Artificial heart valve
| Artificial heart valve | |
 | |
|---|---|
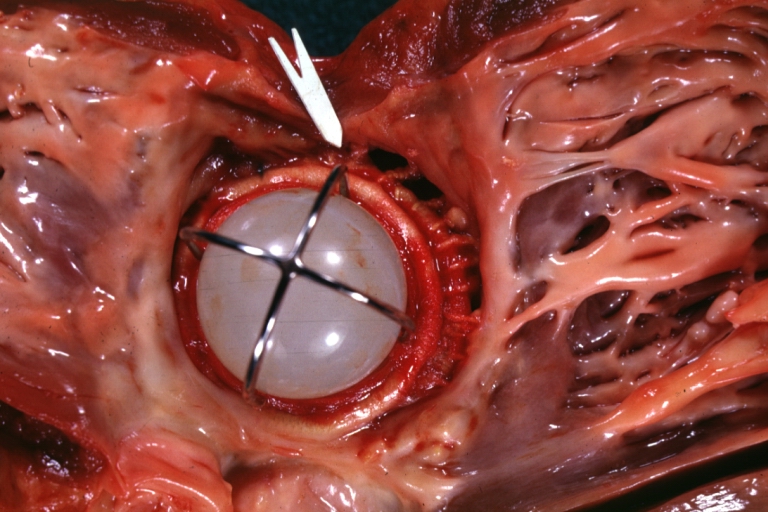
| Mitral Valve Prosthesis with Perivalvular Leak: Gross, natural color, close-up of valve with arrow to site of leakage, probably infected caged ball prosthesis. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Dima Nimri, M.D. [2];Sara Zand, M.D.[3] Arzu Kalayci, M.D. [4]
Overview
An artificial heart valve is a device that is implanted in the heart of patients who suffer from valvular diseases in their heart. When one or two of the four heart valves of the heart have a malfunction, the choice is normally to replace the natural valve with an artificial valve. This requires open-heart surgery. Valves are integral to the normal physiological functioning of the human heart. Natural heart valves are structures which have evolved a form which meets their functional requirements, which is to induce largely unidirectional flow through themselves. Natural heart valves may become dysfunctional due to a variety of pathological causes. Certain heart valve pathologies may necessitate the complete surgical replacement of the natural heart valves with heart valve prostheses.
Types of heart valve prostheses
There are two main types of artificial heart valves: the mechanical and the biological valves.
- Mechanical heart valves
- Percutaneous implantation
- Stent framed
- Not framed
- Sternotomy/Thoracotomy implantation
- Ball and cage
- Tilting disk
- Bi-leaflet
- Tri-leaflet
- Percutaneous implantation
Mechanical valves

Mechanical heart valves are prosthetics designed to replicate the function of the natural valves of the human heart. The human heart contains four valves: tricuspid valve, pulmonic valve, mitral valve and aortic valve. Their main purpose is to maintain unimpeded forward flow through the heart and from the heart into the major blood vessels connected to the heart, the pulmonary artery and the aorta. As a result of a number of disease processes, both acquired and congenital, any one of the four heart valves may malfunction and result in either stenosis (impeded forward flow) and/or backward flow (regurgitation). Either process burdens the heart and may lead to serious problems including heart failure. A mechanical heart valve is intended to replace a diseased heart valve with its prosthetic equivalent.[1]
There are two basic types of valves that can be used for aortic valve replacement, mechanical and tissue valves. Modern mechanical valves can last indefinitely (the equivalent of over 50,000 years in an accelerated valve wear tester). However, current mechanical heart valves all require lifelong treatment with a blood thinner, e.g. warfarin, which requires monthly blood tests to monitor. This process of thinning the blood is called anticoagulation. Tissue heart valves, in contrast, do not require the use of anticoagulant drugs due to the improved blood flow dynamics resulting in less red cell damage and hence less clot formation. Their main weakness however, is their limited lifespan. Traditional tissue valves, made of pig heart valves, will last on average 15 years before they require replacement. (Studies as of November 2006 suggest that they may last longer in recipients under 50, refuting previous understanding)
Types of MHV's
There are three major types of mechanical valves - caged-ball, tilting-disk and bileaflet - with many modifications on these designs.[2]
The first artificial heart valve was the caged-ball, which utilizes a metal cage to house a metal ball. When blood pressure in the chamber of the heart exceeds that of the pressure on the outside of the chamber the ball is pushed against the cage and allows blood to flow. At the completion of the heart's contraction, the pressure inside the chamber drops and is lower than beyond the valve, so the ball moves back against the base of the valve forming a seal. In 1952, Dr. Charles Hufnagel implanted caged-ball heart valves in ten patients (six survived the operation), marking the first long-term success in prosthetic heart valves. A similar valve was invented by Miles "Lowell" Edwards and Albert Starr in 1960 (commonly referred to as the Starr-Edwards Silastic Ball Valve). The first human implant was on Sept 21, 1960. It consisted of a silicone ball enclosed in a cage formed by wires originating from the valve housing. Caged ball valves have a high tendency to forming blood clots, so the patient must have a high degree of anti-coagulation, usually with a target INR of 2.5-3.5. Edwards Lifesciences discontinued production of the Starr-Edwards valve in 2007.
Soon after came tilting-disc valves, which have a single circular occluder controlled by a metal strut. They are made of a metal ring covered by a tissue, into which the suture threads are stitched in order to hold the valve in place. The metal ring holds, by means of two metal supports, a disc which opens and closes as the heart pumps blood through the valve. The disc is usually made of an extremely hard carbon material (pyrolytic carbon), in order to allow the valve to function for years without wearing out. The Medtronic-Hall model is the most common tilting-disc design in the US. In some models of mechanical valves, the disc is divided into two parts, which open and close as a door.
St. Jude Medical is the leader in bileaflet valves, which consist of two semicircular leaflets that rotate about struts attached to the valve housing. This design was introduced in 1979 and while they take care of some of the issues that were seen in the other models, bileaflets are vulnerable to backflow and so it cannot be considered as ideal. Bileaflet valves do, however, provide much more natural blood flow than caged-ball or tilting-disc implants. One of the main advantages of these valves is that they are well tolerated by the body. Only a small amount of blood thinner is needed to be taken by the patient each day in order to prevent clotting of the blood when flowing through the valve.
These bileaflet valves have the advantage that they have a greater effective opening area (2.4-3.2 square cm c.f. 1.5-2.1 for the single-leaflet valves). Also, they are the least thrombogenic of the artificial valves.
Mechanical heart valves are today very reliable and allow the patient to live a normal life. Most mechanical valves last for at least 20 to 30 years.
Durability
Mechanical heart valves are considered to be extremely durable in comparison to their bioprosthetic counterparts. The struts and occluders are made out of either pyrolytic carbon or titanium coated with pyrolytic carbon, and the sewing ring cuff is Teflon, polyester or dacron. The major load arises from transvalvular pressure generated at and after valve closure, and in cases where structural failure does happen, it is usually as a result of occluder impact on the components.
Impact wear and friction wear dictate the loss of material in MHV’s. Impact wear usually occurs in the hinge regions of bileaflets, between the occluder and ring in tilting-discs, and between the ball and cage in caged-ball valves. Friction wear occurs between the occluder and strut in tilting-discs, and between the leaflet pivots and hinge cavities in bileaflets.
MHV’s made out of metal are also susceptible to fatigue failure owing to the polycrystalline characteristic of metals, but this is not an issue with pyrolytic carbon MHV’s because this material is not crystalline in nature.
Cavitation should also be considered when studying degradation of MHV’s.
Fluid mechanics
Many of the complications associated with MHV’s can be explained through fluid mechanics. For example, thrombus formation is a debilitating side effect of high shear stresses created by the design of the valves. An ideal heart valve from an engineering perspective would produce minimal pressure drops, have small regurgitation volumes, minimize turbulence, reduce prevalence of high stresses, and not create flow separations in the vicinity of the valve.
One measure of the quality of a valve is the effective orifice area (EOA), which can be calculated as follows:
<math>EOA(\mathrm{cm}^2) = \frac{Q_{rms}}{51.6\sqrt{\Delta p}}\ </math>
where <math>Q_{rms}</math> is the root mean square systolic/diastolic flow rate (cm³/s) and <math>\Delta p</math> is the mean systolic/diastolic pressure drop (mmHg). This is a measure of how much the prosthesis impedes blood flow through the valve. A higher EOA corresponds to a smaller energy loss. The performance index (PI) normalizes the EOA by valve size and is a size-independent measure of the valve’s resistance characteristics. Bileaflet valves typically have higher PI’s than tilted-disc models, which in turn have higher PI’s than caged-ball models.
As blood flows through a prosthetic heart valve, a sudden pressure drop occurs across the valve due to the reduction in cross-sectional area within the valve housing. This can be quantified through the continuity equation and Bernoulli’s equation:
<math>A_1V_1 = A_2V_2</math>
<math>P_1 + \frac{1}{2} \rho _1 V_1^2 = P_2 + \frac{1}{2} \rho_2 V_2^2</math>
where A represents the cross-sectional area, P is pressure, <math>\rho</math> is density, and V is the velocity. As cross-sectional area decreases in the valve, velocity increases and pressure drops as a result. This effect is more dramatic in caged-ball valves than in tilting-disc and bileaflet valves. A larger systolic pressure is required to drive flow forward in order to compensate for a large pressure drop, so it should be minimized.
Regurgitation is the sum of retrograde flow during the closing motion of the valve and leakage flow after closure. It is directly proportional to valve size and is also dependent on valve type. Typically, caged-ball valves have a low amount of regurgitation as there is very little leakage. Tilting-disc and bileaflet valves are comparable, with the bileaflet valves have a slightly larger regurgitation volume. Bioprosthetics prevail over MHV’s in this case, as they have virtually no regurgitation volume.
Turbulence and high shear stresses are also major issues with MHV’s, as they can fracture the valve housing or components, or induce blood damage. A large flow gradient can lead to these factors, so flow separation and stagnation should be as small as possible. High stresses are created at the edges of the annular jet in caged-ball valves, in narrow regions at the edges of the major orifice jet in tilting-disc valves, and in regions immediately distal to the valve leaflets in bileaflet valves. The implications of blood damage from these stresses are discussed in the next section.
The cavitation phenomenon can also be described using fluid mechanics. This can result from pressure oscillations, flow deceleration, tip cortices, streamline contraction, and squeeze jets [4]. This last cause is the most contributive factor to cavitation. The squeeze jets are formed when the valve is closing and the blood between the occluder and valve housing is “squeezed” out to create a high-speed jet. This in turn creates intense vortices with very low pressures that can lead to cavitation.
Blood damage
One of the major drawbacks of mechanical heart valves is that patients with these implants require consistent anti-coagulation therapy. Clots formed by red blood cell (RBC) and platelet damage can block up blood vessels and lead to very serious consequences. Clotting occurs in one of three basic pathways: tissue factor exposure, platelet activation, or contact activation by foreign materials, and in three steps: initiation, amplification, and propagation.
In the tissue factor exposure path, initiation begins when cells are ruptured and expose tissue factor (TF). Plasma Factor (f) VII binds to TF and sets off a chain reaction which activates fXa and fVa which bind to each other to produce thrombin which in turn activates platelets and fVIII. The platelets activate by binding to the damaged tissue in the initiation phase, and fibrin stabilizes the clot during the propagation phase.
The platelet activation pathway is triggered when stresses reach a level above 6 to 8 Pa (60–80 dyn/cm²). The steps involved with this are less clearly understood, but initiation begins with the binding of vWF from the plasma to GPIb on the platelet. This is followed by a large influx of Ca2+ ions, which activates the platelets. GPIIb-IIIa facilitates platelet-platelet adhesion during amplification. The propagation step is still under study.
Contact activation begins when fXII binds to a negatively charged surface. This in turn activates prekallikrein (PK) and high-molecular-weight kininogen (HK). Eventually, HKa-PK and HKa-fXI complexes form on the surface. In amplification, Hka-FXIa complexes activate fIX to fIXa, which in turn forms thrombin and platelets. Proteins buildup on the surface and facilitate platelet adhesion and tissue growth in the propagation stage.
All MHV models are vulnerable to thrombus formation due to high shear stress, stagnation, and flow separation. The caged-ball designs experience high stresses at the walls that can damage cells, as well as flow separation due to high-velocity reverse flow surrounded by stagnant flow. Tilting-disc valves have flow separation behind the valve struts and disc as a result of a combination of high velocity and stagnant flows. The bileaflet models have high stresses during forward and leakage flows as well as adjacent stagnant flow in the hinge area. As it turns out, the hinge area is the most critical part of bileaflets and is where the thrombus formation is usually prevalent.
In general, blood damage affects valves in both the mitral and aortic positions. High stresses during leakage flow in aortal valves result from higher transvalvular pressures, and high stresses occur during forward flow for mitral valves. Valvular thrombosis is most common in mitral prosthetics. The caged-ball model is better than the other two models in terms of controlling this problem, because it is at a lower risk for thrombosis and it is gradual when it does happen. The bileaflet is more adaptable to this problem than the tilting-disc model because if one leaflet stops working, the other can still function. However, if the hinge is blocked, both leaflets will stop functioning.
Because all models experience high stresses, patients with mechanical heart valve implants require anti-coagulation therapy. Bioprosthetics are less prone to develop blood clotting, but the trade-off concerning durability generally favors their use in patients older than age 55.
Mechanical heart valves can also cause hemolytic anemia with hemolysis of the red blood cells as they pass through the valve.
Contraindicated medications
Mechanical prosthetic heart valve is considered an absolute contraindication to the use of the following medications:
Biological valves
Biological valves are valves of animals, like pigs, which undergo several chemical procedures in order to make them suitable for implantation in the human heart. The porcine (or pig) heart is most similar to the human heart, and therefore represents the best anatomical fit for replacement. Implantation of a porcine valve is a type of Xenotransplantation, or Xenograft, which means a transplant from one species (in this case a pig) to another. There are some risks associated with a Xenograft such as the human body's tendency to reject foreign material. Medication can be used to retard this effect but is not always successful.[3]
Another type of biological valve utilizes biological tissue to make leaflets that are sewn into a metal frame. This tissue is typically harvested from the Pericardial Sac of either Bovine (cows) or Equine (horses). The pericardial sac is particularly well suited for a valve leaflet due to its highly durable physical properties. This type of biological valve is a highly effective means of valve replacement. The tissue is sterilized so that the biological markers are removed, eliminating a response from the host's immune system. The leaflets are flexible and durable and do not require the patient to take blood thinners for the rest of their life.
The most used heart valves in the US and EU are those utilizing tissue leaflets. Mechanical valves are more commonly used in Asia and Latin America. The following companies manufacture tissue heart valves: Edwards Lifesciences, Medtronic, St. Jude Medical, Sorin, ATS, 3F, and CryoLife.
Functional requirements of heart valve prostheses
Many advantages characterize the functioning of natural heart valves:
- Minimal regurgitation - This means that the amount of blood lost upstream as the valve closes is small. For example, closure regurgitation through the mitral valve would result in some blood loss from the left ventricle to the left atrium as the mitral valve closes. Some degree of valvular regurgitation is inevitable and natural (Fixme: Give indicative value). However, several heart valve pathologies (e.g. rheumatic endocarditis) may lead to clinically significant valvular regurgitation. A desirable characteristic of heart valve prostheses is that regurgitation is minimal over the full range of physiological heart function (i.e. complete functional envelope of cardiac output vs. heart rate).
- Minimal transvalvular pressure gradient - Whenever a fluid flows through a restriction, such as a valve, a pressure gradient arises over the restriction. This pressure gradient is a result of the increased resistance to flow through the restriction. Natural heart valves have a low transvalvular pressure gradient as they present a little obstruction to the flow through themselves (Fixme: Give indicative value). A desirable characteristic of heart valve prostheses is that their transvalvular pressure gradient is as tiny as possible.
- Non-thrombogenic - As natural heart valves are lined with an endothelium continuous with the endothelium lining the heart chambers they are not normally thrombogenic. This is important as should thrombus form on the heart valve leaflets and become seeded with bacteria, so-called "bacterial vegetations" will form. Such vegetations are difficult for the body to deal with as the normal physiological defense mechanisms are not present within the valve leaflets because they are avascular and largely composed of connective tissue.
- If bacterial vegetation form on the valve leafets they may continually seed bacteria into the arterial tree which may lead to bacteremia or septicaemia. *Portions of the vegetation may also break off forming septic emboli.
- Septic emboli can lodge anywhere in the arterial tree (e.g. brain, bowel, lungs) causing local infectious foci.
- Even dislodged fragments from non-infectious vegetations can be hazardous as they can lodge in, and block, downstream arteries (e.g. coronary arteries leading to myocardial infarction, cerebral arteries leading to stroke). A desirable characteristic of heart valve prostheses is that they are non or minimally thrombogenic.
- Self-repairing - Although of limited extent compared to well-vascularized tissue (e.g. muscle), the valve leaflets do retain some capacity for repair due to the presence of regenerative cells (e.g. fibroblasts) in the connective tissue from which the leaflets are composed. As the human heart beats approximately 3.4x109 times during a typical human lifespan this limited but nevertheless present repair capacity is critically important. No heart valve prostheses can currently self-repair but replacement tissues grown using stem cell technology may eventually offer such capabilities. (State that they wear).
- Rapid dynamic response - STD
Design challenges of heart valve prostheses
- Thrombogenesis / haemocompatibility
- Mechanisms:
- Forward and backward flow shear
- Static leakage shear
- Presence of foreign material (i.e. intrinsic coagulation cascade)
- Cellular maceration
- Mechanisms:
- Valve-tissue interaction
- Wear
- Blockage
- Getting stuck
- Dynamic responsiveness
- Failure safety
- Valve orifice to anatomical orifice ratio
- Trans-valvular pressure gradient
- Minimal leakages
Typical configuration of a heart valve prosthesis
- Anchor
- Leaflets
MHV manufacturers
Companies that manufacture MHVs include:
- ATS Medical, Inc.
- CarboMedics
- Edwards Lifesciences (formerly Baxter-Edwards Critical Care)
- Medtronic
- St. Jude
Guidelines for management of prosthetic valve disease
| Recommendations for prosthetic valve disease | |
| Mechanical protheses (Class I, Level of Evidence C): | |
|
❑A mechanical prosthesis is recommended according to the desire of the informed patient and NO contraindications to long term anticoagulation | |
| (Class IIa, Level of Evidence C): | |
|
❑A mechanical prosthesis should be considered in patients already on anticoagulation because of a mechanical prosthesis in another valve position | |
| (Class IIa, Level of Evidence B): | |
|
❑A mechanical prosthesis should be considered in patients aged <60 years for prostheses in the aortic position and aged <65 years for prostheses in the mitral position | |
| (Class IIb, Level of Evidence C): | |
|
❑A mechanical prosthesis may be considered in patients already on long-term anticoagulation due to the high risk for thromboembolism | |
| Biological prothesis (Class I, Level of Evidence C): | |
|
❑A bioprosthesis is recommended according to the desire of the informed patient | |
| (Class IIa, Level of Evidence C): | |
|
❑A bioprosthesis should be considered in patients for whom there is a low likelihood and/or a low operative risk of future redo valve surgery | |
| (Class IIb, Level of Evidence B): | |
|
❑A bioprosthesis may be considered in patients already on long-term NOACs for whom are high risk for thromboembolism |
| The above table adopted from 2021 ESC Guideline[4] |
|---|
Abbreviations:
TAVI: Transcatheter aortic valve implantation;
AF: Atrial fibrillation;
NOAC: Non-vitamin K antagonist oral anticoagulant;
| Recommendations for management of antithrombotic therapy after prosthetic valve implantation or valve repair in the perioperative and postoperative periods |
| Management of antithrombotic therapy in the perioperative period (Class I, Level of Evidence C): |
|
❑It is recommended discontinuation of VKA prior to elective surgery to aim for an INR <1.5
❑In patients who have undergone valve surgery with an indication for postoperative therapeutic bridging, it is recommended to initiate either UFH
or LMWH 12-24 h after surgery |
| (Class I, Level of Evidence B): |
|
❑For bridging, therapeutic doses of either UFH or subcutaneous LMWH are recommended |
| (Class I, Level of Evidence C): |
|
❑ Re-initiation of the VKA on the first postoperative day is recommended in patients with mechanical valve |
| (Class IIb, Level of Evidence C): |
|
❑A mechanical prosthesis may be considered in patients already on long-term anticoagulation due to the high risk for thromboembolism |
| Concomitant antiplatelet therapy (Class I, Level of Evidence B): |
|
❑If the risk of stent thrombosis is low, in patients undergone PCI or after ACS requiring long-term OAC, early cessation (≤1 week) of aspirin and continuation of dual therapy with OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 6 months (or up to 12 months in ACS) is recommended |
| (Class IIa, Level of Evidence C): |
|
❑If the risk of stent thrombosis is high, in patients undergone PCI or after ACS requiring both OAC and antiplatelet therapy, triple therapy with aspirin, clopidogrel and OAC for longer than 1 week should be considered with the total duration (≤1 month) |
| (Class IIa, Level of Evidence B): |
|
❑In patients with mechanical heart valve treated with a VKA and low risk for stent thrombosis and HAS-BLED ≥ 3 , clopidogrel alone should be considered
for up to 12 months |
| Surgical valve replacement (Class I, Level of Evidence B): |
|
❑For all patients with an mechanical heart valve prosthesis, OAC using a VKA is recommended lifelong |
| (Class IIa, Level of Evidence B): |
|
❑ In patients with biological heart valve and AF, NOACs should be considered over VKA after 3 months following surgical implantation |
| (Class IIb, Level of Evidence C): |
|
❑ In patients with mechanical heart valve and evidence of atherosclerotic disease and low risk of bleeding, The addition of low-dose aspirin (75-100 mg/
day) to VKA may be considered in selected patients |
| (Class IIa, Level of Evidence C): |
|
❑ Low-dose aspirin (75-100 mg/day) in addition to VKA should be considered after thromboembolism despite an adequate INR |
| (Class III, Level of Evidence B): |
|
❑NOACs are not recommended in patients with a mechanical valve prosthesis |
| Surgical valve repair (Class IIa, Level of Evidence C): |
|
❑OAC with VKA should be considered during the first 3 months after mitral and tricuspid repair |
| Transcatheter aortic valve replacement (Class I, Level of Evidence B): |
|
❑OAC is recommended lifelong for TAVI patients who have other indications for OAC |
| (Class I, Level of Evidence A): |
|
❑Lifelong SAPT is recommended after TAVI in patients with no baseline indication for OAC |
| (Class III, Level of Evidence B): |
|
❑ Routine use OAC is not recommended after TAVI in patients with no baseline indication for OAC |
| The above table adopted from 2021 ESC Guideline[4] |
|---|
Abbreviations:
ACS: Acute coronary syndrome;
AF: Atrial fibrillation;
NOAC: Non-vitamin K antagonist oral anticoagulant;
TAVI: Transcatheter aortic valve implantation;
DAPT: Dual antiplatelet therapy;
INR: International normalized ratio;
LMWH: Low molecular weight heparin;
LV: Left ventricular;
PCI:Percutaneous coronary intervention;
OAC:Oral anticoagulation;
SAPT:Single antiplatelet therap;
UFH: Unfractionated heparin;
VKA:Vitamin K antagonist
| Recommendations for management of prosthetic valve dysfunction |
| Mechanical prosthetic thrombosis (Class I, Level of Evidence B): |
|
❑ In patients with obstructive thrombosis who are critically ill patients without serious comorbidities, urgent or emergency valve replacement is recommended |
| (Class IIa, Level of Evidence B): |
|
❑Fibrinolysis (using recombinant tissue plasminogen activator 10 mg bolus + 90 mg in 90 min with UFH or streptokinase 1500 000 U in 60 min without UFH) should be considered when surgery is very high risk or is not available , or for thrombosis of right-sided prostheses |
| (Class IIa, Level of Evidence C): |
|
❑Surgery should be considered for large (>10 mm) non-obstructive prosthetic thrombus complicated by embolism |
| Bioprosthetic thrombosis (Class I, Level of Evidence C): |
|
❑ In bioprosthetic valve thrombosis, anticoagulation using a VKA and/or UFH is recommended before considering re-intervention |
| (Class IIa, Level of Evidence B): |
|
❑ Anticoagulation should be considered in patients with leaflet thickening and reduced leaflet motion causing elevated gradients, at least until resolution |
| Hemolysis and paravalvular leak (Class I, Level of Evidence C): |
|
❑ Reoperation is considered when a paravalvular leak is related to endocarditis or leading haemolysis requiring repeated blood transfusions or causes severe heart failure symptoms |
| (Class IIa, Level of Evidence B): |
|
❑Transcatheter closure is recommended for suitable paravalvular leaks with clinically significant regurgitation and/or haemolysis in high risk patients for surgery |
| (Class IIa, Level of Evidence C): |
|
❑ Transcatheter or surgical closure of clinically significant paravalvular leaks is considered based on patient risk status, leak morphology, and local expertise |
| Bioprosthetic failure (Class I, Level of Evidence C): |
|
❑Reoperation is recommended in symptomatic patients with severe regurgitation or a significant increase in transprosthetic gradient (after exclusion of valve thrombosis) |
| (Class IIa, Level of Evidence B): |
|
❑ Desion of Transcatheter, transfemoral valve-in-valve implantation in the aortic position should be considered by the Heart Team based on anatomic considerations, features of the prosthesis, and high risk patients for surgery or inoperable patients |
| (Class IIb, Level of Evidence B): |
|
❑ Transcatheter valve-in-valve implantation in the mitral and tricuspid position may be considered in high risk patients for surgery |
| (Class IIa, Level of Evidence C): |
|
❑Reoperation should be considered in asymptomatic patients with significant prosthetic dysfunction if reoperation is low risk |
| The above table adopted from 2021 ESC Guideline[4] |
|---|
Abbreviations:
UFH: Unfractionated heparin;
VKA: Vitamin K antagonist
| Antithrombotic therapy for valve prostheses | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanical heart valve | Bioprosthetic heart valve | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VKA lifelong (Class I) | MVR/TVR | SAVR | TAVI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAD | Other indications for oral anticoagulation | Other indications for oral anticoagulation | Other indications for oral anticoagulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add low-dose ASA in low risk patients for bleeding (Class IIb) | NO | Yes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subtherapeutic INR for major planned invasive procedure | OAC for 3 months (Class IIa) | OAC long-term (Class I) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bridging anticoagulation with UFH or LMWH (not required for minor surgeries) (Class I) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NO | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPT or OAC for 3 months (Class IIa) | OAC long-term (Class I) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NO | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPT long-term (Class I) | OAC long-term (Class I) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The above algorithm adopted from 2021 ESC Guideline[4] |
|---|
Abbreviations:
ASA: acetylsalicylic acid;
AF: Atrial fibrillation;
CAD: Coronary artery disease;
TAVI: Transcatheter aortic valve implantation;
DAPT: Dual antiplatelet therapy;
INR: International normalized ratio;
LMWH: Low molecular weight heparin;
LV: Left ventricular;
SAVR:Surgical aortic valve replacement;
OAC:Oral anticoagulation;
SAPT:Single antiplatelet therap;
UFH: Unfractionated heparin;
VKA:Vitamin K antagonist;
TVR:Tricuspid valve replacement or repair;
MVR:Mitral valve replacement or repair
| Recommendations for anticoagulation for atrial fibrillation in valvular heart disease |
| NOAC (Class I, Level of Evidence A): |
|
❑ Except those with rheumatic mitral stenosis, NOAC is recommended in patients with AF and VHD , or who received a bioprothesis valve > 3 months ago on the basis of CHA2DS2-VASc score |
| VKA (Class I, Level of Evidence C): |
|
❑ Long term VKA oral anticoagulation is recommended in patients with AF and rheumatic MS |
| VKA:(Class IIa, Level of Evidence B) : |
|
❑ Anticoagulation with VKA is reasonable in patients with new onset AF ≤ 3 months after surgical or transcatheter bioprothetic valve replacement |
| NOAC : (Class III: Harm, Level of Evidence B) |
|
❑ NOAC is not recommended in patients with mechanical valve with or without AF, and VKA should be continued for prevention of valve thrombosis formation |
Abbreviations:
NOAC: Novel oral anticoagulant;
VKA: Vitamin-K antagonist;
AF: Artial fibrillation
| The above table adopted from 2020 AHA Guideline[5] |
|---|
| Recommendations for anticoagulant therapy in valvular heart disease |
| NOAC (Class I, Level of Evidence A): |
|
❑ Except those with rheumatic mitral stenosis, NOAC is recommended in patients with AF and VHD , or who received a bioprothesis valve > 3 months ago on the basis of CHA2DS2-VASc score |
| VKA (Class I, Level of Evidence C): |
|
❑ Long term VKA oral anticoagulation is recommended in patients with AF and rheumatic MS |
| VKA:(Class IIa, Level of Evidence B) : |
|
❑ Anticoagulation with VKA is reasonable in patients with new onset AF ≤ 3 months after surgical or transcatheter bioprothetic valve replacement |
| NOAC : (Class III: Harm, Level of Evidence B) |
|
❑ NOAC is not recommended in patients with mechanical valve with or without AF, and VKA should be continued for prevention of valve thrombosis formation |
Abbreviations:
CAD: Coronary artery disease;
VKA: Vitamin-K antagonist;
AF: Artial fibrillation;
MS Mitral stenosis
| The above table adopted from 2020 AHA Guideline[5] |
|---|
References
- ↑ Gott VL, Alejo DE, Cameron DE (December 2003). "Mechanical heart valves: 50 years of evolution". Ann Thorac Surg. 76 (6): S2230–9. doi:10.1016/j.athoracsur.2003.09.002. PMID 14667692.
- ↑ Kostrzewa B, Rybak Z (2013). "[History, present and future of biomaterials used for artificial heart valves]". Polim Med (in Polish). 43 (3): 183–9. PMID 24377185.
- ↑ Kueri S, Kari FA, Fuentes RA, Sievers HH, Beyersdorf F, Bothe W (June 2019). "The Use of Biological Heart Valves". Dtsch Arztebl Int. 116 (25): 423–430. doi:10.3238/arztebl.2019.0423. PMC 6706839 Check
|pmc=value (help). PMID 31423972. - ↑ 4.0 4.1 4.2 4.3 Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W (February 2022). "2021 ESC/EACTS Guidelines for the management of valvular heart disease". Eur Heart J. 43 (7): 561–632. doi:10.1093/eurheartj/ehab395. PMID 34453165 Check
|pmid=value (help). - ↑ 5.0 5.1 Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C (February 2021). "2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation. 143 (5): e35–e71. doi:10.1161/CIR.0000000000000932. PMID 33332149 Check
|pmid=value (help).