doi:10.1093/eurheartj/ehz567. PMID 31433469.
- ↑ Kumar A, Sato K, Banerjee K, Narayanswami J, Betancor J, Menon V, Mohananey D, Anumandla AK, Sawant AC, Krishnaswamy A, Tuzcu EM, Jaber W, Mick S, Svensson LG, Popović ZB, Blackstone EH, Kapadia SR (March 2019). "Hemodynamic durability of transcatheter aortic valves using the updated Valve Academic Research Consortium-2 criteria". Catheter Cardiovasc Interv. 93 (4): 729–738. doi:10.1002/ccd.27927. PMID 30312995.
- ↑ Siemieniuk RA, Agoritsas T, Manja V, Devji T, Chang Y, Bala MM, Thabane L, Guyatt GH (September 2016). "Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis". BMJ. 354: i5130. doi:10.1136/bmj.i5130. PMC 5040923. PMID 27683246.
- ↑ Foroutan F, Guyatt GH, Otto CM, Siemieniuk RA, Schandelmaier S, Agoritsas T, Vandvik PO, Bhagra S, Bagur R (December 2017). "Structural valve deterioration after transcatheter aortic valve implantation". Heart. 103 (23): 1899–1905. doi:10.1136/heartjnl-2017-311329. PMID 28684441.
- ↑ Siontis G, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, Pilgrim T, Petrinic T, Nikolakopoulou A, Salanti G, Søndergaard L, Verma S, Jüni P, Windecker S (October 2019). "Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis". Eur Heart J. 40 (38): 3143–3153. doi:10.1093/eurheartj/ehz275. PMID 31329852. Vancouver style error: initials (help)
- ↑ Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Muñoz-García AJ, Garcia Del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto WY, Clavel MA, de Agustin A, Serra V, Schindler JT, Dahou A, Puri R, Pelletier-Beaumont E, Côté M, Pibarot P, Rodés-Cabau J (March 2018). "Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The TOPAS-TAVI Registry". J Am Coll Cardiol. 71 (12): 1297–1308. doi:10.1016/j.jacc.2018.01.054. PMID 29566812.
- ↑ Foroutan F, Guyatt GH, O'Brien K, Bain E, Stein M, Bhagra S, Sit D, Kamran R, Chang Y, Devji T, Mir H, Manja V, Schofield T, Siemieniuk RA, Agoritsas T, Bagur R, Otto CM, Vandvik PO (September 2016). "Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies". BMJ. 354: i5065. doi:10.1136/bmj.i5065. PMC 5040922. PMID 27683072.
- ↑ Abdel-Wahab M, Neumann FJ, Mehilli J, Frerker C, Richardt D, Landt M, Jose J, Toelg R, Kuck KH, Massberg S, Robinson DR, El-Mawardy M, Richardt G (August 2015). "1-Year Outcomes After Transcatheter Aortic Valve Replacement With Balloon-Expandable Versus Self-Expandable Valves: Results From the CHOICE Randomized Clinical Trial". J Am Coll Cardiol. 66 (7): 791–800. doi:10.1016/j.jacc.2015.06.026. PMID 26271061.
- ↑ Furer A, Chen S, Redfors B, Elmariah S, Pibarot P, Herrmann HC, Hahn RT, Kodali S, Thourani VH, Douglas PS, Alu MC, Fearon WF, Passeri J, Malaisrie SC, Crowley A, McAndrew T, Genereux P, Ben-Yehuda O, Leon MB, Burkhoff D (August 2019). "Effect of Baseline Left Ventricular Ejection Fraction on 2-Year Outcomes After Transcatheter Aortic Valve Replacement: Analysis of the PARTNER 2 Trials". Circ Heart Fail. 12 (8): e005809. doi:10.1161/CIRCHEARTFAILURE.118.005809. PMID 31525069.
- ↑ Elmariah S, Fearon WF, Inglessis I, Vlahakes GJ, Lindman BR, Alu MC, Crowley A, Kodali S, Leon MB, Svensson L, Pibarot P, Hahn RT, Thourani VH, Palacios IF, Miller DC, Douglas PS, Passeri JJ (December 2017). "Transapical Transcatheter Aortic Valve Replacement Is Associated With Increased Cardiac Mortality in Patients With Left Ventricular Dysfunction: Insights From the PARTNER I Trial". JACC Cardiovasc Interv. 10 (23): 2414–2422. doi:10.1016/j.jcin.2017.09.023. PMID 29217004.
- ↑ Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR (June 2015). "5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial". Lancet. 385 (9986): 2485–91. doi:10.1016/S0140-6736(15)60290-2. PMID 25788231.
- ↑ 14.0 14.1 14.2 Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S (October 2010). "Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery". N Engl J Med. 363 (17): 1597–607. doi:10.1056/NEJMoa1008232. PMID 20961243.
- ↑ Kolte D, Khera S, Vemulapalli S, Dai D, Heo S, Goldsweig AM, Aronow HD, Elmariah S, Inglessis I, Palacios IF, Thourani VH, Sharaf BL, Gordon PC, Abbott JD (June 2018). "Outcomes Following Urgent/Emergent Transcatheter Aortic Valve Replacement: Insights From the STS/ACC TVT Registry". JACC Cardiovasc Interv. 11 (12): 1175–1185. doi:10.1016/j.jcin.2018.03.002. PMID 29929641.
- ↑ 16.0 16.1 Vahl TP, Kodali SK, Leon MB (2016). "Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure". J. Am. Coll. Cardiol. 67 (12): 1472–87. doi:10.1016/j.jacc.2015.12.059. PMID 27012409.
- ↑ Schäfer U, Conradi L, Diemert P, Deuschl F, Schofer N, Seiffert M, Lubos E, Schirmer J, Reichenspurner H, Blankenberg S, Treede H (2015). "Symetis ACURATE TAVI: review of the technology, developments and current data with this self-expanding transcatheter heart valve". Minerva Cardioangiol. 63 (5): 359–69. PMID 26198875.
- ↑ Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation. 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help) - ↑ Heart Mirror J 2010; 157-159.
- ↑ CARDIAC INTERVENTIONS TODAY,TAVI using the CoreValve revalving system,July.August 2010
- ↑ Heart Mirror J 2010; 157-159.
- ↑ Bapat VN, Attia RQ, Thomas M (2012). "Distribution of calcium in the ascending aorta in patients undergoing transcatheter aortic valve implantation and its relevance to the transaortic approach". JACC Cardiovasc Interv. 5 (5): 470–6. doi:10.1016/j.jcin.2012.03.006. PMID 22625183.
- ↑ Zajarias A, Cribier AG (2009). "Outcomes and safety of percutaneous aortic valve replacement". Journal of the American College of Cardiology. 53 (20): 1829–36. doi:10.1016/j.jacc.2008.11.059. PMID 19442881. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S (2006). "Percutaneous aortic valve implantation retrograde from the femoral artery". Circulation. 113 (6): 842–50. doi:10.1161/CIRCULATIONAHA.105.582882. PMID 16461813. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O (2010). "Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve". Circulation. 122 (1): 62–9. doi:10.1161/CIRCULATIONAHA.109.907402. PMID 20566953. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Fraccaro C, Napodano M, Tarantini G, Gasparetto V, Gerosa G, Bianco R, Bonato R, Pittarello D, Isabella G, Iliceto S, Ramondo A (2009). "Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system". JACC. Cardiovascular Interventions. 2 (9): 828–33. doi:10.1016/j.jcin.2009.06.016. PMID 19778770. Retrieved 2011-03-23. Unknown parameter
|month=ignored (help) - ↑ Ruge H, Lange R, Bleiziffer S, Hutter A, Mazzitelli D, Will A, Schreiber C, Laborde JC, Bauernschmitt R (2008). "First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: a case report". The Heart Surgery Forum. 11 (5): E323–4. doi:10.1532/HSF98.20081021. PMID 18948247. Retrieved 2011-03-23.
- ↑ Anwaruddin S, Desai ND, Vemulapalli S, Marquis-Gravel G, Li Z, Kosinski A; et al. (2021). "Evaluating Out-of-Hospital 30-Day Mortality After Transfemoral Transcatheter Aortic Valve Replacement: An STS/ACC TVT Analysis". JACC Cardiovasc Interv. 14 (3): 261–274. doi:10.1016/j.jcin.2020.10.027. PMID 33541537 Check
|pmid=value (help). - ↑ 29.0 29.1 Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H (2010). "Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study". Circulation. 121 (7): 870–8. doi:10.1161/CIRCULATIONAHA.109.855866. PMID 20177005. Retrieved 2011-03-22. Unknown parameter
|month=ignored (help) - ↑ Woldendorp K, Indja B, Bannon PG, Fanning JP, Plunkett BT, Grieve SM (2021). "Silent brain infarcts and early cognitive outcomes after transcatheter aortic valve implantation: a systematic review and meta-analysis". Eur Heart J. doi:10.1093/eurheartj/ehab002. PMID 33517376 Check
|pmid=value (help). - ↑ Jilaihawi H, Chin D, Vasa-Nicotera M, Jeilan M, Spyt T, Ng GA, Bence J, Logtens E, Kovac J (2009). "Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis". American Heart Journal. 157 (5): 860–6. doi:10.1016/j.ahj.2009.02.016. PMID 19376312. Retrieved 2011-03-22. Unknown parameter
|month=ignored (help) - ↑ Gerckens U, Latsios G, Mueller R, et al. Left main PCI after trans-subclavian CoreValve implantation. Successful outcome of a combined procedure for management of a rare complication.Clin Res Cardiol. 2009;98:687-690.
- ↑ Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH; et al. (2008). "Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve". JACC Cardiovasc Interv. 1 (3): 310–6. doi:10.1016/j.jcin.2008.04.007. PMID 19463319.
- ↑ Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O; et al. (2008). "Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval". EuroIntervention. 4 (2): 242–9. PMID 19110790.
- ↑ Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R; et al. (2011). "Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation". Am J Cardiol. 107 (5): 747–54. doi:10.1016/j.amjcard.2010.10.054. PMID 21247519.
- ↑ http://www.medpagetoday.com/MeetingCoverage/EUROPACE/27358 accessed on July 1, 2011
- ↑ Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, Trivedi U, Hutchinson N, De Belder AJ, Moat N, Blackman DJ, Levy RD, Manoharan G, Roberts D, Khogali SS, Crean P, Brecker SJ, Baumbach A, Mullen M, Laborde JC, Hildick-Smith D (2011). "Permanent Pacemaker Insertion After CoreValve Transcatheter Aortic Valve Implantation: Incidence and Contributing Factors (the UK CoreValve Collaborative)". Circulation. doi:10.1161/CIRCULATIONAHA.109.927152. PMID 21339482. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help)