ST elevation myocardial infarction pathophysiology: Difference between revisions
Brian Blank (talk | contribs) No edit summary |
No edit summary |
||
| (64 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
{| class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right;" | |||
|- | |- | ||
| | | {{#ev:youtube|https://https://www.youtube.com/watch?v=GeX7-sxxOn4|350}} | ||
| | |||
|- | |- | ||
| | |} | ||
__NOTOC__ | |||
{{ST elevation myocardial infarction}} | |||
{{CMG}}; {{AE}} {{CZ}} | |||
==Overview== | |||
ST elevation myocardial infarction is largely influenced by the role of plaque rupture. | |||
[[ | ==The Role of Plaque Rupture in ST Elevation Myocardial Infarction== | ||
[[Atherosclerosis]], or hardening of the [[arteries]], is the gradual buildup of [[cholesterol]] and [[fibrous tissue]] ([[collagen]] and [[smooth muscle cells]]) throughout the [[vascular]] tree. When there is localized accumulation of [[lipids]] and [[scar tissue]], this is called a "plaque". Somewhat paradoxically, it is not the most severe plaque narrowing that leads to ST elevation MI. [[Pathological]] studies indicate that it is often mild-to-moderate, [[lipid]]-laden, inflamed plaques that are the ones most likely to rupture and cause an ST elevation MI ([[STEMI]]) or a non ST elevation MI ([[NSTEMI]]). <ref name="pmid7634481">{{cite journal |author=Falk E, Shah PK, Fuster V |title=Coronary plaque disruption |journal=Circulation |volume=92 |issue=3 |pages=657–71 |year=1995 |month=August |pmid=7634481 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7634481}}</ref> The role of plaque rupture in STEMI and NSTEMI is supported by studies demonstrating that plaque rupture is present in about 70% and superficial erosion is present in 30% of patients who die suddenly in whom there is documented [[coronary artery disease]]. <ref name="pmid9113930">{{cite journal |author=Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R |title=Coronary risk factors and plaque morphology in men with coronary disease who died suddenly |journal=N. Engl. J. Med. |volume=336 |issue=18 |pages=1276–82 |year=1997 |month=May |pmid=9113930 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=9113930&promo=ONFLNS19}}</ref> Exposure of the [[blood stream]] to the [[Thrombogenicity|thrombogenic]] components of the plaque leads to activation of the [[coagulation cascade]] and [[thrombus]] formation. In STEMI, the [[clot]] completely occludes the epicardial artery, and there is a complete lack of blood flow to the involved territory. This causes transmural injury and ST elevation. In NSTEMI, there is partial obstruction with [[embolus|embolization]]. This causes [[ischemia]] and [[subendocardial]] injury that are manifested by [[ST depression]]. | |||
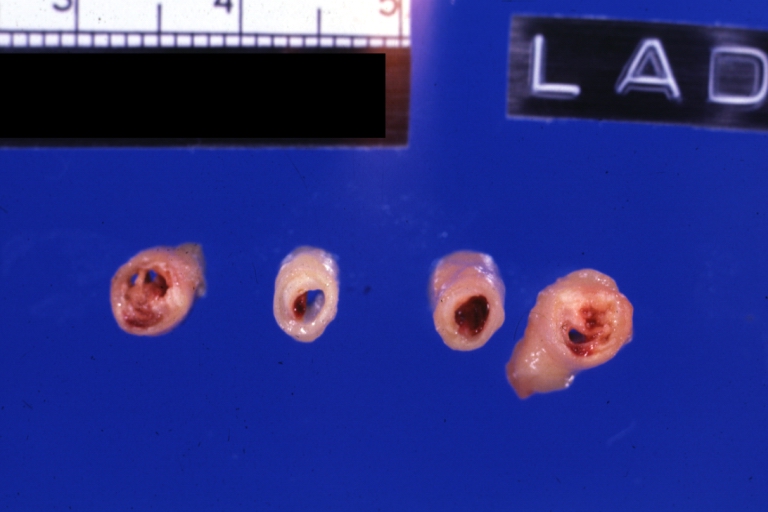
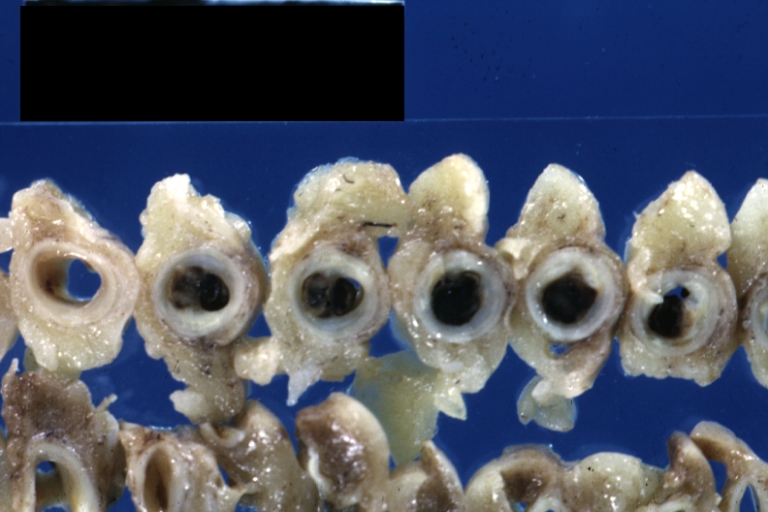
[[ | [[Image:Plaque rupture.jpg|frame|none|500px|Shown here are multiple slices of the LAD. The proximal LAD is located to the left. Plaque rupture with thrombus formation begins in the second slice of the LAD.]] | ||
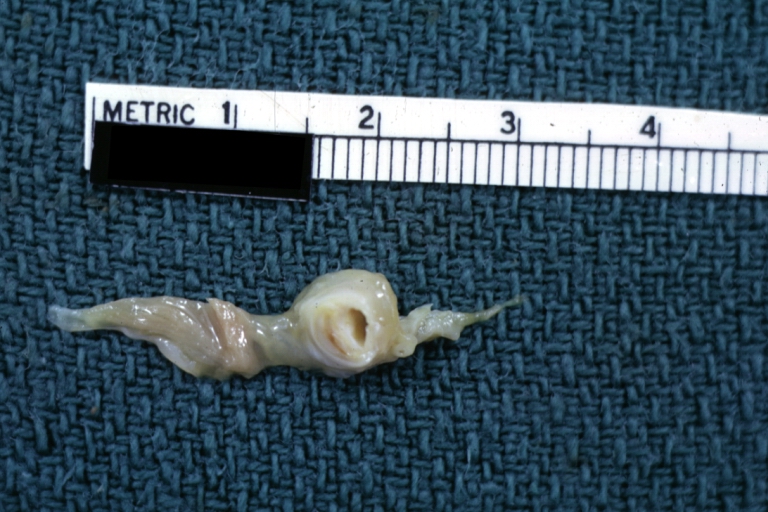
[[ | [[Image:Plaque rupture2 copy.jpg|frame|none|500px|Shown here is a magnified view of the second slice from the left. In yellow is atherosclerotic plaque, in red is clot that has formed inside the ruptured plaque and in the lumen of the coronary artery.]] | ||
| | |||
| | |||
| | |||
| | |||
[[ | ==Pathophysiology of and Risk Factors for Plaque Rupture== | ||
| | #[[Macrophage]] accumulation has been shown to be present to a greater degree in patients with [[acute coronary syndromes]] than in those patients with [[chronic stable angina]] <ref name="pmid8044947">{{cite journal |author=Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT |title=Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture |journal=Circulation |volume=90 |issue=2 |pages=775–8 |year=1994 |month=August |pmid=8044947 |doi= |url=}}</ref> <ref name="pmid8281670">{{cite journal |author=van der Wal AC, Becker AE, van der Loos CM, Das PK |title=Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology |journal=Circulation |volume=89 |issue=1 |pages=36–44 |year=1994 |month=January |pmid=8281670 |doi= |url=}}</ref> These activated [[macrophages]] can release enzymes such as [[metalloproteinases]], [[collagenase|interstitial collagenase]], [[gelatinase]], and stromelysin that degrade [[collagen]], [[elastin]], and [[proteoglycan|proteoglycans]]. <ref name="pmid7664441">{{cite journal |author=Shah PK, Falk E, Badimon JJ, ''et al'' |title=Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture |journal=Circulation |volume=92 |issue=6 |pages=1565–9 |year=1995 |month=September |pmid=7664441 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7664441}}</ref> This [[enzymatic]] degradation in turn leads to breakdown of the [[fibrous]] cap. The thin shoulders or edges of the [[fibrous]] cap appear to be particularly vulnerable to erosion and breakdown. | ||
| | #[[Neovascularization]] of the plaque Moreno et have shown that microvessel density was increased in ruptured plaques when compared with nonruptured plaques (P=0.0001). Furthermore, among lesions with severe [[macrophage]] infiltration at the fibrous cap, [[microvessel]] density was increased (P=0.0001) was well as at the edges or shoulders of the plaque (P=0.0001). [[hemorrhage|Intraplaque hemorrhage]] was also associated with an increase in [[microvessel]] density (P=0.04) as was the presence of thin-cap fibroatheromas (P=0.038). [[Microvessel]] density at the base of the plaque was identified as an independent (P=0.003) correlate of plaque rupture. <ref name="pmid15451780">{{cite journal |author=Moreno PR, Purushothaman KR, Fuster V, ''et al'' |title=Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability |journal=Circulation |volume=110 |issue=14 |pages=2032–8 |year=2004 |month=October |pmid=15451780 |doi=10.1161/01.CIR.0000143233.87854.23 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=15451780}}</ref> | ||
| | # High oscillatory shear stress | ||
| | # [[Vasoconstriction]] | ||
| | # [[Spontaneous coronary dissection]] | ||
| | |||
| | |||
| | |||
| | |||
| | |||
| '' | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| ''' | |||
| | |||
| | |||
| | |||
| | |||
| | |||
==Plaque Rupture== | ==Pathophysiology of and Risk Factors for Thrombosis Following Plaque Rupture== | ||
There are numerous systemic risk factors associated with thrombus formation following plaque rupture: | |||
[[ | #[[Smoking]]: Smoking increases [[platelet aggregation]] and [[plasma]] [[epinephrine]] levels <ref name="pmid7586342">{{cite journal |author=Hung J, Lam JY, Lacoste L, Letchacovski G |title=Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin |journal=Circulation |volume=92 |issue=9 |pages=2432–6 |year=1995 |month=November |pmid=7586342 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7586342}}</ref> | ||
#[[Fibrinogen]]: Elevated levels of [[fibrinogen]] have been associated with [[thrombosis]] including abnormal levels of [[fibrinogen]] <ref name="pmid7845427">{{cite journal |author=Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC |title=Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group |journal=N. Engl. J. Med. |volume=332 |issue=10 |pages=635–41 |year=1995 |month=March |pmid=7845427 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=7845427&promo=ONFLNS19}}</ref> | |||
#[[Von Willebrand factor]] antigen <ref name="pmid7845427">{{cite journal |author=Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC |title=Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group |journal=N. Engl. J. Med. |volume=332 |issue=10 |pages=635–41 |year=1995 |month=March |pmid=7845427 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=7845427&promo=ONFLNS19}}</ref> | |||
#[[Tissue plasminogen activator]] <ref name="pmid7845427">{{cite journal |author=Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC |title=Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group |journal=N. Engl. J. Med. |volume=332 |issue=10 |pages=635–41 |year=1995 |month=March |pmid=7845427 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=7845427&promo=ONFLNS19}}</ref> | |||
#[[Anticardiolipin antibodies]] <ref name="pmid7805207">{{cite journal |author=Vaarala O, Mänttäri M, Manninen V, ''et al'' |title=Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men |journal=Circulation |volume=91 |issue=1 |pages=23–7 |year=1995 |month=January |pmid=7805207 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7805207}}</ref> | |||
# Cross-linked [[fibrin-degradation products]] <ref name="pmid7955179">{{cite journal |author=Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ |title=Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men |journal=Circulation |volume=90 |issue=5 |pages=2236–40 |year=1994 |month=November |pmid=7955179 |doi= |url=}}</ref> | |||
# [[Polymorphisms]] of a [[platelet]] [[glycoprotein]] receptor <ref name="pmid8598867">{{cite journal |author=Weiss EJ, Bray PF, Tayback M, ''et al'' |title=A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis |journal=N. Engl. J. Med. |volume=334 |issue=17 |pages=1090–4 |year=1996 |month=April |pmid=8598867 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=8598867&promo=ONFLNS19}}</ref> | |||
==Gross Pathology Findings in Plaque Rupture== | |||
[http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | [http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
| Line 225: | Line 43: | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
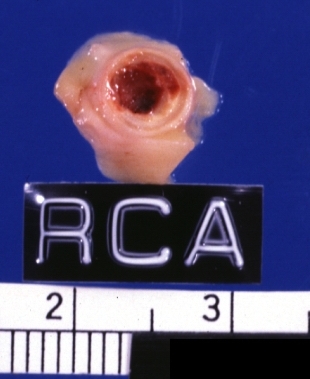
Image:Plaque rupture | Image:Plaque rupture 6.jpg|Left anterior descending coronary artery: Atherosclerosis Plaque Ruptured with Thrombosis: Gross; natural color; four cross sections, close-up view (acute anterior myocardial infarction with rupture) | ||
Image:Plaque rupture | Image:Plaque rupture 7.jpg|Coronary artery: Atherosclerotic Plaque: Gross natural color close-up view of a typical plaque | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
| Line 232: | Line 50: | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
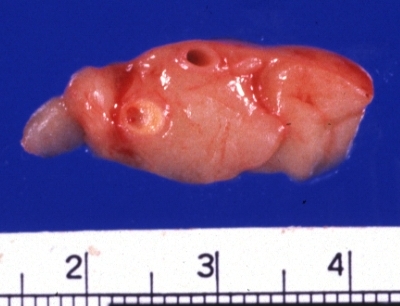
Image:Plaque rupture | Image:Plaque rupture 8.jpg|Coronary Atherosclerosis: Gross, natural color, close-up view of large atherosclerotic plaque with soft atheroma (a quite good example in 54yo male. Smoker with hypertension). This slide shows the left main artery | ||
Image:Plaque rupture | Image:Plaque rupture 9.jpg|Coronary artery: Atherosclerotic Plaque: Gross, natural color, close-up view of plaque with atheroma core causing more than 90% lumen occlusion (an excellent example) | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
| Line 239: | Line 57: | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
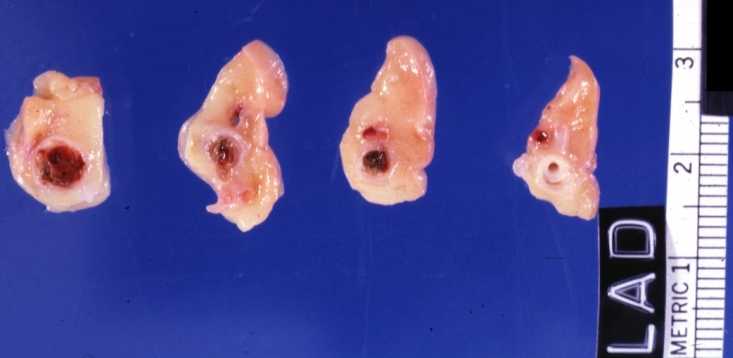
Image:Plaque rupture | Image:Plaque rupture 10.jpg|Coronary artery: Atherosclerotic Plaque with Hemorrhage and Thrombosis: Gross, natural color, cross section, close-up, an excellent example of right coronary artery in 71yo female. | ||
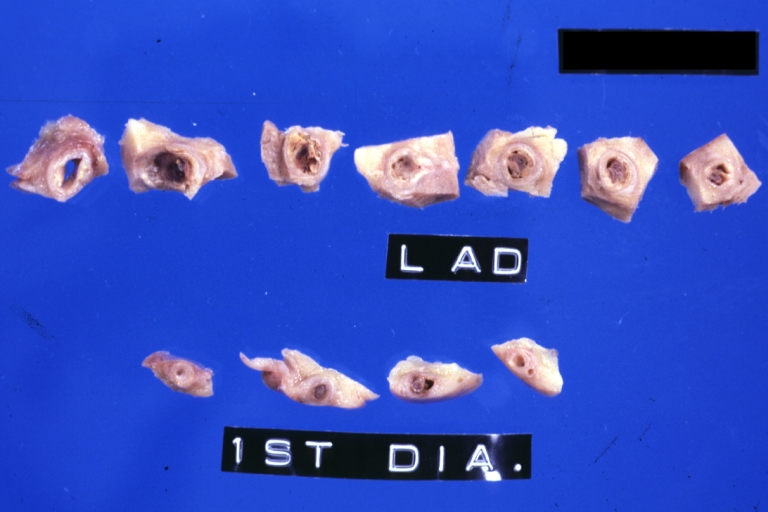
Image:Plaque | Image:Plaque rupture 11.jpg|Coronary artery: Atherosclerotic Plaque with Hemorrhage and Thrombosis: Gross, natural color, cross sections; there is excellent example of hemorrhagic plaque and thrombus at and just below the origin of first diagonal artery. Another one (a more acute one) was in the right coronary artery. | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
| Line 246: | Line 64: | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque | Image:Plaque rupture 12.jpg|Coronary artery: Atherosclerotic Plaque with Thrombus: Gross natural color, close-up of cross section. | ||
Image:Plaque | Image:Plaque rupture 13.jpg|Coronary artery: Atherosclerotic Plaque with Hemorrhage: Gross fixed tissue, cross sections. LAD and 1st diagonal with large plaques and several apparent areas of hemorrhage. | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque rupture | Image:Plaque rupture 14.jpg|Coronary artery: Atherosclerosis: Gross, an excellent close-up atherosclerosis with hemorrhage into plaque. | ||
Image:Plaque rupture | Image:Plaque rupture 15.jpg|Coronary artery: Atherosclerosis: Gross, cross sections coronary artery with hemorrhage into plaque (image shows full length of the artery). | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque rupture | Image:Plaque rupture 16.jpg|Coronary artery: Atherosclerosis: Gross, cross sections of artery showing plaques (an excellent example) | ||
Image:Plaque rupture | Image:Plaque rupture 17.jpg|Coronary artery: Atherosclerosis: Gross natural color in situ cross section with large fibrocalcific plaque with hemorrhage (an excellent example) | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
===Plaque Rupture Histopathological Findings=== | |||
[http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | |||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque rupture | Image:Plaque rupture 1.jpg|Coronary artery: Atherosclerosis: Micro H&E med mag; A good example of plaque rupture with thrombosis. | ||
Image:Plaque rupture | Image:Plaque rupture 2.jpg|Right coronary artery: Ruptured Plaque: Micro low mag H&E; Ruptured plaque with foam cell lesion (near rupture site). | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque rupture | Image:Plaque rupture 3.jpg|Right coronary artery: Atherosclerosis Plaque Ruptured with Thrombus: Micro low mag H&E; an excellent view of ruptured plaque with thrombus and some old fibrin in it. | ||
Image:Plaque rupture | Image:Plaque rupture 4.jpg|Right coronary artery: Atherosclerosis Plaque Ruptured with Thrombus: Micro low mag trichrome. | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque rupture | Image:Plaque rupture 5.jpg|Right coronary artery: Atherosclerosis Plaque Ruptured: Micro low mag H&E; large plaque with hemorrhage; (an excellent example of hemorrhage). | ||
Image:Plaque | Image:Plaque 1.jpg|Coronary artery: Atherosclerosis: Micro H&E low mag injected artery fairly typical uncomplicated atheromatous plaque | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="175" widths="175"> | <gallery heights="175" widths="175"> | ||
Image:Plaque | Image:Plaque 2.jpg|Coronary artery: Atherosclerosis: Micro H&E low mag, injected artery has typical fibrous plaque with small hemorrhage in atheroma. | ||
Image:Plaque | Image:Plaque 3.jpg|Coronary artery: Atherosclerosis: Micro H&E low mag, injected artery is a very good example of marked lumen stenosis due to typical fibrous plaque with calcification | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
==The Time Dependent Wavefront of Necrosis== | ==The Consequence of Plaque Rupture and Vessel Occlusion: The Time Dependent Wavefront of Necrosis== | ||
[[Image:Slow-wavefront.gif|left|Time dependent wavefront of necrosis working its way from the subendocardium to the subepicardium]] | [[Image:Slow-wavefront.gif|left|Time dependent wavefront of necrosis working its way from the subendocardium to the subepicardium]] | ||
Irreversible injury of ischemic myocytes occurs first in the subendocardial zone. With more extended ischemia, a wavefront of cell death moves through the myocardium to involve progressively more of the transmural thickness of the ischemic zone. The precise location, size, and specific morphologic features of an [[acute myocardial infarction]] depend on: | In 1940, Blumgart ligated or tied off the coronary artery in dogs and cats and for the first time demonstrated a wavefront of cell death folllowing [[Blood vessel|vessel]] occlusion <ref>Blumgart HL, Schlesinge MJ, Davis D: Studies on the relation of the clinical manifestations of angina pectoris, coronary thrombosis, and myocardial infarction to the pathologic findings, with particular reference to the significance of collateral circulation. Amer Heart J 19: 1, 1940 </ref> <ref>Blumgart HL, Zoll PM, Freedberg AS, Gilligan DR: The experimental production of intercoronary arterial anastomoses and their functional significance. Circulation 1: 10, 1950 PMID 15401193 </ref> <ref>Blumgart HL, Zoll PM, Kurland CS: Discussion of direct relief of coronary occlusion. Arch Intern Med (Chicago) 104: 862, 1959 PMID 13801751 </ref> <ref>Blumgart HL, Zoll PM. Pathologic physiology of angina pectoris and acute myocardial infarction. Circulation. 1960 Aug;22:301-7. PMID 13801752 </ref> | ||
<ref>Blumgart HL, Zoll PM, Clinical Pathologic Correlations in Coronary Artery Disease, Circulation, Volume XLVII, No 6, June 1973, 1139-43 PMID 4575525 </ref> | |||
Irreversible injury of ischemic myocytes occurs first in the subendocardial zone. With more extended [[ischemia]], a wavefront of cell death moves through the myocardium to involve progressively more of the transmural thickness of the ischemic zone. The precise location, size, and specific morphologic features of an [[acute myocardial infarction]] depend on: | |||
#The location, severity, and rate of development of coronary atherosclerotic obstructions, | #The location, severity, and rate of development of coronary atherosclerotic obstructions, | ||
| Line 312: | Line 129: | ||
#The extent of collateral blood vessels | #The extent of collateral blood vessels | ||
Decrease of ATP levels in myocytes in reaction to ischemia starts within seconds and causes loss of contractility in first two minutes. If ischemia persists, ATP levels reduced to its half level within 10 minutes and to 1/10 within 40 minutes. Irreversible cell injury occurs between 20-40 minutes and microvascular level injury starts if ischemia lasts more than an hour.<ref>Robbins Pathologic Basis of Disease, Kumar V, 7th ed</ref> | Decrease of ATP levels in myocytes in reaction to ischemia starts within seconds and causes loss of [[contractility]] in first two minutes. If [[ischemia]] persists, [[Adenosine triphosphate|ATP]] levels reduced to its half level within 10 minutes and to 1/10 within 40 minutes. Irreversible cell injury occurs between 20-40 minutes and [[Microvascular bed|microvascular]] level injury starts if [[ischemia]] lasts more than an hour.<ref>Robbins Pathologic Basis of Disease, Kumar V, 7th ed</ref> | ||
If impaired [[blood]] flow to the heart lasts long enough, it triggers a process called the [[ischemic cascade]]; the [[Myocardium|heart cells]] die (chiefly through [[necrosis]]) and do not grow back. A [[collagen]] [[scar]] forms in its place. Recent studies indicate that another form of cell death called [[apoptosis]] also plays a role in the process of [[Tissue (biology)|tissue]] damage subsequent to myocardial infarction.<ref name="Krijnen-2002">{{cite journal | author=Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. | title=Apoptosis in myocardial ischaemia and infarction. | journal=J Clin Pathol | year=2002 | volume=55 | issue=11 | pages=801-11 | id=PMID 12401816}}</ref> As a result, the patient's heart can be permanently damaged. This [[scar tissue]] also puts the patient at risk for potentially life threatening [[arrhythmias]]. | |||
==Pathophysiology of ST segment elevation on the electrocardiogram== | |||
In ST segment myocaridal infarction (STEMI), the [[ST segments]] on the [[The electrocardiogram|ECG]] are by definition elevated and there is [[myonecrosis]] (death of [[myocytes]]) as reflected by elevation of [[Cardiac biomarkers|biomarkers]] such as [[creatine kinase]] MB fraction ([[CK-MB]]) or [[troponin]] T or I (tn). The [[ST segments]] are elevated due to full thickness injury of the [[myocardium]]. | |||
The | ==Videos of STEMI pathophysiology== | ||
The following are excellent videos demonstrating the underlying pathophysiology. | |||
{{#ev:youtube|L6EiPLli5x8}} | |||
{{#ev:youtube|cOMzh2hf_Vw}} | |||
{{#ev:youtube|a8Idk4EUYTs}} | |||
==References== | ==References== | ||
| Line 326: | Line 148: | ||
{{refbegin|2}} | {{refbegin|2}} | ||
* Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun 40(6): 633-44. PMID 449273 | * Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun 40(6): 633-44. PMID 449273 | ||
* Hasche ET, Fernandes C, Freedman SB, Jeremy RW. Relation between ischemia time, infarct size, and left ventricular function in humans. Circulation. 1995 Aug 15; 92(4): 710-9. PMID 7641348 | * Hasche ET, Fernandes C, Freedman SB, Jeremy RW. Relation between ischemia time, infarct size, and left ventricular function in humans. Circulation. 1995 Aug 15; 92(4): 710-9. PMID 7641348 | ||
| Line 359: | Line 176: | ||
* Appelbaum E, Kirtane AJ, Clark A, Pride YB, Gelfand EV, Harrigan CJ, Kissinger KV, Manning WJ, Gibson CM. Association of TIMI Myocardial Perfusion Grade and ST-segment resolution with cardiovascular magnetic resonance measures of microvascular obstruction and infarct size following ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2008 Feb 2. [Epub ahead of print] PMID 18246410 | * Appelbaum E, Kirtane AJ, Clark A, Pride YB, Gelfand EV, Harrigan CJ, Kissinger KV, Manning WJ, Gibson CM. Association of TIMI Myocardial Perfusion Grade and ST-segment resolution with cardiovascular magnetic resonance measures of microvascular obstruction and infarct size following ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2008 Feb 2. [Epub ahead of print] PMID 18246410 | ||
* Leshnower BG, Sakamoto H, Hamamoto H, Zeeshan A, Gorman JH 3rd, Gorman RC. Progression of myocardial injury during coronary occlusion in the collateral-deficient heart: a non-wavefront phenomenon. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1799-804. Epub 2007 Jul 20. PMID 17644569 | * Leshnower BG, Sakamoto H, Hamamoto H, Zeeshan A, Gorman JH 3rd, Gorman RC. Progression of myocardial injury during coronary occlusion in the collateral-deficient heart: a non-wavefront phenomenon. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1799-804. Epub 2007 Jul 20. PMID 17644569 | ||
{{refend}} | {{refend}} | ||
{{WikiDoc Help Menu}} | |||
{{WikiDoc Sources}} | |||
[[Category:Disease]] | |||
[[Category:Cardiology]] | [[Category:Cardiology]] | ||
[[Category:Ischemic heart diseases]] | |||
[[Category:Intensive care medicine]] | |||
[[Category:Emergency medicine]] | |||
[[Category:Mature chapter]] | |||
Latest revision as of 21:17, 25 January 2023
| https://https://www.youtube.com/watch?v=GeX7-sxxOn4%7C350}} |
|
ST Elevation Myocardial Infarction Microchapters |
|
Differentiating ST elevation myocardial infarction from other Diseases |
|
Diagnosis |
|
Treatment |
|
|
Case Studies |
|
ST elevation myocardial infarction pathophysiology On the Web |
|
ST elevation myocardial infarction pathophysiology in the news |
|
Directions to Hospitals Treating ST elevation myocardial infarction |
|
Risk calculators and risk factors for ST elevation myocardial infarction pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
ST elevation myocardial infarction is largely influenced by the role of plaque rupture.
The Role of Plaque Rupture in ST Elevation Myocardial Infarction
Atherosclerosis, or hardening of the arteries, is the gradual buildup of cholesterol and fibrous tissue (collagen and smooth muscle cells) throughout the vascular tree. When there is localized accumulation of lipids and scar tissue, this is called a "plaque". Somewhat paradoxically, it is not the most severe plaque narrowing that leads to ST elevation MI. Pathological studies indicate that it is often mild-to-moderate, lipid-laden, inflamed plaques that are the ones most likely to rupture and cause an ST elevation MI (STEMI) or a non ST elevation MI (NSTEMI). [1] The role of plaque rupture in STEMI and NSTEMI is supported by studies demonstrating that plaque rupture is present in about 70% and superficial erosion is present in 30% of patients who die suddenly in whom there is documented coronary artery disease. [2] Exposure of the blood stream to the thrombogenic components of the plaque leads to activation of the coagulation cascade and thrombus formation. In STEMI, the clot completely occludes the epicardial artery, and there is a complete lack of blood flow to the involved territory. This causes transmural injury and ST elevation. In NSTEMI, there is partial obstruction with embolization. This causes ischemia and subendocardial injury that are manifested by ST depression.

Pathophysiology of and Risk Factors for Plaque Rupture
- Macrophage accumulation has been shown to be present to a greater degree in patients with acute coronary syndromes than in those patients with chronic stable angina [3] [4] These activated macrophages can release enzymes such as metalloproteinases, interstitial collagenase, gelatinase, and stromelysin that degrade collagen, elastin, and proteoglycans. [5] This enzymatic degradation in turn leads to breakdown of the fibrous cap. The thin shoulders or edges of the fibrous cap appear to be particularly vulnerable to erosion and breakdown.
- Neovascularization of the plaque Moreno et have shown that microvessel density was increased in ruptured plaques when compared with nonruptured plaques (P=0.0001). Furthermore, among lesions with severe macrophage infiltration at the fibrous cap, microvessel density was increased (P=0.0001) was well as at the edges or shoulders of the plaque (P=0.0001). Intraplaque hemorrhage was also associated with an increase in microvessel density (P=0.04) as was the presence of thin-cap fibroatheromas (P=0.038). Microvessel density at the base of the plaque was identified as an independent (P=0.003) correlate of plaque rupture. [6]
- High oscillatory shear stress
- Vasoconstriction
- Spontaneous coronary dissection
Pathophysiology of and Risk Factors for Thrombosis Following Plaque Rupture
There are numerous systemic risk factors associated with thrombus formation following plaque rupture:
- Smoking: Smoking increases platelet aggregation and plasma epinephrine levels [7]
- Fibrinogen: Elevated levels of fibrinogen have been associated with thrombosis including abnormal levels of fibrinogen [8]
- Von Willebrand factor antigen [8]
- Tissue plasminogen activator [8]
- Anticardiolipin antibodies [9]
- Cross-linked fibrin-degradation products [10]
- Polymorphisms of a platelet glycoprotein receptor [11]
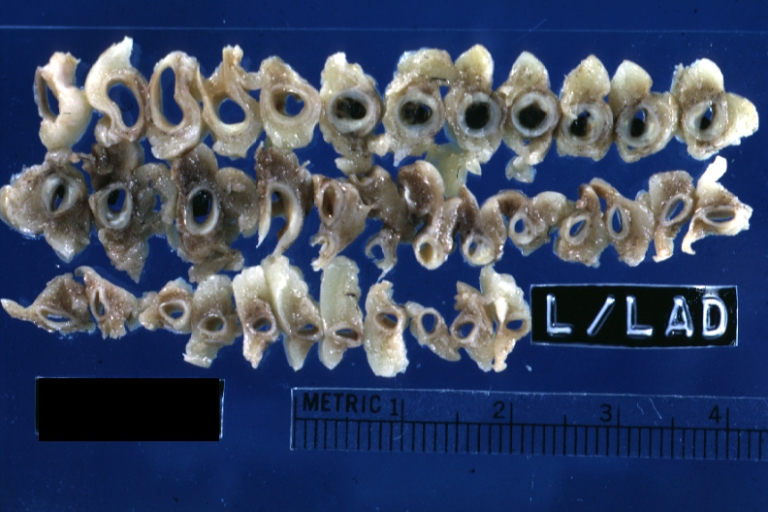
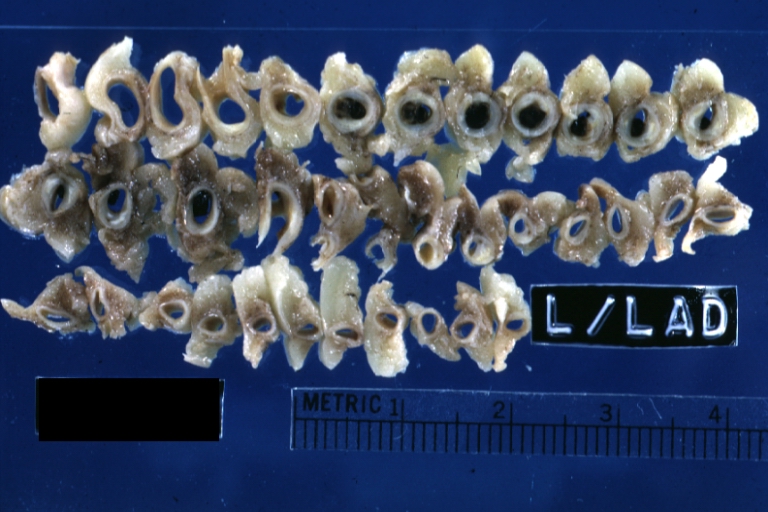
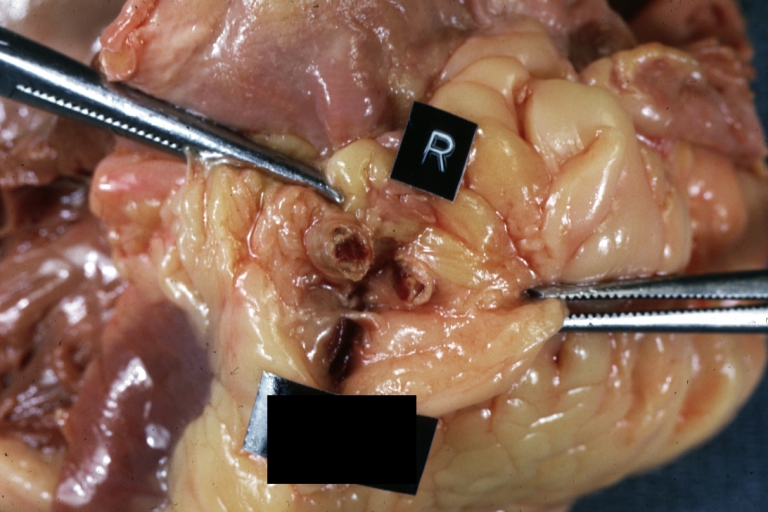
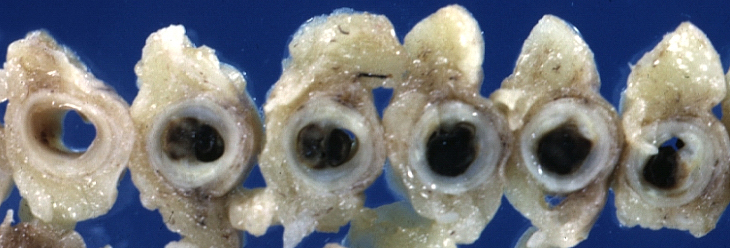
Gross Pathology Findings in Plaque Rupture
-
Left anterior descending coronary artery: Atherosclerosis Plaque Ruptured with Thrombosis: Gross; natural color; four cross sections, close-up view (acute anterior myocardial infarction with rupture)
-
Coronary artery: Atherosclerotic Plaque: Gross natural color close-up view of a typical plaque
-
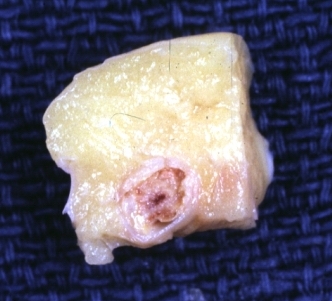
Coronary Atherosclerosis: Gross, natural color, close-up view of large atherosclerotic plaque with soft atheroma (a quite good example in 54yo male. Smoker with hypertension). This slide shows the left main artery
-
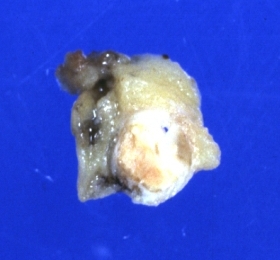
Coronary artery: Atherosclerotic Plaque: Gross, natural color, close-up view of plaque with atheroma core causing more than 90% lumen occlusion (an excellent example)
-
Coronary artery: Atherosclerotic Plaque with Hemorrhage and Thrombosis: Gross, natural color, cross section, close-up, an excellent example of right coronary artery in 71yo female.
-
Coronary artery: Atherosclerotic Plaque with Hemorrhage and Thrombosis: Gross, natural color, cross sections; there is excellent example of hemorrhagic plaque and thrombus at and just below the origin of first diagonal artery. Another one (a more acute one) was in the right coronary artery.
-
Coronary artery: Atherosclerotic Plaque with Thrombus: Gross natural color, close-up of cross section.
-
Coronary artery: Atherosclerotic Plaque with Hemorrhage: Gross fixed tissue, cross sections. LAD and 1st diagonal with large plaques and several apparent areas of hemorrhage.
-
Coronary artery: Atherosclerosis: Gross, an excellent close-up atherosclerosis with hemorrhage into plaque.
-
Coronary artery: Atherosclerosis: Gross, cross sections coronary artery with hemorrhage into plaque (image shows full length of the artery).
-
Coronary artery: Atherosclerosis: Gross, cross sections of artery showing plaques (an excellent example)
-
Coronary artery: Atherosclerosis: Gross natural color in situ cross section with large fibrocalcific plaque with hemorrhage (an excellent example)
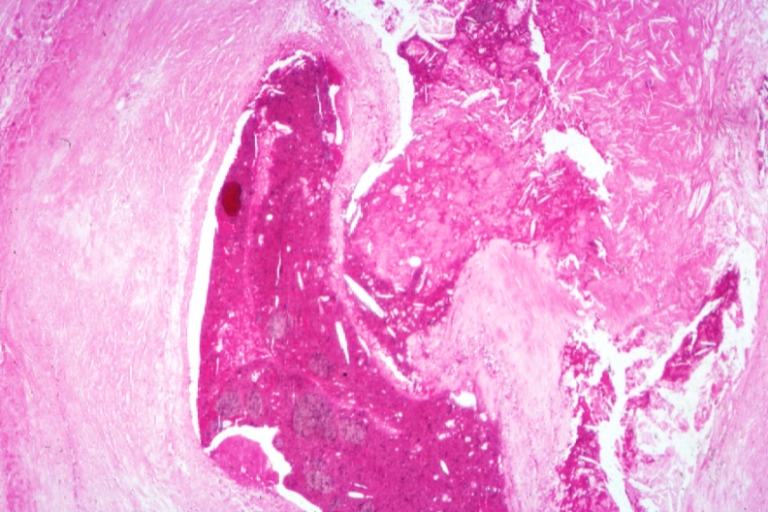
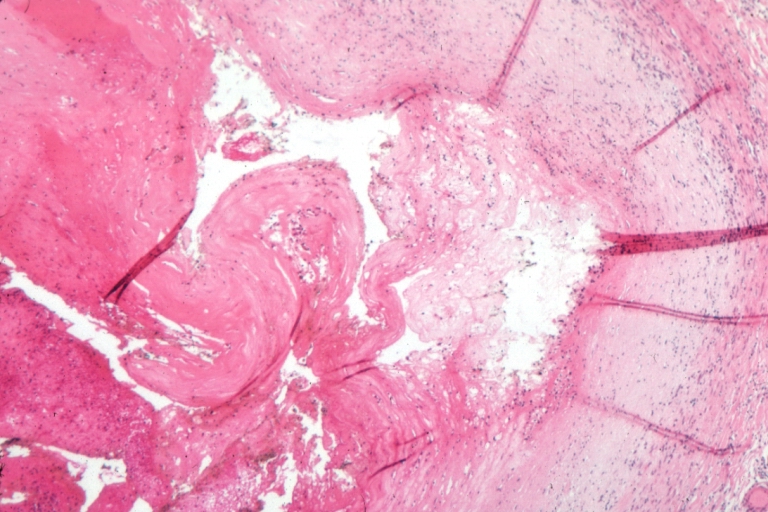
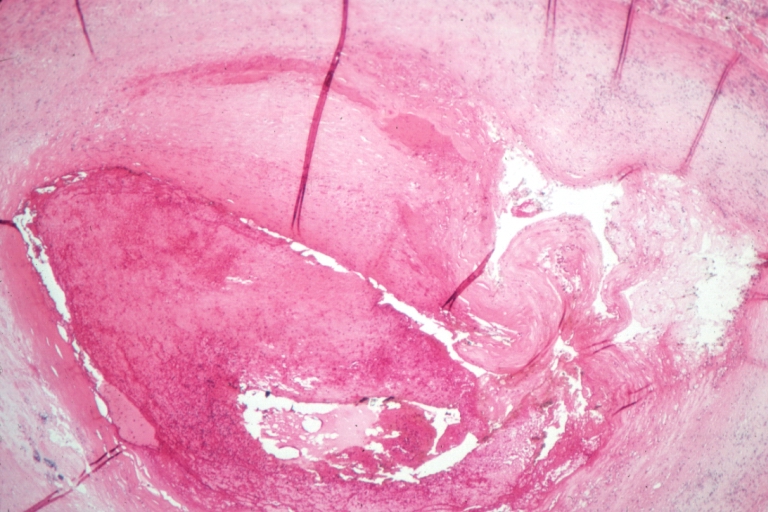
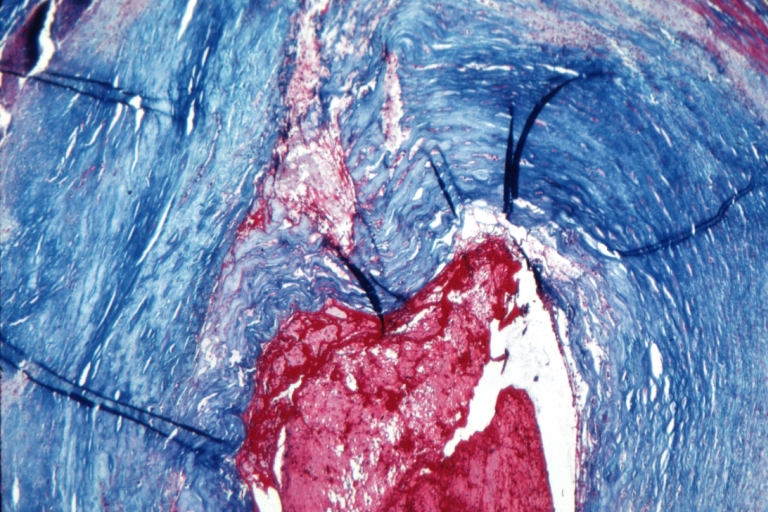
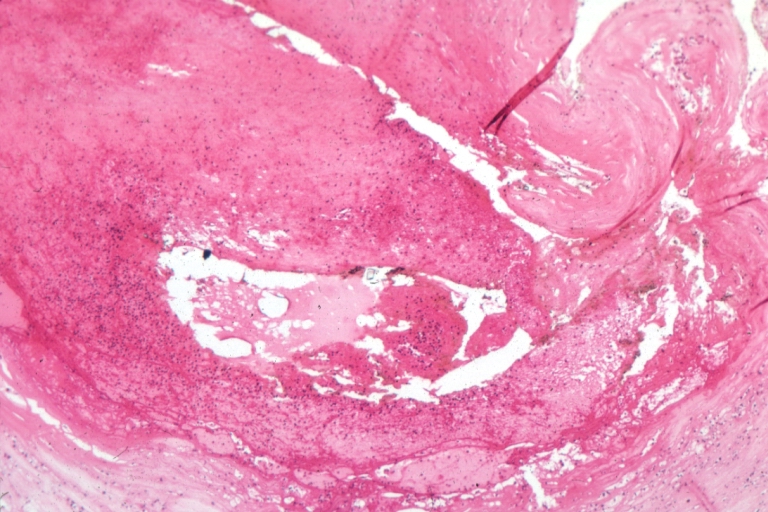
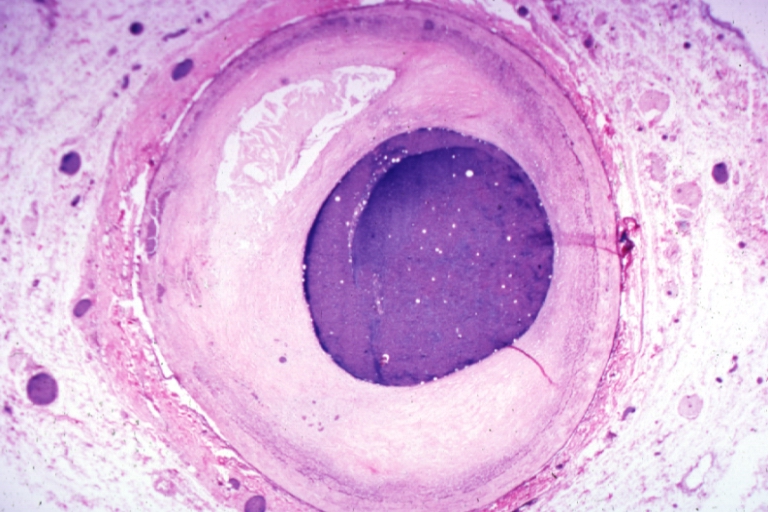
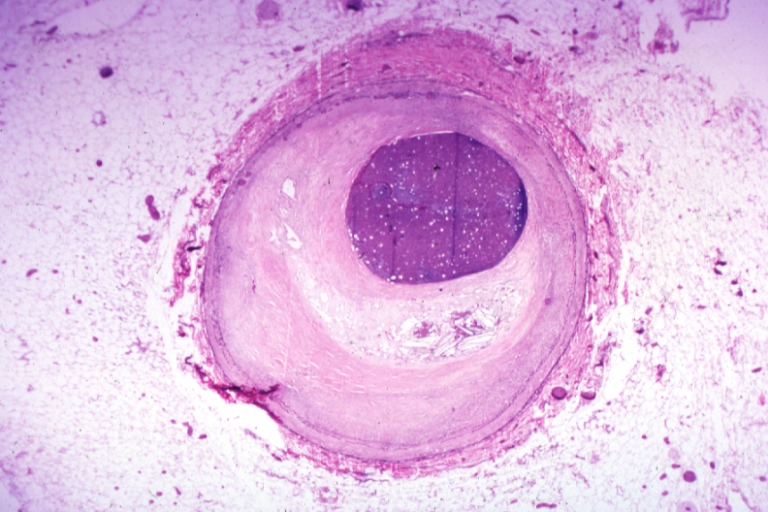
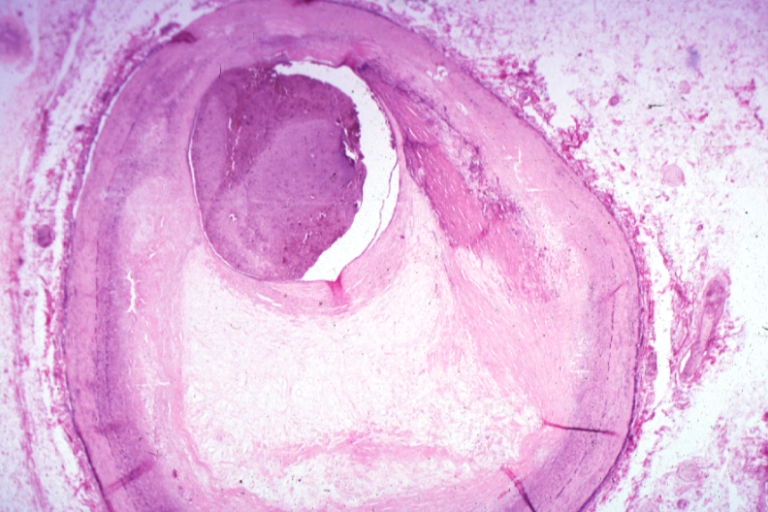
Plaque Rupture Histopathological Findings
-
Coronary artery: Atherosclerosis: Micro H&E med mag; A good example of plaque rupture with thrombosis.
-
Right coronary artery: Ruptured Plaque: Micro low mag H&E; Ruptured plaque with foam cell lesion (near rupture site).
-
Right coronary artery: Atherosclerosis Plaque Ruptured with Thrombus: Micro low mag H&E; an excellent view of ruptured plaque with thrombus and some old fibrin in it.
-
Right coronary artery: Atherosclerosis Plaque Ruptured with Thrombus: Micro low mag trichrome.
-
Right coronary artery: Atherosclerosis Plaque Ruptured: Micro low mag H&E; large plaque with hemorrhage; (an excellent example of hemorrhage).
-
Coronary artery: Atherosclerosis: Micro H&E low mag injected artery fairly typical uncomplicated atheromatous plaque
-
Coronary artery: Atherosclerosis: Micro H&E low mag, injected artery has typical fibrous plaque with small hemorrhage in atheroma.
-
Coronary artery: Atherosclerosis: Micro H&E low mag, injected artery is a very good example of marked lumen stenosis due to typical fibrous plaque with calcification
The Consequence of Plaque Rupture and Vessel Occlusion: The Time Dependent Wavefront of Necrosis
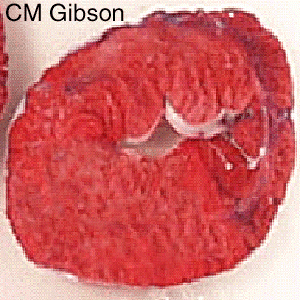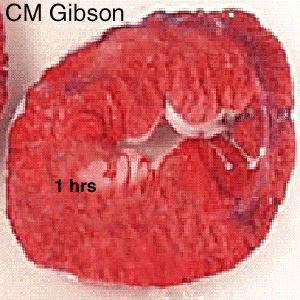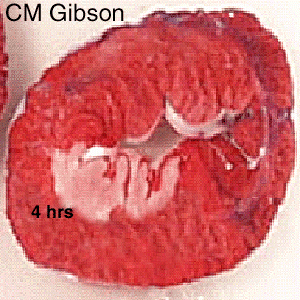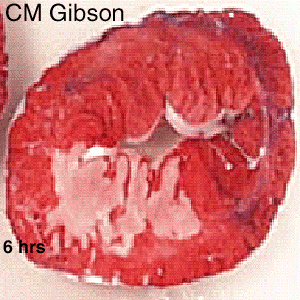
In 1940, Blumgart ligated or tied off the coronary artery in dogs and cats and for the first time demonstrated a wavefront of cell death folllowing vessel occlusion [12] [13] [14] [15] [16]
Irreversible injury of ischemic myocytes occurs first in the subendocardial zone. With more extended ischemia, a wavefront of cell death moves through the myocardium to involve progressively more of the transmural thickness of the ischemic zone. The precise location, size, and specific morphologic features of an acute myocardial infarction depend on:
- The location, severity, and rate of development of coronary atherosclerotic obstructions,
- The size of the vascular bed perfused by the obstructed vessels
- The duration of the coronary artery occlusion
- The metabolic / oxygen needs of the myocardium at risk,
- The extent of collateral blood vessels
Decrease of ATP levels in myocytes in reaction to ischemia starts within seconds and causes loss of contractility in first two minutes. If ischemia persists, ATP levels reduced to its half level within 10 minutes and to 1/10 within 40 minutes. Irreversible cell injury occurs between 20-40 minutes and microvascular level injury starts if ischemia lasts more than an hour.[17]
If impaired blood flow to the heart lasts long enough, it triggers a process called the ischemic cascade; the heart cells die (chiefly through necrosis) and do not grow back. A collagen scar forms in its place. Recent studies indicate that another form of cell death called apoptosis also plays a role in the process of tissue damage subsequent to myocardial infarction.[18] As a result, the patient's heart can be permanently damaged. This scar tissue also puts the patient at risk for potentially life threatening arrhythmias.
Pathophysiology of ST segment elevation on the electrocardiogram
In ST segment myocaridal infarction (STEMI), the ST segments on the ECG are by definition elevated and there is myonecrosis (death of myocytes) as reflected by elevation of biomarkers such as creatine kinase MB fraction (CK-MB) or troponin T or I (tn). The ST segments are elevated due to full thickness injury of the myocardium.
Videos of STEMI pathophysiology
The following are excellent videos demonstrating the underlying pathophysiology. {{#ev:youtube|L6EiPLli5x8}} {{#ev:youtube|cOMzh2hf_Vw}} {{#ev:youtube|a8Idk4EUYTs}}
References
- ↑ Falk E, Shah PK, Fuster V (1995). "Coronary plaque disruption". Circulation. 92 (3): 657–71. PMID 7634481. Unknown parameter
|month=ignored (help) - ↑ Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R (1997). "Coronary risk factors and plaque morphology in men with coronary disease who died suddenly". N. Engl. J. Med. 336 (18): 1276–82. PMID 9113930. Unknown parameter
|month=ignored (help) - ↑ Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT (1994). "Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture". Circulation. 90 (2): 775–8. PMID 8044947. Unknown parameter
|month=ignored (help) - ↑ van der Wal AC, Becker AE, van der Loos CM, Das PK (1994). "Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology". Circulation. 89 (1): 36–44. PMID 8281670. Unknown parameter
|month=ignored (help) - ↑ Shah PK, Falk E, Badimon JJ; et al. (1995). "Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture". Circulation. 92 (6): 1565–9. PMID 7664441. Unknown parameter
|month=ignored (help) - ↑ Moreno PR, Purushothaman KR, Fuster V; et al. (2004). "Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability". Circulation. 110 (14): 2032–8. doi:10.1161/01.CIR.0000143233.87854.23. PMID 15451780. Unknown parameter
|month=ignored (help) - ↑ Hung J, Lam JY, Lacoste L, Letchacovski G (1995). "Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin". Circulation. 92 (9): 2432–6. PMID 7586342. Unknown parameter
|month=ignored (help) - ↑ 8.0 8.1 8.2 Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC (1995). "Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group". N. Engl. J. Med. 332 (10): 635–41. PMID 7845427. Unknown parameter
|month=ignored (help) - ↑ Vaarala O, Mänttäri M, Manninen V; et al. (1995). "Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men". Circulation. 91 (1): 23–7. PMID 7805207. Unknown parameter
|month=ignored (help) - ↑ Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ (1994). "Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men". Circulation. 90 (5): 2236–40. PMID 7955179. Unknown parameter
|month=ignored (help) - ↑ Weiss EJ, Bray PF, Tayback M; et al. (1996). "A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis". N. Engl. J. Med. 334 (17): 1090–4. PMID 8598867. Unknown parameter
|month=ignored (help) - ↑ Blumgart HL, Schlesinge MJ, Davis D: Studies on the relation of the clinical manifestations of angina pectoris, coronary thrombosis, and myocardial infarction to the pathologic findings, with particular reference to the significance of collateral circulation. Amer Heart J 19: 1, 1940
- ↑ Blumgart HL, Zoll PM, Freedberg AS, Gilligan DR: The experimental production of intercoronary arterial anastomoses and their functional significance. Circulation 1: 10, 1950 PMID 15401193
- ↑ Blumgart HL, Zoll PM, Kurland CS: Discussion of direct relief of coronary occlusion. Arch Intern Med (Chicago) 104: 862, 1959 PMID 13801751
- ↑ Blumgart HL, Zoll PM. Pathologic physiology of angina pectoris and acute myocardial infarction. Circulation. 1960 Aug;22:301-7. PMID 13801752
- ↑ Blumgart HL, Zoll PM, Clinical Pathologic Correlations in Coronary Artery Disease, Circulation, Volume XLVII, No 6, June 1973, 1139-43 PMID 4575525
- ↑ Robbins Pathologic Basis of Disease, Kumar V, 7th ed
- ↑ Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. (2002). "Apoptosis in myocardial ischaemia and infarction". J Clin Pathol. 55 (11): 801–11. PMID 12401816.
Additional Resources
- Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun 40(6): 633-44. PMID 449273
- Hasche ET, Fernandes C, Freedman SB, Jeremy RW. Relation between ischemia time, infarct size, and left ventricular function in humans. Circulation. 1995 Aug 15; 92(4): 710-9. PMID 7641348
- Gibson CM, Kirtane AJ, Morrow DA, Palabrica TM, Murphy SA, Stone PH, Scirica BM, Jennings LK, Herrmann HC, Cohen DJ, McCabe CH, Braunwald E; TIMI Study Group. Association between thrombolysis in myocardial infarction myocardial perfusion grade, biomarkers, and clinical outcomes among patients with moderate- to high-risk acute coronary syndromes: observations from the randomized trial to evaluate the relative PROTECTion against post-PCI microvascular dysfunction and post-PCI ischemia among antiplatelet and antithrombotic agents-Thrombolysis In Myocardial Infarction 30 (PROTECT-TIMI 30). Am Heart J. 2006 Oct; 152 (4): 756-61. PMID 16996854
- Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992 Jul; 86(1): 81-90. PMID 1617793
- Gibson CM, Cannon CP, Murphy SA, Marble SJ, Barron HV, Braunwald E; TIMI Study Group. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002 Apr 23; 105 (16): 1909-13. PMID 11997276
- Kandzari DE, Tcheng JE, Gersh BJ, Cox DA, Stuckey T, Turco M, Mehran R, Garcia E, Zimetbaum P, McGlaughlin MG, Lansky AJ, Costantini CO, Grines CL, Stone GW; CADILLAC Investigators. Relationship between infarct artery location, epicardial flow, and myocardial perfusion after primary percutaneous revascularization in acute myocardial infarction. Am Heart J. 2006 Jun; 151(6): 1288-95. PMID 16781238
- Elsman P, van 't Hof AW, de Boer MJ, Hoorntje JC, Suryapranata H, Dambrink JH, Zijlstra F; Zwolle Myocardial Infarction Study Group. Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J. 2004 May; 25(10): 854-8. PMID 15140533
- Ortiz-Pérez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007 Jul;28(14):1670-2. Epub 2007 Jun 22 PMID 17586811
- Maehara A, Mintz GS, Bui AB, Walter OR, Castagna MT, Canos D, Pichard AD, Satler LF, Waksman R, Suddath WO, Laird JR Jr, Kent KM, Weissman NJ. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol. 2002 Sep 4;40 (5): 904-10. PMID 12225714
- Gibson CM, Murphy SA, Kirtane AJ, Giugliano RP, Cannon CP, Antman EM, Braunwald E; TIMI Study Group. Association of duration of symptoms at presentation with angiographic and clinical outcomes after fibrinolytic therapy in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004 Sep 1; 44 (5): 980-7. PMID 15337207
- D. Garcia-Dorado, P. Theroux, M. Desco, J. Solares, J. Elizaga, F. Fernandez-Aviles, J. Alonso and J. Soriano, Cell-to-cell interaction: a mechanism to explain wave-front progression of myocardial necrosis. Am J Physiol Heart Circ Physiol 256: H1266-H1273, 1989; 0363-6135/89 $5.00 PMID 2719127
- Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, Stuckey T, Tcheng JE, Mehran R, Lansky AJ, Grines CL, Stone GW. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007 Jul; 28(14): 1709-16. Epub 2007 Jun 7. PMID 17556348
- Brener S. Insights into the pathophysiology of ST-elevation myocardial infarction. American Heart Journal, Volume 151, Issue 6, Pages S4 - S10, 2006 PMID 16777509
- Biasucci LM, Leo M, De Maria GL. Local and Systemic Mechanisms of Plaque Rupture. Angiology. 2008 Jun 10. [Epub ahead of print] PMID 1854458
- El-Menyar AA. Cytokines and coronary artery disease: the state of the art. Crit Pathw Cardiol. 2008 Jun; 7(2): 139-51. PMID 18520532
- Kaneda H. Coronary plaque rupture and vessel remodeling. Am J Cardiol 2008 May 15; 101 (10): 1519; PMID 18471472
- Sorajja P, Gersh BJ, Mehran R, Lansky AJ, Krucoff MW, Webb J, Cox DA, Brodie BR, Stone GW. Impact of collateral flow on myocardial reperfusion and infarct size in patients undergoing primary angioplasty for acute myocardial infarction. Am Heart J. 2007 Aug;154(2):379-84. PMID 17643592
- Kitabata H, Kubo T, Akasaka T.Identification of multiple plaque ruptures by optical coherence tomography in a patient with acute myocardial infarction: a three-vessel study. Heart 2008; 94: 544; doi:10.1136/hrt.2007.124339 PMID 18411345
- Hong MK, Mintz GS, Lee CW, Park KM, Lee BK, Kim YH, Kang DH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. Plaque ruptures in stable angina pectoris compared with acute coronary syndrome. Int J Cardiol. 2007 Jan 2; 114(1): 78-82. Epub 2006 May 18. PMID 1671298
- Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007 Sep 4;50(10):933-9. Epub 2007 Aug 20. PMID 17765119
- Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation. 2002 Aug 13; 106(7): 804-8. PMID 12176951
- Hong MK, Mintz GS, Lee CW, Lee BK, Yang TH, Kim YH, Song JM, Han KH, Kang DH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol. 2005 Jul 19; 46 (2): 261-5. PMID 16022952
- Kusama I, Hibi K, Kosuge M, Nozawa N, Ozaki H, Yano H, Sumita S, Tsukahara K, Okuda J, Ebina T, Umemura S, Kimura K. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J Am Coll Cardiol. 2007 Sep 25;50(13):1230-7. Epub 2007 Sep 10. PMID 17888839
- Tanaka N, Ehara M, Surmely JF, Matsubara T, Terashima M, Tsuchikane E, Katoh O, Suzuki T. Images in cardiovascular medicine. Sixty-four-multislice computed tomography image of a ruptured coronary plaque. Circulation. 2006 Oct 3; 114 (14): e519-20. PMID 17015797
- Fujii K, Mintz GS, Carlier SG, Costa JR Jr, Kimura M, Sano K, Tanaka K, Costa RA, Lui J, Stone GW, Moses JW, Leon MB. Intravascular ultrasound profile analysis of ruptured coronary plaques. Am J Cardiol. 2006 Aug 15;98(4):429-35. Epub 2006 Jun 19. PMID 16893692
- Gilard M, Rioufol G, Zeller M, Cottin Y, Rochette L, Finet G. Reliability and limitations of angiography in the diagnosis of coronary plaque rupture: an intravascular ultrasound study Arch Cardiovasc Dis. 2008 Feb;101(2):114-20. PMID 18398396
- Appelbaum E, Kirtane AJ, Clark A, Pride YB, Gelfand EV, Harrigan CJ, Kissinger KV, Manning WJ, Gibson CM. Association of TIMI Myocardial Perfusion Grade and ST-segment resolution with cardiovascular magnetic resonance measures of microvascular obstruction and infarct size following ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2008 Feb 2. [Epub ahead of print] PMID 18246410
- Leshnower BG, Sakamoto H, Hamamoto H, Zeeshan A, Gorman JH 3rd, Gorman RC. Progression of myocardial injury during coronary occlusion in the collateral-deficient heart: a non-wavefront phenomenon. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1799-804. Epub 2007 Jul 20. PMID 17644569