ST elevation myocardial infarction physical examination
|
ST Elevation Myocardial Infarction Microchapters |
|
Differentiating ST elevation myocardial infarction from other Diseases |
|
Diagnosis |
|
Treatment |
|
|
Case Studies |
|
ST elevation myocardial infarction physical examination On the Web |
|
FDA on ST elevation myocardial infarction physical examination |
|
CDC on ST elevation myocardial infarction physical examination |
|
ST elevation myocardial infarction physical examination in the news |
|
Blogs on ST elevation myocardial infarction physical examination |
|
Directions to Hospitals Treating ST elevation myocardial infarction |
|
Risk calculators and risk factors for ST elevation myocardial infarction physical examination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
The physical examination in patients who have suspected acute myocardial infarction may reveal arrhythmia, evidence of heart failure, a new murmur, or cardiovascular compromise and shock. A systems focused examination is probably most appropriate at the time of presentation so as to not delay decisions regarding and implementation of reperfusion therapy. Following these initial stages of management, a more through examination is then warranted. Throughout the patient's course, detailed serial examinations should be performed in an effort to remain vigilant for the development of mechanical complications of acute MI. The approach to the physical examination in the patient with ST elevation MI is divided into two phases: The initial physical examination and then the more thorough examination of the patient after the initial assessment and treatment of the patient.
Initial Physical Examination at the Time of Presentation
Assess the Appropriateness of the Patient for Reperfusion Therapy
The focus of the initial physical examination should be directed towards evaluating the appropriateness of the patient for reperfusion therapy. In particular, contraindications fibrinolytic therapy should be thoroughly evaluated. The presence of aortic dissection should be excluded as should the presence of pericarditis. Thus if the patient has complaints such as back pain or interscapular pain that would suggest aortic dissection, the blood pressure should be evaluated in both arms and any discrepancy should be noted. Likewise, the presence of aortic insufficiency should be noted during cardiac auscultation. Careful auscultation of the heart to be performed to also assure that there is no pericardial friction rub to suggest the presence of pericarditis.
Likewise, the presence of the focal neurologic defect should be noted. A careful assessment of the patient's risk of bleeding should be assessed, including the assessment for occult bleeding from the G.I. tract if there is a suspicion.
If the patient is to undergo primary percutaneous coronary intervention (PCI) then careful assessment of the femoral pulses should be made. The strength of the pulses and the presence of bruits should be assessed. In particular, it should be anticipated that an intra-aortic balloon pump may need to be placed. If the choice is to proceed from the right femoral artery, then the ability to place an intra-aortic balloon pump in the left femoral artery should be noted.
Assess the Patient for the Presence of Right Ventricular Myocardial Infarction
Administration of nitrates are an important part of the initial management of patients with ST elevation MI. Patients who are treated with nitrates may become hypotensive in the presence of a right ventricular infarction. Therefore a careful assessment for signs of right ventricular myocardial infarction should be performed. If right heart failure is present, elevated jugular venous pressure and hepatojugular reflux, or swelling of the legs due to peripheral edema may be found on physical examination. The presence of ST segment elevation on right sided leads (V4R) are critical in establishing the diagnosis of RV infarction. In the patient with ST segment elevation in inferior leads, right sided leads should be obtained to quickly aid in the diagnosis of RV infarction, particularly if signs of RV failure or hypotension are present.
Assess the Patient for the Presence of Hypoperfusion and Left Ventricular Failure
Administration of beta blockers, angiotensin converting enzyme inhibitors and other vasoactive medications are part of the initial management of acute MI. Therefore, at the time of the initial evaluation, a careful assessment of tissue perfusion should be made. Classically, patients with acute MI move around in the bed so that they can find a more comfortable position. This is in contrast to patients with angina who often lie still. However, in the presence of cardiogenic shock, the patient may lie still. As a result of intense vasoconstriction from heightened sympathetic tone, systemic blood pressure may not reveal the presence of a shock like state. In the presence of intense vasoconstriction, the skin is often cool, clammy, mottled and pale. The lips and the nail beds may be cyanotic. In cases of severe hypoperfusion the patient may be either confused and/or disoriented. Rales on the lung exam are are indicative of left heart failure. The presence of tachycardia may also suggest a low perfusion state.
Rarely, a cardiac bulge with a pace different from the pulse rhythm can be felt on precordial examination. Various abnormalities can be found on auscultation, such as a third and fourth heart sound, systolic murmurs, paradoxical splitting of the second heart sound, a pericardial friction rub and rales over the lung.[1]
Complete Phsyical Examination of the Patient After the Initial Rapid Assessment
The purpose of the physical examination following initial presentation is to monitor the patient for the development of mechanical complications and to monitor the patient's response to therapeutic interventions. The presence of indwelling catheters is no replacement for a careful daily physical examination. In fact, careful physical examination should often suffice in replacing the need for hemodynamic monitoring in many patients. Careful attention to the pulse, blood pressure, the neck veins, the presence of rales, and the urine output should all be used to guide therapeutic interventions. [1]
Vitals
Temperature
Some patients may have low-grade fever (38–39 °C) and an elevated white blood cell count in the setting of acute MI.
Pulse
The heart rate will vary depending upon infarct artery location and the presence of left ventricular dysfunction. Upon presentation many patients are anxious and in pain. This may result in a hyperadrenergic state which in turn may result in the sinus tachycardia. Patients with left ventricular failure will obviously develop sinus tachycardia. Inferior myocardial infarction is often associated with various degrees of heart block and bradycardia (see complications). The presence of pulsus alternans (an alternating strong and weak pulse) may reflect the presence of left ventricular dysfunction. Both mitral regurgitation and the development of a new ventricular septal defect may be associated with a widened pulse pressure (a more abrupt rise and fall in the pulse) (see complications).
Blood Pressure
It is critical to obtain a complete prior history to place the patient's blood pressure in the appropriate context. Normotension in a patient who was previously hypertensive can be reflective of poor LV function. Again as noted in the above section on the pulse, upon presentation many patients are anxious and in pain and this hyperadrenergic state may result in a hypertensive response in a patient who was previously normotensive. A brief, non-sustained elevation of the blood pressure in a patient who is ordinarily not hypertensive, should not preculed the administration of a fibrinolytic agent.
It should be noted that the blood pressure during the initial course of myocardial infarction generally does not reflect what a patient's blood pressure will ultimately be at the time of discharge. In general, it is not uncommon for patients with an inferior MI to be hypotensive at some point early in their admission due to transient autonomic dysfunction. Likewise, it is not uncommon for a patient with an anterior MI to be hypertensive in the early phase infarction due to an early surge in adrenergic tone.
Hypotension in the early course of acute myocardial infarction has two broad causes. One is cardiogenic shock (see complications). These patients will have a systolic blood pressure below 90 mm Hg and will have evidence of end-organ hypoperfusion. In general, they will be tachycardic. Cardiogenic shock is more often seen in patients with infarction involving the left ventricle. In contrast, patients with activation of the Bezold-Jarisch reflex will in general not be tachycardic and may instead be bradycardic. This reflex activation is more common in patients with an inferior myocardial infarction. Whereas cardiogenic shock will persist for a protracted period of time, the Bezold-Jarisch reflex will generally resolve in a shorter period of time, and is responsive to fluids, atropine (0.5 to 1.0 mg IV) and elevation of the legs (Trendelenburg position).
Cardiac Exam
-
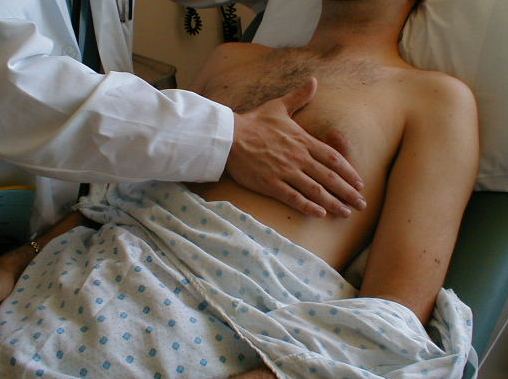
Palpation of the Precordium.
-
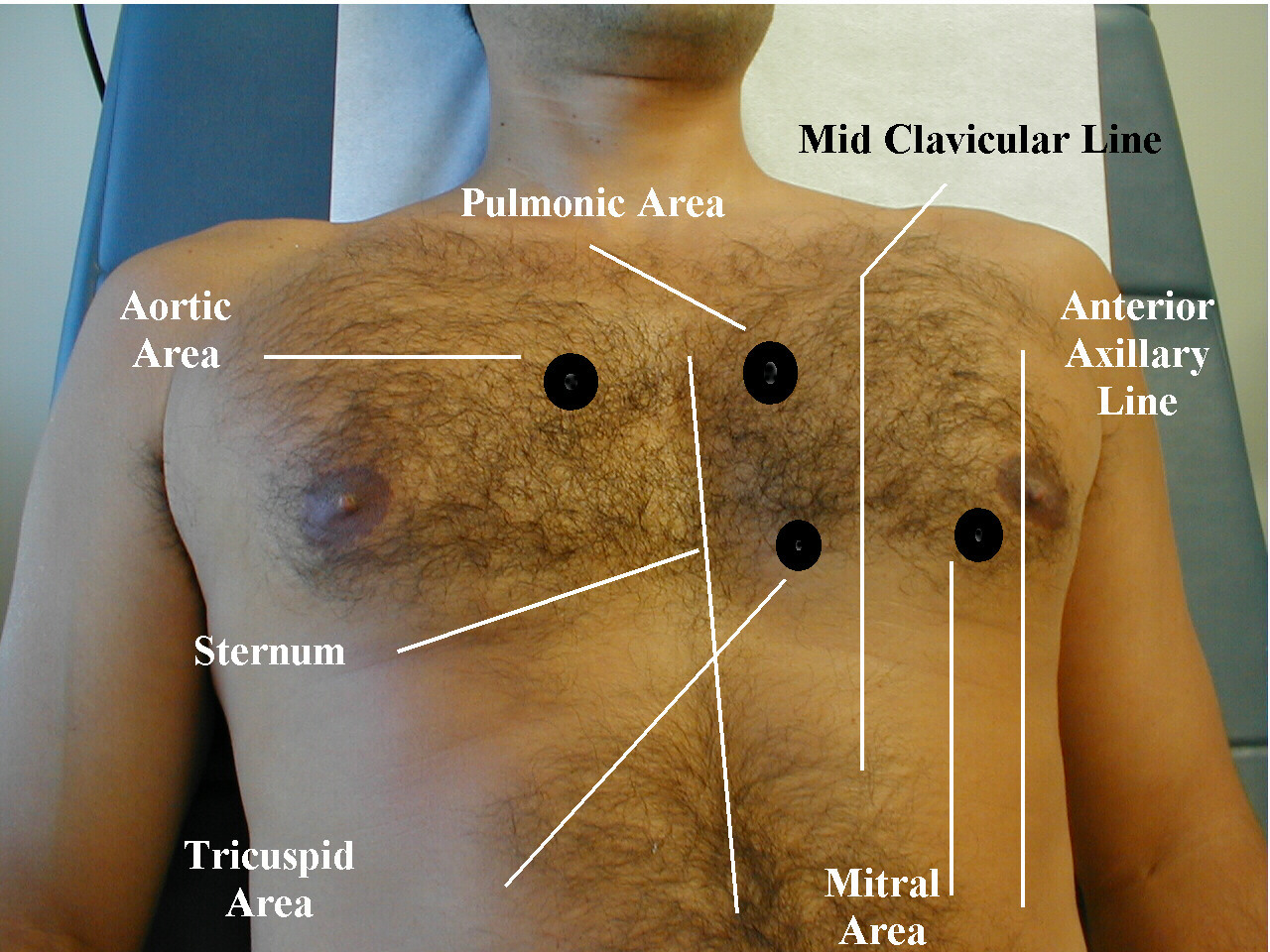
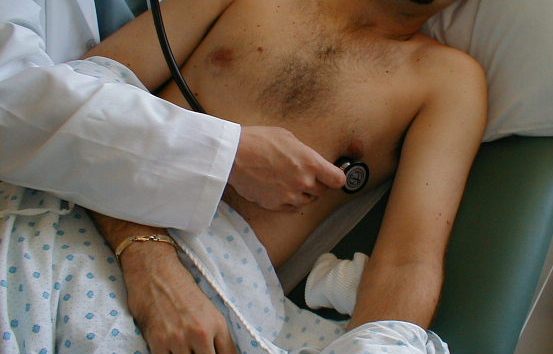
Auscultation of the Heart.
-
Listening for Extra Heart Sounds.
(Images courtesy of Charlie Goldberg, M.D., UCSD)
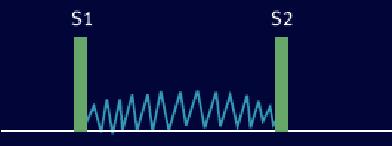
Heart Sounds [2]
Third Heart Sound (S3) [2]
This sound is called a protodiastolic gallop or ventricular gallop , a type of gallop rhythm occurs when the left ventricle is not very compliant, and at the beginning of diastole the rush of blood into the left ventricle suddenly is halted, resulting in a vibration of the ventricle and surrounding structures. It is caused by the sudden slowing of blood rushing in from the atrium into the ventricle as it is relaxing.
Differential Diagnosis of Causes of an S3:
It is associated with heart failure, caused by conditions which have:
- Rapid ventricular filling
- Mitral regurgitation - this is when one of the heart valves that usually stops blood going from the left ventricle to the left atria fails, allowing blood into the atria during systole. This means they will be overfilled when they come to contract, leading to the rapid ventricular filling.
- Elevated left atrial and left ventricular filling pressures, usually a result of a stiffened and dilated left ventricle
- Ventricular septal defect - this is a hole in the wall between the two ventricles, which allows rapid filling from the other ventricle.
- Elevated atrial pressure
- High-output states
- Poor Left Ventricular Function
- Post-MI - the death of tissue in the ventricular wall due to loss of blood supply causes areas which do not move as well, if at all (hypokinetic and akinetic), meaning they do not relax quickly enough so the ventricular filling is relatively too quick.
- Dilated cardiomyopathy - the ventricular walls are abnormal for a variety of reasons, and become thin and stiff so do not relax well.
- Hypertrophic obstructive cardiomyopathy
- Cardiomegaly of a variety of causes
In conditions affecting the pericardium or diseases that primarily affect the heart muscle (restrictive cardiomyopathies) a similar sound can be heard, but is usually more high-pitched and is called a 'pericardial knock'.
The S3 can also be confused with a widely split S2, or a mitral opening snap, but these sounds are typically of much higher pitch and occur closer to the onset of S2.
Fourth Heart Sound S4 [2]
S4 (or fourth heart sound) occurs just before S1 (thus right at the end of one whole cycle), giving a cadence like the word 'Tennessee'. It is never normal. If the problem lies with the left ventricle, the gallop rhythm will be heard best at the cardiac apex (the point of the two ventricles). It will become more apparent with exercise, with the patient lying on their left-hand side, or with the patient holding expiration. If the culprit is the right ventricle, the abnormal rhythm will be most evident on the lower left hand side of the sternum, and will get louder with exercise and quick, deep inspiration <[3]. The fourth heart sound S4 is sometimes audible in healthy children, but when audible in an adult is called a presystolic gallop or atrial gallop. This gallop is a sign of a pathologic state, usually a failing left ventricle. This sound occurs just after atrial contraction ("atrial kick") and is the sound of blood being forced into a stiff/hypertrophic left ventricle. The combined presence of S3 and S4 is a quadruple gallop. At rapid heart rates, S3 and S4 may merge to produce asummation gallop.
It is caused by the atria contracting forcefully in an effort to overcome an abnormally-stiff ventricle.
Differential Diagnosis of Causes of an S4:
The S4 rhythm is associated with disorders that increases the stiffness of the ventricle, including:
- Long-standing hypertension
- Aortic stenosis
- Overload of the ventricle
- Fibrosis of the ventricle
- Left ventricular hypertrophy
- Coronary Artery Disease
- Hypertrophic Cardiomyopathy
- Pulmonary Hypertension
Murmurs [2]
It is important to thoroughly evaluate and record the presence of any murmur at baseline so that the development of any new murmur which may reflect a mechanical complication) can be promptly noted. The intensity of the murmur may vary depending upon the size and compliance of the cardiac chambers involved.[4][5][6][7]
Grading the loudness of a murmur from 1 to 6 is a well described and widely used method [8] Systolic thrills usually are associated with murmurs of grade 4 or louder.
Mitral Regurgitation
Mitral regurgitation can develop as a result of either papillary muscle dysfunction or as a result a dilation of the mitral apparatus secondary to left ventricular dilation. [9][10] Mitral regurgitation generally results in a blowing holosystolic murmur best heard at the apex. If there has been rupture of the head of the papillary muscle, a thrill may be palpated (see complications). [11]
Ventricular Septal Defect
The development of a ventricular septal defect is associated with a holosystolic murmur and thrill which are most prominent along the left and right sternal border (see complications).
Tricuspid Regurgitation
Either right ventricular infarction or rupture of a right ventricular papillary muscle may result in tricuspid regurgitation. This is a systolic murmur that is best heard along the left sternal border. In general, it is intensified by inspiration. Tricuspid regurgitation may be associated with a prominent c-v wave in the jugular venous pulse.
-
Holosystolic murmur can be found in mitral regurgitation, ventricular septal defect and tricuspid regurgitation
Pericardial Friction Rub
A pericardial friction rub is often best heard over the left sternal border, but many also be prominent at the apex of the heart. In general, a rub is heard in both systole and diastole. If it is heard only in systole, it may be mistaken for a systolic murmur. In general a rub is more scratchy and squeaky in nature compared with a murmur, which is more blowing or rumbling in character.[12]
References
- ↑ 1.0 1.1 S. Garas et al.. Myocardial Infarction. eMedicine. Retrieved November 22, 2006.
- ↑ 2.0 2.1 2.2 2.3 Heart sound examples
- ↑ Tavel ME (1996). "The appearance of gallop rhythm after exercise stress testing". Clin Cardiol. 19 (11): 887–91. PMID 8914783. Unknown parameter
|month=ignored (help) - ↑ Cheng TO (1969). "Some new observations on the syndrome of papillary muscle dysfunction". Am. J. Med. 47 (6): 924–45. PMID 5308102. Unknown parameter
|month=ignored (help) - ↑ CRAIGE E (1960). "Phonocardiography in interventricular septal defects". Am. Heart J. 60: 51–60. PMID 13812602. Unknown parameter
|month=ignored (help) - ↑ Mineo K, Cummings J, Josephson R, Nanda NC (2001). "Acquired left ventricular outflow tract obstruction during acute myocardial infarction: diagnosis of a new cardiac murmur". Am J Geriatr Cardiol. 10 (5): 283–5. PMID 11528289.
- ↑ Ronan JA, Steelman RB, DeLeon AC, Waters TJ, Perloff JK, Harvey WP (1971). "The clinical diagnosis of acute severe mitral insufficiency". Am. J. Cardiol. 27 (3): 284–90. PMID 5101319. Unknown parameter
|month=ignored (help) - ↑ Freeman AR, Levine SA. Clinical significance of systolic murmurs: study of 1000 consecutive "noncardiac" cases. Ann Intern Med. 1933, 6: 1371–85.
- ↑ Birnbaum Y, Chamoun AJ, Conti VR, Uretsky BF (2002). "Mitral regurgitation following acute myocardial infarction". Coron. Artery Dis. 13 (6): 337–44. PMID 12436029. Unknown parameter
|month=ignored (help) - ↑ Bursi F, Enriquez-Sarano M, Jacobsen SJ, Roger VL (2006). "Mitral regurgitation after myocardial infarction: a review". Am. J. Med. 119 (2): 103–12. doi:10.1016/j.amjmed.2005.08.025. PMID 16443408. Unknown parameter
|month=ignored (help) - ↑ Maisel AS, Gilpin EA, Klein L, Le Winter M, Henning H, Collins D (1986). "The murmur of papillary muscle dysfunction in acute myocardial infarction: clinical features and prognostic implications". Am. Heart J. 112 (4): 705–11. PMID 3766369. Unknown parameter
|month=ignored (help) - ↑ Chizner MA (2008). "Cardiac auscultation: rediscovering the lost art". Curr Probl Cardiol. 33 (7): 326–408. doi:10.1016/j.cpcardiol.2008.03.003. PMID 18513577. Unknown parameter
|month=ignored (help)