Retinoblastoma pathophysiology: Difference between revisions
m (Bot: Removing from Primary care) |
|||
| (165 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Retinoblastoma}} | {{Retinoblastoma}} | ||
{{CMG}}; {{AE}} {{Sahar}} {{Simrat}} | |||
==Overview== | ==Overview== | ||
Retinoblastoma is a [[neoplasm]] which is caused by the inactivation of [[RB1]] [[gene]], a [[tumor suppressor gene]], located on the long arm of the [[chromosome 13]]. [[Mutation]] in both [[alleles]] of the [[RB1]] [[gene]] is necessary for the inactivation of the [[gene]]. This [[disorder]] may occur in the [[familial]] or sporadic form. ([[Rb]]) [[gene]] product limits the [[cell]] progression from the [[G1 phase]] to the [[S phase]] of the [[cell cycle]]. Loss of this active, functional [[protein]] ([[Rb]]) causes [[cell cycle]] [[dysregulation]] and subsequent overgrowth and [[tumor]] formation. | |||
== Pathophysiology == | |||
===Pathogenesis=== | |||
*Retinoblastoma is a [[neoplasm]] which is caused by the inactivation of [[RB1]] [[gene]], a [[tumor suppressor gene]].<ref name="pmid3175621">{{cite journal |vauthors=Dunn JM, Phillips RA, Becker AJ, Gallie BL |title=Identification of germline and somatic mutations affecting the retinoblastoma gene |journal=Science |volume=241 |issue=4874 |pages=1797–800 |date=September 1988 |pmid=3175621 |doi= |url=}}</ref> | |||
*Normally, [[RB1]] [[gene]] is necessary for the normal [[differentiation]] and growth of [[retinal]] [[stem cells]] and its [[mutation]] results in unregulated growth of these [[cells]] and [[development]] of the [[tumor]]. | |||
*[[Mutation]] in both [[alleles]] of the [[RB1]] [[gene]] is necessary for the inactivation of the [[gene]].<ref name="pmid2601691">{{cite journal |vauthors=Dunn JM, Phillips RA, Zhu X, Becker A, Gallie BL |title=Mutations in the RB1 gene and their effects on transcription |journal=Mol. Cell. Biol. |volume=9 |issue=11 |pages=4596–604 |date=November 1989 |pmid=2601691 |pmc=363605 |doi= |url=}}</ref> | |||
*This [[disorder]] may occur in the [[familial]] or sporadic form. | |||
*In the [[familial]] form (48% of the cases), the first [[mutation]] occurs during [[germ cell]] division and the second [[mutation]] occurs later during the division of [[Somatic cell|somatic cells]].<ref name="pmid15637391">{{cite journal |vauthors=Garber JE, Offit K |title=Hereditary cancer predisposition syndromes |journal=J. Clin. Oncol. |volume=23 |issue=2 |pages=276–92 |date=January 2005 |pmid=15637391 |doi=10.1200/JCO.2005.10.042 |url=}}</ref> | |||
*In the sporadic form, both [[mutations]] occur during the lifetime of the individual. | |||
*([[Rb]]) [[gene]] product limits the [[Cell (biology)|cell]] progression from the [[G1 phase]] to the [[S phase]] of the [[cell cycle]].<ref name="GoodrichWang1991">{{cite journal|last1=Goodrich|first1=David W.|last2=Wang|first2=Nan Ping|last3=Qian|first3=Yue-Wei|last4=Lee|first4=Eva Y.-H.P.|last5=Lee|first5=Wen-Hwa|title=The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle|journal=Cell|volume=67|issue=2|year=1991|pages=293–302|issn=00928674|doi=10.1016/0092-8674(91)90181-W}}</ref> | |||
*Active form of [[RB]] [[protein]] prevent the interaction of [[E2F]], a [[transcription factor]]. Loss of this active, functional [[protein]] ([[Rb]]) causes [[Transcription (genetics)|transcribing]] the [[gene]] and subsequent [[cell cycle]] dysregulation, overgrowth and [[tumor]] formation. | |||
==Genetics== | |||
*[[Retinoblastoma]] occurs due to [[Mutation|mutational]] inactivation of [[RB1]] [[gene]] located on the [[chromosome 13]].<ref name="pmid5279523">{{cite journal |vauthors=Knudson AG |title=Mutation and cancer: statistical study of retinoblastoma |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=68 |issue=4 |pages=820–3 |date=April 1971 |pmid=5279523 |pmc=389051 |doi= |url=}}</ref> | |||
*The [[RB1]] [[gene]] acts as [[tumor suppressor gene]].<ref name="pmid2877398">{{cite journal |vauthors=Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP |title=A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma |journal=Nature |volume=323 |issue=6089 |pages=643–6 |date=1986 |pmid=2877398 |doi=10.1038/323643a0 |url=}}</ref> | |||
*Two [[Mutation|mutational]] events are needed for the [[development]] of retinoblastoma. | |||
*In [[familial]] form, with [[autosomal dominant]] [[inheritance]], one [[mutation]] occurs in the [[germline]] and the second one during the [[somatic]] division of the [[retinal]] [[cells]]. | |||
*In the acquired form, both [[mutations]] occur during [[somatic]] divisions. | |||
*Another [[gene]] which has been associated with the [[pathogenesis]] of retinoblastoma is MYCN [[gene]].<ref name="pmid29915469">{{cite journal |vauthors=Fabian ID, Rosser E, Sagoo MS |title=Epidemiological and genetic considerations in retinoblastoma |journal=Community Eye Health |volume=31 |issue=101 |pages=29–30 |date=2018 |pmid=29915469 |pmc=5998388 |doi= |url=}}</ref> | |||
*Retinoblastoma may also occur as part of [[13q deletion syndrome]].<ref name="ClarkAvishay2015">{{cite journal|last1=Clark|first1=Robin D.|last2=Avishay|first2=Stefanie G.|title=Retinoblastoma: Genetic Counseling and Testing|year=2015|pages=77–88|doi=10.1007/978-3-662-43451-2_8}}</ref> | |||
**This [[13q deletion syndrome|syndrome]] is the result of the [[deletion]] of the long arm of [[chromosome 13]]. | |||
**[[Symptoms]] may vary according to the size of the [[deletion]], but it may lead to [[developmental delay]] as well. | |||
**[[Child|Children]] with [[chromosome]] 13q14 [[Deletion (genetics)|deletions]] may develop retinoblastoma at a later age and they develop a unilateral [[tumor]]. | |||
*[[Mosaicism]], presence of [[RB1]] [[gene mutation]] in some [[cells]] of the affected person, may occur in retinoblastoma. | |||
**[[Patient|Patients]] with [[Mosaicism|mosaic mutation]] often have unilateral retinoblastoma, later onset of the [[tumor]], and no [[family history]] of the [[disease]]. | |||
==Associated Conditions== | |||
*Heritable form of this [[disorder]] is associated with the development of non-ocular [[malignancies]] including:<ref name="TseBrennan2015">{{cite journal|last1=Tse|first1=Brian C.|last2=Brennan|first2=Rachel C.|last3=Rodriguez-Galindo|first3=Carlos|last4=Wilson|first4=Matthew W.|title=Non-ocular Tumors|year=2015|pages=201–208|doi=10.1007/978-3-662-43451-2_19}}</ref> | |||
**Different types of [[Sarcoma]] | |||
**[[Small cell lung cancer]] | |||
**[[Bladder cancer]] | |||
**[[Breast cancer]] | |||
**[[Glioblastoma]] | |||
== | ==Gross Pathology== | ||
*[[Macroscopic]] appearance of the [[tumor]] varies according to the [[Cancer staging|staging]] of the [[tumor]].<ref name="pmid24881618">{{cite journal |vauthors=Das D, Bhattacharjee K, Barthakur SS, Tahiliani PS, Deka P, Bhattacharjee H, Deka A, Paul R |title=A new rosette in retinoblastoma |journal=Indian J Ophthalmol |volume=62 |issue=5 |pages=638–41 |date=May 2014 |pmid=24881618 |pmc=4065523 |doi=10.4103/0301-4738.129786 |url=}}</ref> | |||
*The [[tumor]] is white and has areas of [[calcification]] and [[necrosis]]. | |||
*The presence of [[calcium]] is more noticeable when the [[tumor]] is treated via prior [[chemotherapy]] or [[radiotherapy]]. | |||
*The [[tumor]] can be [[Classification|classified]] into five sub-groups according to its growth pattern:<ref name="SinghMurphree2015">{{cite book | last = Singh | first = Arun | title = Clinical ophthalmic oncology : retinoblastoma | publisher = Springer | location = Heidelberg | year = 2015 | isbn = 978-3-662-43451-2 }}</ref> | |||
**[[Endophyte|Endophytic]] | |||
**Exophytic | |||
**Mixed | |||
**Diffuse infiltrative | |||
**[[Necrotic]] variant | |||
These [[growth]] patterns are described in the table below: | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 600px" align="center" | |||
| valign="top" | | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Growth patterns}} | |||
! style="background: #4479BA; width: 400px;" | {{fontcolor|#FFF|Features}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
:Endophytic | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*Growth occurs inwards into the [[vitreous]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
:Exophytic | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*Growth occurs outwards towards [[choroid]] | |||
*Associated with non-rhegmatogenous [[retinal detachment]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
:Mixed | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*Most common type | |||
*Mixed components of endophytic and exophytic are seen | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
:Diffuse Infiltrative | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*More commonly seen among older [[Child|children]] | |||
*Diffuse growth of the [[tumor]] without an obvious [[retinal]] [[mass]] | |||
*Frequently involves [[anterior chamber]] and causes pseudohypopyon of [[Tumor cell|tumor cells]] | |||
*Clinically can be mistaken for an [[inflammatory process]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
:Necrotic | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*May present as an [[inflammatory process]] and can be mistaken for [[orbital cellulitis]] with [[chemosis]] and [[proptosis]] | |||
*Associated with increased risk of [[metastasis]] | |||
|- | |||
|} | |||
{| | |||
|[[image:Retinoblastoma gross pathology.jpeg|thumb|400px|Gross pathology of retinoblastoma, Case courtesy of A.Prof Frank Gaillard, Radiopaedia.org, rID: 9461]] | |||
<br style="clear:left" /> | |||
|- | |||
|} | |||
==Microscopic Pathology== | |||
* [[Microscopic|Microscopically]], retinoblastoma is characterized by:<ref name="pmid22288967">{{cite journal |vauthors=Kashyap S, Sethi S, Meel R, Pushker N, Sen S, Bajaj MS, Chandra M, Ghose S |title=A histopathologic analysis of eyes primarily enucleated for advanced intraocular retinoblastoma from a developing country |journal=Arch. Pathol. Lab. Med. |volume=136 |issue=2 |pages=190–3 |date=February 2012 |pmid=22288967 |doi=10.5858/arpa.2010-0759-OA |url=}}</ref> | |||
**Small [[Hyperchromicity|hyperchromatic]] [[cells]] with a high [[nuclear]] to [[cytoplasmic]] ratio | |||
**Large areas of [[necrosis]] | |||
**Multifocal area of [[Calcification|calcifications]] | |||
* Retinoblastoma [[histopathology]] is a combination of undifferentiated [[Cell (biology)|cells]] and areas of [[tumor]] [[differentiation]] shown as rosettes and fleurettes.<ref name="Chévez-BarriosEagle2015">{{cite journal|last1=Chévez-Barrios|first1=Patricia|last2=Eagle|first2=Ralph C.|last3=Marback|first3=Eduardo F.|title=Histopathologic Features and Prognostic Factors|year=2015|pages=167–183|doi=10.1007/978-3-662-43451-2_16}}</ref> | |||
*The most differentiated part is formed from a bouquet-like aggregates of [[Cell (biology)|cells]] called fleurettes, where [[Mitosis|mitoses]] or [[necrosis]] are not present. | |||
**These [[Cell (biology)|cells]] resemble the [[photoreceptors]] and are arranged similar to [[Flower|flowers]]. | |||
*The rosettes are composed of [[Cell (biology)|cells]] with varying degrees of differentiation. | |||
*There are two types of rosettes: | |||
**Flexner–Wintersteiner rosette: Composed of a ring of [[cells]] surrounding a clear center resembling the [[Retina|subretinal]] space. | |||
**Homer Wright rosette: Comprises of a rim of [[cells]] with a [[lumen]] filled by [[cytoplasmic]] prolongations of the [[tumor]] [[cells]]. | |||
* Retinoblastoma may be [[Classification|classified]] according to the degree of [[differentiation]] to well/poor-differentiated. | |||
**Well-differentiated [[tumor]] is > 50% Homer-Wright (HW) rosettes. | |||
**Poor-differentiated [[tumor]] is < 50% Flexner-Wintersteiner (FW) rosettes. | |||
==Immunohistochemistry== | |||
*There is no specific [[immunohistochemical]] [[marker]] for the [[diagnosis]] of retinoblastoma.<ref name="pmid16049534">{{cite journal |vauthors=Odashiro AN, Pereira PR, de Souza Filho JP, Cruess SR, Burnier MN |title=Retinoblastoma in an adult: case report and literature review |journal=Can. J. Ophthalmol. |volume=40 |issue=2 |pages=188–91 |date=April 2005 |pmid=16049534 |doi=10.1016/S0008-4182(05)80032-8 |url=}}</ref><ref name="pmid23166876">{{cite journal |vauthors=Zhang Z, Shi JT, Wang NL, Ma JM |title=Retinoblastoma in a young adult mimicking Coats' disease |journal=Int J Ophthalmol |volume=5 |issue=5 |pages=625–9 |date=2012 |pmid=23166876 |pmc=3484701 |doi=10.3980/j.issn.2222-3959.2012.05.16 |url=}}</ref> | |||
*The most commonly applied [[marker]] is neuron specific enolase (NSE). | |||
*Other useful [[Marker|markers]] are: | |||
**[[Synaptophysin]] | |||
**[[CD56]] | |||
**[[Glial fibrillary acidic protein]] ([[GFAP]]) | |||
*Although there is no specific [[biomarker]] for the [[diagnosis]] of retinoblastoma, it may be needed for the [[diagnosis]] of undifferentiated form of the [[tumor]].<ref name="pmid25378879">{{cite journal |vauthors=Yousef YA, Istetieh J, Nawaiseh I, Al-Hussaini M, Alrawashdeh K, Jaradat I, Sultan I, Mehyar M |title=Resistant retinoblastoma in a 23-year-old patient |journal=Oman J Ophthalmol |volume=7 |issue=3 |pages=138–40 |date=September 2014 |pmid=25378879 |pmc=4220401 |doi=10.4103/0974-620X.142597 |url=}}</ref><ref name="pmid6856254">{{cite journal |vauthors=Takahashi T, Tamura S, Inoue M, Isayama Y, Sashikata T |title=Retinoblastoma in a 26-year-old adult |journal=Ophthalmology |volume=90 |issue=2 |pages=179–83 |date=February 1983 |pmid=6856254 |doi= |url=}}</ref> | |||
*[[Immunocytochemistry|IHC]] may be useful for the identification of [[photoreceptors]] and [[glial cells]] in the retinoblastoma. | |||
*[[Immunocytochemistry|IHC]] may also be useful in identifying the level of differentiation of the [[tumor]] by detecting red and green [[cones]] found in the rosettes and fleurettes and blue [[cones]] which do not form rosettes and fleurettes. | |||
==References== | ==References== | ||
{{Reflist|2}} | |||
| |||
[[Category:Medicine]] | |||
[[Category: | |||
[[Category:Oncology]] | [[Category:Oncology]] | ||
[[Category: | [[Category:Up-To-Date]] | ||
[[Category:Surgery]] | |||
Latest revision as of 23:59, 29 July 2020
|
Retinoblastoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Retinoblastoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Retinoblastoma pathophysiology |
|
Risk calculators and risk factors for Retinoblastoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sahar Memar Montazerin, M.D.[2] Simrat Sarai, M.D. [3]
Overview
Retinoblastoma is a neoplasm which is caused by the inactivation of RB1 gene, a tumor suppressor gene, located on the long arm of the chromosome 13. Mutation in both alleles of the RB1 gene is necessary for the inactivation of the gene. This disorder may occur in the familial or sporadic form. (Rb) gene product limits the cell progression from the G1 phase to the S phase of the cell cycle. Loss of this active, functional protein (Rb) causes cell cycle dysregulation and subsequent overgrowth and tumor formation.
Pathophysiology
Pathogenesis
- Retinoblastoma is a neoplasm which is caused by the inactivation of RB1 gene, a tumor suppressor gene.[1]
- Normally, RB1 gene is necessary for the normal differentiation and growth of retinal stem cells and its mutation results in unregulated growth of these cells and development of the tumor.
- Mutation in both alleles of the RB1 gene is necessary for the inactivation of the gene.[2]
- This disorder may occur in the familial or sporadic form.
- In the familial form (48% of the cases), the first mutation occurs during germ cell division and the second mutation occurs later during the division of somatic cells.[3]
- In the sporadic form, both mutations occur during the lifetime of the individual.
- (Rb) gene product limits the cell progression from the G1 phase to the S phase of the cell cycle.[4]
- Active form of RB protein prevent the interaction of E2F, a transcription factor. Loss of this active, functional protein (Rb) causes transcribing the gene and subsequent cell cycle dysregulation, overgrowth and tumor formation.
Genetics
- Retinoblastoma occurs due to mutational inactivation of RB1 gene located on the chromosome 13.[5]
- The RB1 gene acts as tumor suppressor gene.[6]
- Two mutational events are needed for the development of retinoblastoma.
- In familial form, with autosomal dominant inheritance, one mutation occurs in the germline and the second one during the somatic division of the retinal cells.
- In the acquired form, both mutations occur during somatic divisions.
- Another gene which has been associated with the pathogenesis of retinoblastoma is MYCN gene.[7]
- Retinoblastoma may also occur as part of 13q deletion syndrome.[8]
- This syndrome is the result of the deletion of the long arm of chromosome 13.
- Symptoms may vary according to the size of the deletion, but it may lead to developmental delay as well.
- Children with chromosome 13q14 deletions may develop retinoblastoma at a later age and they develop a unilateral tumor.
- Mosaicism, presence of RB1 gene mutation in some cells of the affected person, may occur in retinoblastoma.
- Patients with mosaic mutation often have unilateral retinoblastoma, later onset of the tumor, and no family history of the disease.
Associated Conditions
- Heritable form of this disorder is associated with the development of non-ocular malignancies including:[9]
- Different types of Sarcoma
- Small cell lung cancer
- Bladder cancer
- Breast cancer
- Glioblastoma
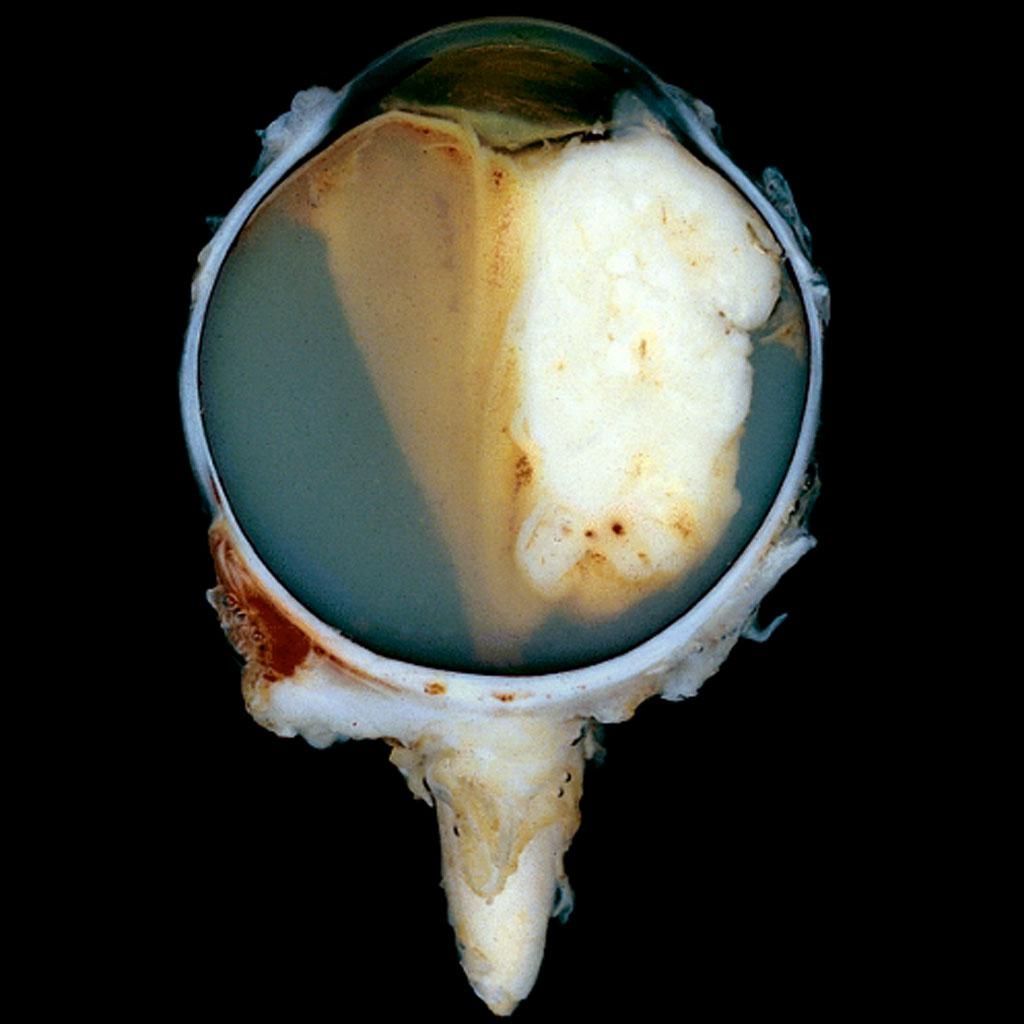
Gross Pathology
- Macroscopic appearance of the tumor varies according to the staging of the tumor.[10]
- The tumor is white and has areas of calcification and necrosis.
- The presence of calcium is more noticeable when the tumor is treated via prior chemotherapy or radiotherapy.
- The tumor can be classified into five sub-groups according to its growth pattern:[11]
- Endophytic
- Exophytic
- Mixed
- Diffuse infiltrative
- Necrotic variant
These growth patterns are described in the table below:
| Growth patterns | Features |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
Microscopic Pathology
- Microscopically, retinoblastoma is characterized by:[12]
- Small hyperchromatic cells with a high nuclear to cytoplasmic ratio
- Large areas of necrosis
- Multifocal area of calcifications
- Retinoblastoma histopathology is a combination of undifferentiated cells and areas of tumor differentiation shown as rosettes and fleurettes.[13]
- The most differentiated part is formed from a bouquet-like aggregates of cells called fleurettes, where mitoses or necrosis are not present.
- These cells resemble the photoreceptors and are arranged similar to flowers.
- The rosettes are composed of cells with varying degrees of differentiation.
- There are two types of rosettes:
- Flexner–Wintersteiner rosette: Composed of a ring of cells surrounding a clear center resembling the subretinal space.
- Homer Wright rosette: Comprises of a rim of cells with a lumen filled by cytoplasmic prolongations of the tumor cells.
- Retinoblastoma may be classified according to the degree of differentiation to well/poor-differentiated.
Immunohistochemistry
- There is no specific immunohistochemical marker for the diagnosis of retinoblastoma.[14][15]
- The most commonly applied marker is neuron specific enolase (NSE).
- Other useful markers are:
- Although there is no specific biomarker for the diagnosis of retinoblastoma, it may be needed for the diagnosis of undifferentiated form of the tumor.[16][17]
- IHC may be useful for the identification of photoreceptors and glial cells in the retinoblastoma.
- IHC may also be useful in identifying the level of differentiation of the tumor by detecting red and green cones found in the rosettes and fleurettes and blue cones which do not form rosettes and fleurettes.
References
- ↑ Dunn JM, Phillips RA, Becker AJ, Gallie BL (September 1988). "Identification of germline and somatic mutations affecting the retinoblastoma gene". Science. 241 (4874): 1797–800. PMID 3175621.
- ↑ Dunn JM, Phillips RA, Zhu X, Becker A, Gallie BL (November 1989). "Mutations in the RB1 gene and their effects on transcription". Mol. Cell. Biol. 9 (11): 4596–604. PMC 363605. PMID 2601691.
- ↑ Garber JE, Offit K (January 2005). "Hereditary cancer predisposition syndromes". J. Clin. Oncol. 23 (2): 276–92. doi:10.1200/JCO.2005.10.042. PMID 15637391.
- ↑ Goodrich, David W.; Wang, Nan Ping; Qian, Yue-Wei; Lee, Eva Y.-H.P.; Lee, Wen-Hwa (1991). "The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle". Cell. 67 (2): 293–302. doi:10.1016/0092-8674(91)90181-W. ISSN 0092-8674.
- ↑ Knudson AG (April 1971). "Mutation and cancer: statistical study of retinoblastoma". Proc. Natl. Acad. Sci. U.S.A. 68 (4): 820–3. PMC 389051. PMID 5279523.
- ↑ Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP (1986). "A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma". Nature. 323 (6089): 643–6. doi:10.1038/323643a0. PMID 2877398.
- ↑ Fabian ID, Rosser E, Sagoo MS (2018). "Epidemiological and genetic considerations in retinoblastoma". Community Eye Health. 31 (101): 29–30. PMC 5998388. PMID 29915469.
- ↑ Clark, Robin D.; Avishay, Stefanie G. (2015). "Retinoblastoma: Genetic Counseling and Testing": 77–88. doi:10.1007/978-3-662-43451-2_8.
- ↑ Tse, Brian C.; Brennan, Rachel C.; Rodriguez-Galindo, Carlos; Wilson, Matthew W. (2015). "Non-ocular Tumors": 201–208. doi:10.1007/978-3-662-43451-2_19.
- ↑ Das D, Bhattacharjee K, Barthakur SS, Tahiliani PS, Deka P, Bhattacharjee H, Deka A, Paul R (May 2014). "A new rosette in retinoblastoma". Indian J Ophthalmol. 62 (5): 638–41. doi:10.4103/0301-4738.129786. PMC 4065523. PMID 24881618.
- ↑ Singh, Arun (2015). Clinical ophthalmic oncology : retinoblastoma. Heidelberg: Springer. ISBN 978-3-662-43451-2.
- ↑ Kashyap S, Sethi S, Meel R, Pushker N, Sen S, Bajaj MS, Chandra M, Ghose S (February 2012). "A histopathologic analysis of eyes primarily enucleated for advanced intraocular retinoblastoma from a developing country". Arch. Pathol. Lab. Med. 136 (2): 190–3. doi:10.5858/arpa.2010-0759-OA. PMID 22288967.
- ↑ Chévez-Barrios, Patricia; Eagle, Ralph C.; Marback, Eduardo F. (2015). "Histopathologic Features and Prognostic Factors": 167–183. doi:10.1007/978-3-662-43451-2_16.
- ↑ Odashiro AN, Pereira PR, de Souza Filho JP, Cruess SR, Burnier MN (April 2005). "Retinoblastoma in an adult: case report and literature review". Can. J. Ophthalmol. 40 (2): 188–91. doi:10.1016/S0008-4182(05)80032-8. PMID 16049534.
- ↑ Zhang Z, Shi JT, Wang NL, Ma JM (2012). "Retinoblastoma in a young adult mimicking Coats' disease". Int J Ophthalmol. 5 (5): 625–9. doi:10.3980/j.issn.2222-3959.2012.05.16. PMC 3484701. PMID 23166876.
- ↑ Yousef YA, Istetieh J, Nawaiseh I, Al-Hussaini M, Alrawashdeh K, Jaradat I, Sultan I, Mehyar M (September 2014). "Resistant retinoblastoma in a 23-year-old patient". Oman J Ophthalmol. 7 (3): 138–40. doi:10.4103/0974-620X.142597. PMC 4220401. PMID 25378879.
- ↑ Takahashi T, Tamura S, Inoue M, Isayama Y, Sashikata T (February 1983). "Retinoblastoma in a 26-year-old adult". Ophthalmology. 90 (2): 179–83. PMID 6856254.