Sandbox: treatment: Difference between revisions
| Line 45: | Line 45: | ||
*The VAD regimen ([[vincristine]], [[doxorubicin|adriamycin]], and dexamethasone) has shown efficacy ranging from 60 to 80%, in previously treated and untreated patients. This regimen is beneficial in the manner that it avoids early exposure to alkylating agents thus reducing the risks of [[myelosuppression]] and further leukemia's, without compromising on efficacy.<ref name="Alexanian-1990">{{Cite journal | last1 = Alexanian | first1 = R. | last2 = Barlogie | first2 = B. | last3 = Tucker | first3 = S. | title = VAD-based regimens as primary treatment for multiple myeloma. | journal = Am J Hematol | volume = 33 | issue = 2 | pages = 86-9 | month = Feb | year = 1990 | doi = | PMID = 2301376 }}</ref><ref name="Segeren-1999">{{Cite journal | last1 = Segeren | first1 = CM. | last2 = Sonneveld | first2 = P. | last3 = van der Holt | first3 = B. | last4 = Baars | first4 = JW. | last5 = Biesma | first5 = DH. | last6 = Cornellissen | first6 = JJ. | last7 = Croockewit | first7 = AJ. | last8 = Dekker | first8 = AW. | last9 = Fibbe | first9 = WE. | title = Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. | journal = Br J Haematol | volume = 105 | issue = 1 | pages = 127-30 | month = Apr | year = 1999 | doi = | PMID = 10233375 }}</ref><ref name="Anderson-1995">{{Cite journal | last1 = Anderson | first1 = H. | last2 = Scarffe | first2 = JH. | last3 = Ranson | first3 = M. | last4 = Young | first4 = R. | last5 = Wieringa | first5 = GS. | last6 = Morgenstern | first6 = GR. | last7 = Fitzsimmons | first7 = L. | last8 = Ryder | first8 = D. | title = VAD chemotherapy as remission induction for multiple myeloma. | journal = Br J Cancer | volume = 71 | issue = 2 | pages = 326-30 | month = Feb | year = 1995 | doi = | PMID = 7841049 }}</ref> | *The VAD regimen ([[vincristine]], [[doxorubicin|adriamycin]], and dexamethasone) has shown efficacy ranging from 60 to 80%, in previously treated and untreated patients. This regimen is beneficial in the manner that it avoids early exposure to alkylating agents thus reducing the risks of [[myelosuppression]] and further leukemia's, without compromising on efficacy.<ref name="Alexanian-1990">{{Cite journal | last1 = Alexanian | first1 = R. | last2 = Barlogie | first2 = B. | last3 = Tucker | first3 = S. | title = VAD-based regimens as primary treatment for multiple myeloma. | journal = Am J Hematol | volume = 33 | issue = 2 | pages = 86-9 | month = Feb | year = 1990 | doi = | PMID = 2301376 }}</ref><ref name="Segeren-1999">{{Cite journal | last1 = Segeren | first1 = CM. | last2 = Sonneveld | first2 = P. | last3 = van der Holt | first3 = B. | last4 = Baars | first4 = JW. | last5 = Biesma | first5 = DH. | last6 = Cornellissen | first6 = JJ. | last7 = Croockewit | first7 = AJ. | last8 = Dekker | first8 = AW. | last9 = Fibbe | first9 = WE. | title = Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. | journal = Br J Haematol | volume = 105 | issue = 1 | pages = 127-30 | month = Apr | year = 1999 | doi = | PMID = 10233375 }}</ref><ref name="Anderson-1995">{{Cite journal | last1 = Anderson | first1 = H. | last2 = Scarffe | first2 = JH. | last3 = Ranson | first3 = M. | last4 = Young | first4 = R. | last5 = Wieringa | first5 = GS. | last6 = Morgenstern | first6 = GR. | last7 = Fitzsimmons | first7 = L. | last8 = Ryder | first8 = D. | title = VAD chemotherapy as remission induction for multiple myeloma. | journal = Br J Cancer | volume = 71 | issue = 2 | pages = 326-30 | month = Feb | year = 1995 | doi = | PMID = 7841049 }}</ref> | ||
* | *One of the commonly used induction regimens include [[dexamethasone]] with [[lenalidomide]] and [[bortezomib]] for a period of 8 months based on the side effect profile and associated co-morbidities.<ref name="Mateos-2010">{{Cite journal | last1 = Mateos | first1 = MV. | last2 = Richardson | first2 = PG. | last3 = Schlag | first3 = R. | last4 = Khuageva | first4 = NK. | last5 = Dimopoulos | first5 = MA. | last6 = Shpilberg | first6 = O. | last7 = Kropff | first7 = M. | last8 = Spicka | first8 = I. | last9 = Petrucci | first9 = MT. | title = Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. | journal = J Clin Oncol | volume = 28 | issue = 13 | pages = 2259-66 | month = May | year = 2010 | doi = 10.1200/JCO.2009.26.0638 | PMID = 20368561 }}</ref><ref name="Rajkumar-2010">{{Cite journal | last1 = Rajkumar | first1 = SV. | last2 = Jacobus | first2 = S. | last3 = Callander | first3 = NS. | last4 = Fonseca | first4 = R. | last5 = Vesole | first5 = DH. | last6 = Williams | first6 = ME. | last7 = Abonour | first7 = R. | last8 = Siegel | first8 = DS. | last9 = Katz | first9 = M. | title = Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. | journal = Lancet Oncol | volume = 11 | issue = 1 | pages = 29-37 | month = Jan | year = 2010 | doi = 10.1016/S1470-2045(09)70284-0 | PMID = 19853510 }}</ref> | ||
*[[Lenalidomide]] is orally administered and increases the risk for deep vein thrombosis, while [[bortezomib]] is given IV and preferred in patients with renal | *[[Lenalidomide]] is orally administered and increases the risk for deep vein thrombosis, while [[bortezomib]] is given IV and preferred in patients with abnormal renal function tests.<ref name="Facon-2006">{{Cite journal | last1 = Facon | first1 = T. | last2 = Mary | first2 = JY. | last3 = Pégourie | first3 = B. | last4 = Attal | first4 = M. | last5 = Renaud | first5 = M. | last6 = Sadoun | first6 = A. | last7 = Voillat | first7 = L. | last8 = Dorvaux | first8 = V. | last9 = Hulin | first9 = C. | title = Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. | journal = Blood | volume = 107 | issue = 4 | pages = 1292-8 | month = Feb | year = 2006 | doi = 10.1182/blood-2005-04-1588 | PMID = 16174762 }}</ref><ref name="Shustik-2007">{{Cite journal | last1 = Shustik | first1 = C. | last2 = Belch | first2 = A. | last3 = Robinson | first3 = S. | last4 = Rubin | first4 = SH. | last5 = Dolan | first5 = SP. | last6 = Kovacs | first6 = MJ. | last7 = Grewal | first7 = KS. | last8 = Walde | first8 = D. | last9 = Barr | first9 = R. | title = A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. | journal = Br J Haematol | volume = 136 | issue = 2 | pages = 203-11 | month = Jan | year = 2007 | doi = 10.1111/j.1365-2141.2006.06405.x | PMID = 17233817 }}</ref> | ||
*[[Bortezomib]] is given IV and preferred in patients with renal | *[[Bortezomib]] is given IV and preferred in patients with abnormal renal function tests.<ref name="Facon-2006">{{Cite journal | last1 = Facon | first1 = T. | last2 = Mary | first2 = JY. | last3 = Pégourie | first3 = B. | last4 = Attal | first4 = M. | last5 = Renaud | first5 = M. | last6 = Sadoun | first6 = A. | last7 = Voillat | first7 = L. | last8 = Dorvaux | first8 = V. | last9 = Hulin | first9 = C. | title = Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. | journal = Blood | volume = 107 | issue = 4 | pages = 1292-8 | month = Feb | year = 2006 | doi = 10.1182/blood-2005-04-1588 | PMID = 16174762 }}</ref><ref name="Shustik-2007">{{Cite journal | last1 = Shustik | first1 = C. | last2 = Belch | first2 = A. | last3 = Robinson | first3 = S. | last4 = Rubin | first4 = SH. | last5 = Dolan | first5 = SP. | last6 = Kovacs | first6 = MJ. | last7 = Grewal | first7 = KS. | last8 = Walde | first8 = D. | last9 = Barr | first9 = R. | title = A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. | journal = Br J Haematol | volume = 136 | issue = 2 | pages = 203-11 | month = Jan | year = 2007 | doi = 10.1111/j.1365-2141.2006.06405.x | PMID = 17233817 }}</ref> | ||
*Dexamethasone is used alone at a dose of 40 mg for 4 days consecutively, and equals efficacy to that shown by a combination with VAD ([[vincristine]], [[doxorubicin|adriamycin]], and dexamethasone).<ref name="Facon-2006">{{Cite journal | last1 = Facon | first1 = T. | last2 = Mary | first2 = JY. | last3 = Pégourie | first3 = B. | last4 = Attal | first4 = M. | last5 = Renaud | first5 = M. | last6 = Sadoun | first6 = A. | last7 = Voillat | first7 = L. | last8 = Dorvaux | first8 = V. | last9 = Hulin | first9 = C. | title = Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. | journal = Blood | volume = 107 | issue = 4 | pages = 1292-8 | month = Feb | year = 2006 | doi = 10.1182/blood-2005-04-1588 | PMID = 16174762 }}</ref><ref name="Shustik-2007">{{Cite journal | last1 = Shustik | first1 = C. | last2 = Belch | first2 = A. | last3 = Robinson | first3 = S. | last4 = Rubin | first4 = SH. | last5 = Dolan | first5 = SP. | last6 = Kovacs | first6 = MJ. | last7 = Grewal | first7 = KS. | last8 = Walde | first8 = D. | last9 = Barr | first9 = R. | title = A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. | journal = Br J Haematol | volume = 136 | issue = 2 | pages = 203-11 | month = Jan | year = 2007 | doi = 10.1111/j.1365-2141.2006.06405.x | PMID = 17233817 }}</ref> | *Dexamethasone is used alone at a dose of 40 mg for 4 days consecutively, and equals efficacy to that shown by a combination with VAD ([[vincristine]], [[doxorubicin|adriamycin]], and dexamethasone).<ref name="Facon-2006">{{Cite journal | last1 = Facon | first1 = T. | last2 = Mary | first2 = JY. | last3 = Pégourie | first3 = B. | last4 = Attal | first4 = M. | last5 = Renaud | first5 = M. | last6 = Sadoun | first6 = A. | last7 = Voillat | first7 = L. | last8 = Dorvaux | first8 = V. | last9 = Hulin | first9 = C. | title = Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. | journal = Blood | volume = 107 | issue = 4 | pages = 1292-8 | month = Feb | year = 2006 | doi = 10.1182/blood-2005-04-1588 | PMID = 16174762 }}</ref><ref name="Shustik-2007">{{Cite journal | last1 = Shustik | first1 = C. | last2 = Belch | first2 = A. | last3 = Robinson | first3 = S. | last4 = Rubin | first4 = SH. | last5 = Dolan | first5 = SP. | last6 = Kovacs | first6 = MJ. | last7 = Grewal | first7 = KS. | last8 = Walde | first8 = D. | last9 = Barr | first9 = R. | title = A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. | journal = Br J Haematol | volume = 136 | issue = 2 | pages = 203-11 | month = Jan | year = 2007 | doi = 10.1111/j.1365-2141.2006.06405.x | PMID = 17233817 }}</ref> | ||
*Thalidomide: A number of trials were conducted to assess the efficacy of thalidomide as an independent induction agent. All of them found improved response rates with thalidomide and no reduction in stem cell load.<ref name="Rajkumar-2008">{{Cite journal | last1 = Rajkumar | first1 = SV. | last2 = Rosiñol | first2 = L. | last3 = Hussein | first3 = M. | last4 = Catalano | first4 = J. | last5 = Jedrzejczak | first5 = W. | last6 = Lucy | first6 = L. | last7 = Olesnyckyj | first7 = M. | last8 = Yu | first8 = Z. | last9 = Knight | first9 = R. | title = Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. | journal = J Clin Oncol | volume = 26 | issue = 13 | pages = 2171-7 | month = May | year = 2008 | doi = 10.1200/JCO.2007.14.1853 | PMID = 18362366 }}</ref><ref name="Barlogie-2006">{{Cite journal | last1 = Barlogie | first1 = B. | last2 = Tricot | first2 = G. | last3 = Anaissie | first3 = E. | last4 = Shaughnessy | first4 = J. | last5 = Rasmussen | first5 = E. | last6 = van Rhee | first6 = F. | last7 = Fassas | first7 = A. | last8 = Zangari | first8 = M. | last9 = Hollmig | first9 = K. | title = Thalidomide and hematopoietic-cell transplantation for multiple myeloma. | journal = N Engl J Med | volume = 354 | issue = 10 | pages = 1021-30 | month = Mar | year = 2006 | doi = 10.1056/NEJMoa053583 | PMID = 16525139 }}</ref><ref name="Palumbo-2008">{{Cite journal | last1 = Palumbo | first1 = A. | last2 = Bringhen | first2 = S. | last3 = Liberati | first3 = AM. | last4 = Caravita | first4 = T. | last5 = Falcone | first5 = A. | last6 = Callea | first6 = V. | last7 = Montanaro | first7 = M. | last8 = Ria | first8 = R. | last9 = Capaldi | first9 = A. | title = Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. | journal = Blood | volume = 112 | issue = 8 | pages = 3107-14 | month = Oct | year = 2008 | doi = 10.1182/blood-2008-04-149427 | PMID = 18505783 }}</ref> | *Thalidomide: A number of trials were conducted to assess the efficacy of thalidomide as an independent induction agent. All of them found improved response rates with thalidomide and no reduction in stem cell load.<ref name="Rajkumar-2008">{{Cite journal | last1 = Rajkumar | first1 = SV. | last2 = Rosiñol | first2 = L. | last3 = Hussein | first3 = M. | last4 = Catalano | first4 = J. | last5 = Jedrzejczak | first5 = W. | last6 = Lucy | first6 = L. | last7 = Olesnyckyj | first7 = M. | last8 = Yu | first8 = Z. | last9 = Knight | first9 = R. | title = Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. | journal = J Clin Oncol | volume = 26 | issue = 13 | pages = 2171-7 | month = May | year = 2008 | doi = 10.1200/JCO.2007.14.1853 | PMID = 18362366 }}</ref><ref name="Barlogie-2006">{{Cite journal | last1 = Barlogie | first1 = B. | last2 = Tricot | first2 = G. | last3 = Anaissie | first3 = E. | last4 = Shaughnessy | first4 = J. | last5 = Rasmussen | first5 = E. | last6 = van Rhee | first6 = F. | last7 = Fassas | first7 = A. | last8 = Zangari | first8 = M. | last9 = Hollmig | first9 = K. | title = Thalidomide and hematopoietic-cell transplantation for multiple myeloma. | journal = N Engl J Med | volume = 354 | issue = 10 | pages = 1021-30 | month = Mar | year = 2006 | doi = 10.1056/NEJMoa053583 | PMID = 16525139 }}</ref><ref name="Palumbo-2008">{{Cite journal | last1 = Palumbo | first1 = A. | last2 = Bringhen | first2 = S. | last3 = Liberati | first3 = AM. | last4 = Caravita | first4 = T. | last5 = Falcone | first5 = A. | last6 = Callea | first6 = V. | last7 = Montanaro | first7 = M. | last8 = Ria | first8 = R. | last9 = Capaldi | first9 = A. | title = Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. | journal = Blood | volume = 112 | issue = 8 | pages = 3107-14 | month = Oct | year = 2008 | doi = 10.1182/blood-2008-04-149427 | PMID = 18505783 }}</ref> | ||
Revision as of 13:15, 23 September 2015
|
Multiple myeloma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox: treatment On the Web |
|
American Roentgen Ray Society Images of Sandbox: treatment |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Haytham Allaham, M.D. [2]
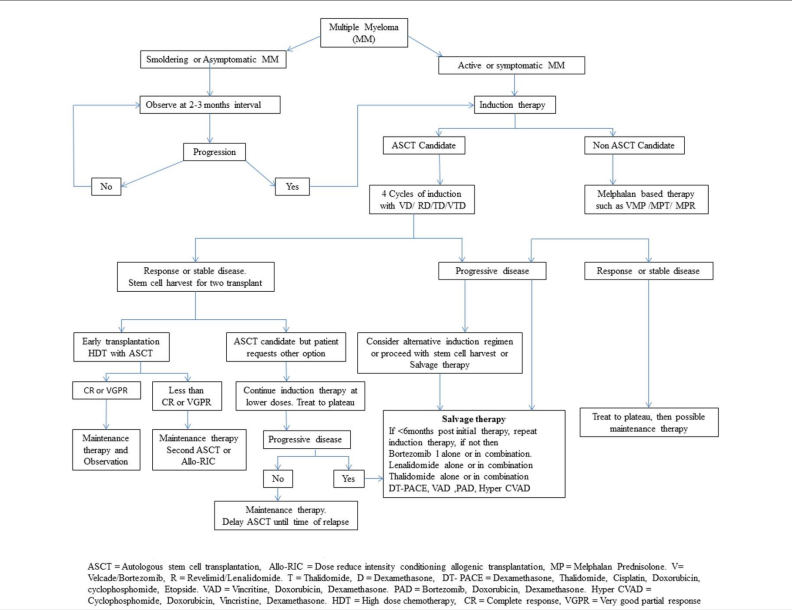
Overview
Pharmacological regimes for patients with active (symptomatic) multiple myeloma include steroid therapy, immune modulator therapy, and chemotherapy.[1][2] Whereas patients with smoldering (asymptomatic) multiple myeloma are managed by observation and undergoing follow up tests every 3 to 6 months.[3][4] The optimal therapy for active multiple myeloma depends on whether or not a patient is eligible for bone marrow transplantation.[5][6] Pharmacologic medical therapy for active multiple myeloma patients who are eligible for a bone marrow transplant include either dexamethasone, lenalidomide, bortezomib, thalidomide, carfilzomib, cyclophosphamide, vincristine, or doxorubicin.[7][8] In addition to the aforementioned agents, pharmacological regimes used for treatment of active multiple myeloma patients who are not eligible for a bone marrow transplant include either melphalan or prednisone.[9][10] Alkylating agents are not recommended among transplant eligible patients, as the toxicity of such agents makes the harvest process of bone marrow stem cell difficult later in the course of the disease.[11][12]
Medical Therapy
Smoldering multiple myeloma
- Patients with smoldering (asymptomatic) multiple myeloma are managed by observation and follow up tests every 3 to 6 months.[13][14]
- Follow up tests for asymptomatic multiple myeloma patients include:[15][16]
- Laboratory studies such as complete blood count, electrolytes, 24 hour urine collection, electrophoresis, and quantitative immunoglobulin analysis
- Imaging studies such as skeletal survey, MRI, and PET scan
- Flow cytometry as needed
- Bone marrow aspiration and biopsy as needed
- Treatment should be differed until such patients develop symptoms.[17][18]
Active multiple myeoloma
- The optimal therapy for active multiple myeloma depends on whether or not a patient is eligible for bone marrow transplantation.[19][20]
- Deciding whether a patient is a candidate for bone marrow transplantation depends on a number of key factors that include:[21][22]
- Type of chromosomal mutation
- Age
- Presence of current associated diseases
- Patient's liver function
- Patient's kidney function
Initial therapy
- Preferred pharmacological regimes for treating bone marrow transplant eligible patients include:[23][24]
- Bortezomib/dexamethasone
- Bortezomib/dexamethasone/lenalidomide
- Dexamethasone/lenalidomide
- Bortezomib/dexamethasone/doxorubicin
- Bortezomib/dexamethasone/thalidomide
- Bortezomib/dexamethasone/cyclophosphamide
- Bortezomib/dexamethasone
- Low dose dexamethasone/lenalidomide
- Melphalan/prednisone/thalidomide
- Melphalan/prednisone/bortezomib
- The VAD regimen (vincristine, adriamycin, and dexamethasone) has shown efficacy ranging from 60 to 80%, in previously treated and untreated patients. This regimen is beneficial in the manner that it avoids early exposure to alkylating agents thus reducing the risks of myelosuppression and further leukemia's, without compromising on efficacy.[27][28][29]
- One of the commonly used induction regimens include dexamethasone with lenalidomide and bortezomib for a period of 8 months based on the side effect profile and associated co-morbidities.[30][31]
- Lenalidomide is orally administered and increases the risk for deep vein thrombosis, while bortezomib is given IV and preferred in patients with abnormal renal function tests.[32][33]
- Bortezomib is given IV and preferred in patients with abnormal renal function tests.[32][33]
- Dexamethasone is used alone at a dose of 40 mg for 4 days consecutively, and equals efficacy to that shown by a combination with VAD (vincristine, adriamycin, and dexamethasone).[32][33]
- Thalidomide: A number of trials were conducted to assess the efficacy of thalidomide as an independent induction agent. All of them found improved response rates with thalidomide and no reduction in stem cell load.[34][35][36]
- Alkylating agents are equal in efficacy when combined with VAD regimens, two of the most common ones being melphalan and oral cyclophosphamide plus prednisolone.[37]
Supportive therapy
- Supportive therapy is recommended based on patient's symptoms and medication's side effect.[38][39]
- Supportive therapy for active multiple myeloma includes:[40][41]
- Bisphosphonates to prevent osteoporosis
- Erythropoietin to prevent anemia
- Vaccines to prevent recurrent infections
- Blood thinners to prevent blood clots
- Plasmapheresis to prevent hyperviscosity and renal failure
Maintenance therapy
- After a few months of induction therapy the advantages of continuing the same therapy seems to be limited.[42][43]
- Therefore, this phase is being followed up with maintenance therapy with one of the newer agents such as thalidomide, lenalidomide or bortezomib.[44][45]
- However, further clinical trials are needed to establish the efficacy of each of these agents.[46][47]
Gallery
References
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Alexanian, R.; Barlogie, B.; Tucker, S. (1990). "VAD-based regimens as primary treatment for multiple myeloma". Am J Hematol. 33 (2): 86–9. PMID 2301376. Unknown parameter
|month=ignored (help) - ↑ Segeren, CM.; Sonneveld, P.; van der Holt, B.; Baars, JW.; Biesma, DH.; Cornellissen, JJ.; Croockewit, AJ.; Dekker, AW.; Fibbe, WE. (1999). "Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma". Br J Haematol. 105 (1): 127–30. PMID 10233375. Unknown parameter
|month=ignored (help) - ↑ Anderson, H.; Scarffe, JH.; Ranson, M.; Young, R.; Wieringa, GS.; Morgenstern, GR.; Fitzsimmons, L.; Ryder, D. (1995). "VAD chemotherapy as remission induction for multiple myeloma". Br J Cancer. 71 (2): 326–30. PMID 7841049. Unknown parameter
|month=ignored (help) - ↑ Mateos, MV.; Richardson, PG.; Schlag, R.; Khuageva, NK.; Dimopoulos, MA.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, MT. (2010). "Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial". J Clin Oncol. 28 (13): 2259–66. doi:10.1200/JCO.2009.26.0638. PMID 20368561. Unknown parameter
|month=ignored (help) - ↑ Rajkumar, SV.; Jacobus, S.; Callander, NS.; Fonseca, R.; Vesole, DH.; Williams, ME.; Abonour, R.; Siegel, DS.; Katz, M. (2010). "Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial". Lancet Oncol. 11 (1): 29–37. doi:10.1016/S1470-2045(09)70284-0. PMID 19853510. Unknown parameter
|month=ignored (help) - ↑ 32.0 32.1 32.2 Facon, T.; Mary, JY.; Pégourie, B.; Attal, M.; Renaud, M.; Sadoun, A.; Voillat, L.; Dorvaux, V.; Hulin, C. (2006). "Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy". Blood. 107 (4): 1292–8. doi:10.1182/blood-2005-04-1588. PMID 16174762. Unknown parameter
|month=ignored (help) - ↑ 33.0 33.1 33.2 Shustik, C.; Belch, A.; Robinson, S.; Rubin, SH.; Dolan, SP.; Kovacs, MJ.; Grewal, KS.; Walde, D.; Barr, R. (2007). "A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7". Br J Haematol. 136 (2): 203–11. doi:10.1111/j.1365-2141.2006.06405.x. PMID 17233817. Unknown parameter
|month=ignored (help) - ↑ Rajkumar, SV.; Rosiñol, L.; Hussein, M.; Catalano, J.; Jedrzejczak, W.; Lucy, L.; Olesnyckyj, M.; Yu, Z.; Knight, R. (2008). "Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma". J Clin Oncol. 26 (13): 2171–7. doi:10.1200/JCO.2007.14.1853. PMID 18362366. Unknown parameter
|month=ignored (help) - ↑ Barlogie, B.; Tricot, G.; Anaissie, E.; Shaughnessy, J.; Rasmussen, E.; van Rhee, F.; Fassas, A.; Zangari, M.; Hollmig, K. (2006). "Thalidomide and hematopoietic-cell transplantation for multiple myeloma". N Engl J Med. 354 (10): 1021–30. doi:10.1056/NEJMoa053583. PMID 16525139. Unknown parameter
|month=ignored (help) - ↑ Palumbo, A.; Bringhen, S.; Liberati, AM.; Caravita, T.; Falcone, A.; Callea, V.; Montanaro, M.; Ria, R.; Capaldi, A. (2008). "Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial". Blood. 112 (8): 3107–14. doi:10.1182/blood-2008-04-149427. PMID 18505783. Unknown parameter
|month=ignored (help) - ↑ "Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group". J Clin Oncol. 16 (12): 3832–42. 1998. PMID 9850028. Unknown parameter
|month=ignored (help) - ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide active multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Treatment guide smoldering multiple myeloma. National comprehensive cancer network(2015) http://www.nccn.org/patients/guidelines/myeloma/#44/z Accessed on September, 20th 2015
- ↑ Gupta M, Pal RAGK, Tikoo D. Multiple myeloma: the disease and its treatment. Int J Basic Clin Pharmacol 2013;2:103-21