Reperfusion injury pathophysiology: Difference between revisions
No edit summary |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 19: | Line 19: | ||
During this phase mainly the dysregulation of [[Metabolic pathway|metabolic pathways]] occurs and in the [[Reperfusion|reperfusion phase]] there will be a generation of [[free radicals]]. | During this phase mainly the dysregulation of [[Metabolic pathway|metabolic pathways]] occurs and in the [[Reperfusion|reperfusion phase]] there will be a generation of [[free radicals]]. | ||
* [[Ischemia]] when the [[blood]] supply to the [[Tissue (biology)|tissues]] decreases with respect to the demand required to function properly. This results in [[deficiency]] in [[oxygen]], [[glucose]] and various other substrates required for [[cellular metabolism]]. As previously dais the derangement or dysregulation of metabolic function begins in this phase. Due to less [[oxygen]] supply [[cellular metabolism]] shifts to [[anaerobic]] [[glycolysis]] causing the [[glycogen]] to breakdown resulting in the production of 2 ATP and a [[lactic acid]]. This decrease in tissue PH starts further inhibits the [[Adenosine triphosphate|ATP generation]] by negative feed back mechanism. [[Adenosine triphosphate|ATP]] gets broken down into [[Adenosine diphosphate|ADP]], [[Adenosine monophosphate|AMP]] and [[Inosine monophosphate|IMP]]. This finally gets converted to [[adenosine]], [[inosine]], [[hypoxanthine]] and [[xanthine]]. | * [[Ischemia]] when the [[blood]] supply to the [[Tissue (biology)|tissues]] decreases with respect to the demand required to function properly. This results in [[deficiency]] in [[oxygen]], [[glucose]] and various other substrates required for [[cellular metabolism]]. As previously dais the derangement or dysregulation of metabolic function begins in this phase<ref name="pmid10972541">{{cite journal |vauthors=Allen DG, Xiao XH |title=Activity of the Na+/H+ exchanger contributes to cardiac damage following ischaemia and reperfusion |journal=Clin. Exp. Pharmacol. Physiol. |volume=27 |issue=9 |pages=727–33 |date=September 2000 |pmid=10972541 |doi=10.1046/j.1440-1681.2000.03329.x |url=}}</ref>. Due to less [[oxygen]] supply [[cellular metabolism]] shifts to [[anaerobic]] [[glycolysis]] causing the [[glycogen]] to breakdown resulting in the production of 2 ATP and a [[lactic acid]]. This decrease in tissue PH starts further inhibits the [[Adenosine triphosphate|ATP generation]] by negative feed back mechanism. [[Adenosine triphosphate|ATP]] gets broken down into [[Adenosine diphosphate|ADP]], [[Adenosine monophosphate|AMP]] and [[Inosine monophosphate|IMP]]. This finally gets converted to [[adenosine]], [[inosine]], [[hypoxanthine]] and [[xanthine]]. | ||
* Lack of [[Adenosine triphosphate|ATP]] at the cellular level causes impairment in the function of ionic pumps - [[Na+/K+-ATPase|Na+/K+]] and Ca<sup>2</sup>+ pumps. As a result [[cytosolic]] sodium rises which in turn withdraws water to maintain the [[Osmosis|osmotic]] [[equilibrium]] consequently resulting in the [[cellular]] [[Swelling (medical)|swelling]]. To maintain ionic balance [[Potassium ion channels|potassium ion]] escape from the cell. [[Calcium]] is released from the [[Mitochondrion|mitochondria]] to the [[cytoplasm]] and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent [[Proteases|cytosolic proteases]]. These converts the enzyme [[xanthine dehydrogenase]] to [[xanthine oxidase]]. Phospholipases activated during [[ischemia]] promotes membrane degradation and increases level of [[Fatty acid|free fatty acids]] | * Lack of [[Adenosine triphosphate|ATP]] at the cellular level causes impairment in the function of ionic pumps - [[Na+/K+-ATPase|Na+/K+]] and Ca<sup>2</sup>+ pumps. As a result [[cytosolic]] sodium rises which in turn withdraws water to maintain the [[Osmosis|osmotic]] [[equilibrium]] consequently resulting in the [[cellular]] [[Swelling (medical)|swelling]]<ref name="pmid12399448">{{cite journal |vauthors=Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L |title=Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice |journal=Physiol. Genomics |volume=11 |issue=3 |pages=263–72 |date=December 2002 |pmid=12399448 |doi=10.1152/physiolgenomics.00110.2002 |url=}}</ref>. To maintain ionic balance [[Potassium ion channels|potassium ion]] escape from the cell. [[Calcium]] is released from the [[Mitochondrion|mitochondria]] to the [[cytoplasm]] and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent [[Proteases|cytosolic proteases]]. These converts the enzyme [[xanthine dehydrogenase]] to [[xanthine oxidase]]. Phospholipases activated during [[ischemia]] promotes membrane degradation and increases level of [[Fatty acid|free fatty acids]] | ||
* [[Ischemia]] also induces expression of a large number of [[genes]] and [[Transcription factor|transcription factors]], which play a major role in the damage to the tissues. | * [[Ischemia]] also induces expression of a large number of [[genes]] and [[Transcription factor|transcription factors]], which play a major role in the damage to the tissues<ref name="pmid20348484">{{cite journal |vauthors=Safronova O, Morita I |title=Transcriptome remodeling in hypoxic inflammation |journal=J. Dent. Res. |volume=89 |issue=5 |pages=430–44 |date=May 2010 |pmid=20348484 |doi=10.1177/0022034510366813 |url=}}</ref><ref name="pmid11317684">{{cite journal |vauthors=Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ |title=Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events in hemorrhagic shock |journal=Arch Orthop Trauma Surg |volume=121 |issue=4 |pages=219–22 |date=2001 |pmid=11317684 |doi=10.1007/s004020000211 |url=}}</ref>. | ||
** Transcription factors | ** Transcription factors | ||
*** Activating protein-1 ([[AP-1 (transcription factor)|AP-1]]) | *** Activating protein-1 ([[AP-1 (transcription factor)|AP-1]]) | ||
| Line 35: | Line 35: | ||
==== Reactive oxygen species ==== | ==== Reactive oxygen species ==== | ||
The [[ROS]] play major role in the [[tissue]] damage related to [[ischemia]] [[reperfusion injury]]. Once the [[ischemic]] tissue is reperfused the molecular [[oxygen]] catalyzes the conversion of [[hypoxanthine]] to [[uric acid]] and liberating the [[Superoxide|superoxide anion]] (O<sub>2</sub><sup>-</sup>). This superoxide gets further converted to (H<sub>2</sub>O<sub>2</sub>) and the [[hydroxyl radical]] ([[Hydroxyl radical|OH<sup>•</sup>)]]. This OH ion causes the peroxidation [[Lipid|lipids]] in the [[Cell membrane|cell membranes]] resulting in the production and release of proinflammatory [[Eicosanoid|eicosanoids]] and ultimately [[cell death]]. | The [[ROS]] play major role in the [[tissue]] damage related to [[ischemia]] [[reperfusion injury]]. Once the [[ischemic]] tissue is reperfused the molecular [[oxygen]] catalyzes the conversion of [[hypoxanthine]] to [[uric acid]] and liberating the [[Superoxide|superoxide anion]] (O<sub>2</sub><sup>-</sup>). This superoxide gets further converted to (H<sub>2</sub>O<sub>2</sub>) and the [[hydroxyl radical]] ([[Hydroxyl radical|OH<sup>•</sup>)]]. This OH ion causes the peroxidation [[Lipid|lipids]] in the [[Cell membrane|cell membranes]] resulting in the production and release of proinflammatory [[Eicosanoid|eicosanoids]] and ultimately [[cell death]]<ref name="pmid10226957">{{cite journal |vauthors=Yokoyama K, Kimura M, Nakamura K, Nakamura K, Itoman M |title=Time course of post-ischemic superoxide generation in venous effluent from reperfused rabbit hindlimbs |journal=J Reconstr Microsurg |volume=15 |issue=3 |pages=215–21 |date=April 1999 |pmid=10226957 |doi=10.1055/s-2007-1000094 |url=}}</ref>. | ||
Reperfusion Injury | Reperfusion Injury | ||
[[File: Reperfusion Injury Mech.jpg|thumb|Reperfusion injury ( Reperfusion phase). Various | [[File: Reperfusion Injury Mech.jpg|thumb|Reperfusion injury ( Reperfusion phase). Various steps and intermediates formed and involved in the pathogenesis of the Reperfusion phase of Ischemia-reperfusion injury. [https://www.ncbi.nlm.nih.gov/books/NBK534267/figure/ch18f2/?report=objectonly]]] | ||
During the Ischemia-reperfusion injury ROS also activate [[Endothelium|endothelial cells]], which further produces numerous [[Cell adhesion molecule|adhesion molecules.]] | During the Ischemia-reperfusion injury ROS also activate [[Endothelium|endothelial cells]], which further produces numerous [[Cell adhesion molecule|adhesion molecules.]]<ref name="pmid16507884">{{cite journal |vauthors=Pacher P, Nivorozhkin A, Szabó C |title=Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol |journal=Pharmacol. Rev. |volume=58 |issue=1 |pages=87–114 |date=March 2006 |pmid=16507884 |pmc=2233605 |doi=10.1124/pr.58.1.6 |url=}}</ref> | ||
* [[E-selectin]] | * [[E-selectin]] | ||
| Line 51: | Line 51: | ||
* ''[[Arachidonic acid]] (substrate for [[Prostaglandin|prostaglandins]])'' | * ''[[Arachidonic acid]] (substrate for [[Prostaglandin|prostaglandins]])'' | ||
** Prostaglandins usually have a [[Vasodilatory|vasodilatory effect]] hat provides protective effect during [[Ischemia]] [[reperfusion injury]]. But they have a short life so their fast depletion leads to [[vasoconstriction]] ultimately leading to reduced [[blood]] flow and exacerbation of [[ischemia]]. | ** Prostaglandins usually have a [[Vasodilatory|vasodilatory effect]] hat provides protective effect during [[Ischemia]] [[reperfusion injury]]. But they have a short life so their fast depletion leads to [[vasoconstriction]] ultimately leading to reduced [[blood]] flow and exacerbation of [[ischemia]]<ref name="pmid10693639">{{cite journal |vauthors=Neumann UP, Kaisers U, Langrehr JM, Glanemann M, Müller AR, Lang M, Jörres A, Settmacher U, Bechstein WO, Neuhaus P |title=Administration of prostacyclin after liver transplantation: a placebo controlled randomized trial |journal=Clin Transplant |volume=14 |issue=1 |pages=70–4 |date=February 2000 |pmid=10693639 |doi=10.1034/j.1399-0012.2000.140113.x |url=}}</ref>. | ||
* ''[[Thromboxane]]'' | * ''[[Thromboxane]]'' | ||
**[[Thromboxane A2|Plasma thromboxane A<sub>2</sub>]] level rises within minutes after [[reperfusion]], resulting in [[vasoconstriction]] and [[platelet aggregation]]. This usually coincide with rapid rise in [[Pulmonary artery hypertension|pulmonary artery pressure]] and a subsequent increase in [[Lung|pulmonary]] [[Microvascular bed|microvascular]] permeability. | **[[Thromboxane A2|Plasma thromboxane A<sub>2</sub>]] level rises within minutes after [[reperfusion]], resulting in [[vasoconstriction]] and [[platelet aggregation]]. This usually coincide with rapid rise in [[Pulmonary artery hypertension|pulmonary artery pressure]] and a subsequent increase in [[Lung|pulmonary]] [[Microvascular bed|microvascular]] permeability<ref name="pmid7582995">{{cite journal |vauthors=Katircioğlu SF, Küçükaksu DS, Bozdayi M, Taşdemir O, Bayazit K |title=Beneficial effects of prostacyclin treatment on reperfusion of the myocardium |journal=Cardiovasc Surg |volume=3 |issue=4 |pages=405–8 |date=August 1995 |pmid=7582995 |doi=10.1016/0967-2109(95)94159-t |url=}}</ref>. | ||
* ''[[Leukotriene|Leukotrienes]]'' | * ''[[Leukotriene|Leukotrienes]]'' | ||
**[[Leukotriene|Leukotrienes]] are also synthesized from arachidonic acid. [[Leukotriene]]<nowiki/>s acts directly in the [[endothelial cells]], [[smooth muscle]] and indirectly on the [[neutrophils]]. The [[Leukotriene|leukotrienes]] C<sub>4</sub>, D<sub>4,</sub> and E<sub>4</sub> alters the endothelial [[cytoskeleton]], resulting in increased [[vascular]] permeability and [[smooth muscle]] contraction, and finally leading to [[vasoconstriction]]. | **[[Leukotriene|Leukotrienes]] are also synthesized from arachidonic acid. [[Leukotriene]]<nowiki/>s acts directly in the [[endothelial cells]], [[smooth muscle]] and indirectly on the [[neutrophils]]. The [[Leukotriene|leukotrienes]] C<sub>4</sub>, D<sub>4,</sub> and E<sub>4</sub> alters the endothelial [[cytoskeleton]], resulting in increased [[vascular]] permeability and [[smooth muscle]] contraction, and finally leading to [[vasoconstriction]]<ref name="pmid10610833">{{cite journal |vauthors=Rowlands TE, Gough MJ, Homer-Vanniasinkam S |title=Do prostaglandins have a salutary role in skeletal muscle ischaemia-reperfusion injury? |journal=Eur J Vasc Endovasc Surg |volume=18 |issue=5 |pages=439–44 |date=November 1999 |pmid=10610833 |doi=10.1053/ejvs.1999.0929 |url=}}</ref><ref name="pmid8169854">{{cite journal |vauthors=Mangino MJ, Murphy MK, Anderson CB |title=Effects of the arachidonate 5-lipoxygenase synthesis inhibitor A-64077 in intestinal ischemia-reperfusion injury |journal=J. Pharmacol. Exp. Ther. |volume=269 |issue=1 |pages=75–81 |date=April 1994 |pmid=8169854 |doi= |url=}}</ref>. | ||
==== Nitric oxide ==== | ==== Nitric oxide ==== | ||
[[L-arginine]] is the substrate for the synthesis of [[Nitric oxide]] with the help of [[nitric oxide]] synthase enzyme. The [[nitric oxide synthase]] enzyme is usually of 3 types | [[L-arginine]] is the substrate for the synthesis of [[Nitric oxide]] with the help of [[nitric oxide]] synthase enzyme. The [[nitric oxide synthase]] enzyme is usually of 3 types<ref name="pmid15961106">{{cite journal |vauthors=Khanna A, Cowled PA, Fitridge RA |title=Nitric oxide and skeletal muscle reperfusion injury: current controversies (research review) |journal=J. Surg. Res. |volume=128 |issue=1 |pages=98–107 |date=September 2005 |pmid=15961106 |doi=10.1016/j.jss.2005.04.020 |url=}}</ref> | ||
* CNOS- Constitutive [[Nitric oxide synthase|nitric oxide synthase enzyme]] | * CNOS- Constitutive [[Nitric oxide synthase|nitric oxide synthase enzyme]] | ||
| Line 65: | Line 65: | ||
* ENO S- [[Endothelial|Endothelia]]<nowiki/>l nitric oxide synthase enzyme | * ENO S- [[Endothelial|Endothelia]]<nowiki/>l nitric oxide synthase enzyme | ||
In the first 15 minutes of ischemia [[Nitric oxide|NO]] level rises due to transient ENOS activation. As said this elevation is transient so ultimately after a few minutes there will be a general decline in [[Endothelium|endothelial function]] resulting in the fall of NO production. The reduction in ENOS levels during ischemia [[reperfusion injury]] are also predisposed to [[vasoconstriction]], the response mainly seen in [[Reperfusion injury|IRI]]. | In the first 15 minutes of ischemia [[Nitric oxide|NO]] level rises due to transient ENOS activation. As said this elevation is transient so ultimately after a few minutes there will be a general decline in [[Endothelium|endothelial function]] resulting in the fall of NO production. The reduction in ENOS levels during ischemia [[reperfusion injury]] are also predisposed to [[vasoconstriction]], the response mainly seen in [[Reperfusion injury|IRI]]<ref name="pmid17559881">{{cite journal |vauthors=Cowled PA, Khanna A, Laws PE, Field JB, Varelias A, Fitridge RA |title=Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury |journal=J. Surg. Res. |volume=141 |issue=2 |pages=267–76 |date=August 2007 |pmid=17559881 |doi=10.1016/j.jss.2006.11.021 |url=}}</ref>. | ||
[[File:Neutrophils involved in tissue destruction.jpg|thumb|400x400px|Neutrophils, attachment, rolling and extravasation. Explained the role of neutrophils and the various steps involved in their | [[File:Neutrophils involved in tissue destruction.jpg|thumb|400x400px|Neutrophils, attachment, rolling and extravasation. Explained the role of neutrophils and the various steps involved in their extravasation so as to contribute to Ischemia-reperfusion injury. [https://www.ncbi.nlm.nih.gov/books/NBK534267/figure/ch18f3/?report=objectonly]]] | ||
==== Endothelin ==== | ==== Endothelin ==== | ||
These are peptide [[Vasoconstrictor|vasoconstrictors]] mainly produced from the [[endothelium]]. They mainly mediate [[vasoconstriction]] through Ca<sup>2+</sup>-mediated [[vasoconstriction]]. [[Endothelin-1|Endothelin -1]] levels increase during [[Ischemia-reperfusion injury|ischemia reperfusion injury]] in both the phases of [[ischemia]] as well as [[reperfusion]], that mainly help in [[capillary]] vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting [[vasoconstriction]] and increasing [[vascular permeability]]. | These are peptide [[Vasoconstrictor|vasoconstrictors]] mainly produced from the [[endothelium]]. They mainly mediate [[vasoconstriction]] through Ca<sup>2+</sup>-mediated [[vasoconstriction]]. [[Endothelin-1|Endothelin -1]] levels increase during [[Ischemia-reperfusion injury|ischemia reperfusion injury]] in both the phases of [[ischemia]] as well as [[reperfusion]], that mainly help in [[capillary]] vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting [[vasoconstriction]] and increasing [[vascular permeability]]<ref name="pmid19135850">{{cite journal |vauthors=Kiriş I, Narin C, Gülmen S, Yilmaz N, Sütçü R, Kapucuoğlu N |title=Endothelin receptor antagonism by tezosentan attenuates lung injury induced by aortic ischemia-reperfusion |journal=Ann Vasc Surg |volume=23 |issue=3 |pages=382–91 |date=2009 |pmid=19135850 |doi=10.1016/j.avsg.2008.10.003 |url=}}</ref>. | ||
==== Cytokines ==== | ==== Cytokines ==== | ||
| Line 75: | Line 75: | ||
* [[Tumor necrosis factor-alpha|TNF-a]] | * [[Tumor necrosis factor-alpha|TNF-a]] | ||
** Elevated levels detected during [[cerebral]] and [[skeletal]] IRI. it can also induce the generation of ROS and enhance the susceptibility of [[vascular]] [[endothelium]] to neutrophil-mediated injury by increasing the expression of [[ICAM-1]] which helps in binding of [[Neutrophil|neutrophils]] to the [[endothelium]]. | ** Elevated levels detected during [[cerebral]] and [[skeletal]] IRI<ref name="pmid20509932">{{cite journal |vauthors=Lutz J, Thürmel K, Heemann U |title=Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation |journal=J Inflamm (Lond) |volume=7 |issue= |pages=27 |date=May 2010 |pmid=20509932 |pmc=2894818 |doi=10.1186/1476-9255-7-27 |url=}}</ref>. it can also induce the generation of ROS and enhance the susceptibility of [[vascular]] [[endothelium]] to neutrophil-mediated injury by increasing the expression of [[ICAM-1]] which helps in binding of [[Neutrophil|neutrophils]] to the [[endothelium]]<ref name="pmid9357467">{{cite journal |vauthors=Tassiopoulos AK, Carlin RE, Gao Y, Pedoto A, Finck CM, Landas SK, Tice DG, Marx W, Hakim TS, McGraw DJ |title=Role of nitric oxide and tumor necrosis factor on lung injury caused by ischemia/reperfusion of the lower extremities |journal=J. Vasc. Surg. |volume=26 |issue=4 |pages=647–56 |date=October 1997 |pmid=9357467 |doi=10.1016/s0741-5214(97)70065-x |url=}}</ref>. | ||
* [[IL-1|IL-1, IL-6, IL-8]] | * [[IL-1|IL-1, IL-6, IL-8]] | ||
** IL-6 is a proinflammatory [[cytokine]] produces in large amounts in hypo perfused [[Tissue (biology)|tissues]]. | ** IL-6 is a proinflammatory [[cytokine]] produces in large amounts in hypo perfused [[Tissue (biology)|tissues]]. | ||
** IL-8 is a [[neutrophil]] [[Chemotaxis|chemotactic]] and activating factor and mainly results in the [[diapedesis]] of activated [[Neutrophil|neutrophils]] through the [[endothelium]]. | ** IL-8 is a [[neutrophil]] [[Chemotaxis|chemotactic]] and activating factor and mainly results in the [[diapedesis]] of activated [[Neutrophil|neutrophils]] through the [[endothelium]]. | ||
* PAF | * PAF | ||
** It enhances the binding of [[Neutrophil|neutrophils]] to the [[endothelial cells]]. | ** It enhances the binding of [[Neutrophil|neutrophils]] to the [[endothelial cells]]<ref name="pmid8812764">{{cite journal |vauthors=Durán WN, Milazzo VJ, Sabido F, Hobson RW |title=Platelet-activating factor modulates leukocyte adhesion to endothelium in ischemia-reperfusion |journal=Microvasc. Res. |volume=51 |issue=1 |pages=108–115 |date=January 1996 |pmid=8812764 |doi=10.1006/mvre.1996.0011 |url=}}</ref><ref name="pmid11990391">{{cite journal |vauthors=Börjesson A, Wang X, Sun Z, Inghammar M, Truedsson L, Andersson R |title=Early treatment with lexipafant, a platelet-activating factor-receptor antagonist, is not sufficient to prevent pulmonary endothelial damage after intestinal ischaemia and reperfusion in rats |journal=Dig Liver Dis |volume=34 |issue=3 |pages=190–6 |date=March 2002 |pmid=11990391 |doi=10.1016/s1590-8658(02)80192-x |url=}}</ref>. | ||
These [[Cytokine|cytokines]] mainly generate systemic inflammatory response ultimately leads to multi [[organ failure]]. | These [[Cytokine|cytokines]] mainly generate systemic inflammatory response ultimately leads to multi [[organ failure]]. | ||
==== Neutrophils and endothelial interactions ==== | ==== Neutrophils and endothelial interactions ==== | ||
[[Neutrophil|Neutrophils]] plays Important role in the tissue damage. Activated neutrophils secrete [[Protease|proteases]], [[metalloproteinase]], that results in the degradation of [[basement membrane]] and contributes to tissue damage. [[Selectin|Selectins]] are expressed on the surface of [[leucocytes]], [[endothelial cells]] and [[platelets]]. Selectins play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent [[adhesion]] and [[extravasation]]. [[L-selectin]] are present on surface of [[Neutrophil|neutrophils]] and help in the reversible attachment of neutrophils to [[endothelial cells]]. [[Antibody]]-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration. | [[Neutrophil|Neutrophils]] plays Important role in the tissue damage<ref name="pmid11396626">{{cite journal |vauthors=Martinez-Mier G, Toledo-Pereyra LH, McDuffie JE, Warner RL, Ward PA |title=Neutrophil depletion and chemokine response after liver ischemia and reperfusion |journal=J Invest Surg |volume=14 |issue=2 |pages=99–107 |date=2001 |pmid=11396626 |doi=10.1080/08941930152024228 |url=}}</ref>. Activated neutrophils secrete [[Protease|proteases]], [[metalloproteinase]], that results in the degradation of [[basement membrane]] and contributes to tissue damage. [[Selectin|Selectins]]<ref name="pmid11108771">{{cite journal |vauthors=Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES |title=Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke |journal=Stroke |volume=31 |issue=12 |pages=3047–53 |date=December 2000 |pmid=11108771 |doi= |url=}}</ref> are expressed on the surface of [[leucocytes]], [[endothelial cells]] and [[platelets]]. Selectins<ref name="pmid17454392">{{cite journal |vauthors=Calvey CR, Toledo-Pereyra LH |title=Selectin inhibitors and their proposed role in ischemia and reperfusion |journal=J Invest Surg |volume=20 |issue=2 |pages=71–85 |date=2007 |pmid=17454392 |doi=10.1080/08941930701250212 |url=}}</ref> play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent [[adhesion]] and [[extravasation]]. [[L-selectin]] are present on surface of [[Neutrophil|neutrophils]] and help in the reversible attachment of neutrophils to [[endothelial cells]]. [[Antibody]]-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration. | ||
==== Complement activation ==== | ==== Complement activation ==== | ||
Contributes in the pathogenesis of IRI. [[Reperfusion]] is usually associated with depletion of [[Complement|complement proteins]], [[factor B]] that will indicates the turning on of alternate complement pathway. The C5b-9 also gets deposited into the endothelial cell after [[ischemia]] leading to [[Osmotic lysis|osmotic lysis.]] | Contributes in the pathogenesis of IRI. [[Reperfusion]] is usually associated with depletion of [[Complement|complement proteins]], [[factor B]] that will indicates the turning on of alternate complement pathway<ref name="pmid2167024">{{cite journal |vauthors=Rubin BB, Smith A, Liauw S, Isenman D, Romaschin AD, Walker PM |title=Complement activation and white cell sequestration in postischemic skeletal muscle |journal=Am. J. Physiol. |volume=259 |issue=2 Pt 2 |pages=H525–31 |date=August 1990 |pmid=2167024 |doi=10.1152/ajpheart.1990.259.2.H525 |url=}}</ref>. The C5b-9<ref name="pmid16078298">{{cite journal |vauthors=Harkin DW, Marron CD, Rother RP, Romaschin A, Rubin BB, Lindsay TF |title=C5 complement inhibition attenuates shock and acute lung injury in an experimental model of ruptured abdominal aortic aneurysm |journal=Br J Surg |volume=92 |issue=10 |pages=1227–34 |date=October 2005 |pmid=16078298 |doi=10.1002/bjs.4938 |url=}}</ref> also gets deposited into the endothelial cell after [[ischemia]] leading to [[Osmotic lysis|osmotic lysis.]] | ||
<br /> | <br /> | ||
==Main organs affected in reperfusion injury== | |||
* '''Central Nervous System''' | * '''Central Nervous System''' | ||
| Line 97: | Line 97: | ||
* '''Cardiovascular system''' | * '''Cardiovascular system''' | ||
** In the [[cardiovascular system]], the most common complications studied are [[arrhythmias]], and [[myocardial stunning]] and myocardial cells death also. According to various studies done so far, Impaired [[microvascular function]] is the main reason behind the [[Stunned myocardium|myocardial stunning]]. | ** In the [[cardiovascular system]], the most common complications studied are [[arrhythmias]], and [[myocardial stunning]] and myocardial cells death also. According to various studies done so far, Impaired [[microvascular function]] is the main reason behind the [[Stunned myocardium|myocardial stunning]]. | ||
==References== | ==References== | ||
Latest revision as of 21:56, 21 August 2020
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4] Associate Editor(s)-in-Chief: Anjan K. Chakrabarti, M.D. [5] Shivam Singla, M.D.[6] Kashish Goel,M.D.,
Overview
The component playing a major role in the pathophysiology of Ischemia-reperfusion injury is Reactive oxygen species (ROS) causing damage to cellular and biological membranes. Neutrophils also play an important role in initiating and propagating much of the damage involved in the process of Ischemia-reperfusion injury. Ischemia is the phase that precedes the restoration of blood flow to that organ or tissue, resulting in the built-up of xanthine oxidase and hypoxanthine that upon the restoration of blood flow leads to the formation of ROS. Neutrophils also potentiate the effect of Ischemia-reperfusion injury through microvascular injury by releasing various proteolytic enzymes and ROS. Most of the experimental studies carried out in helping understand the mechanism of Ischemia reperfusion injury are mainly on the cat, dog, and horses.
Pathophysiology
Mainly divided into 2 phases
1) Ischemic phase
2) Reperfusion Phase
Ischemic Phase
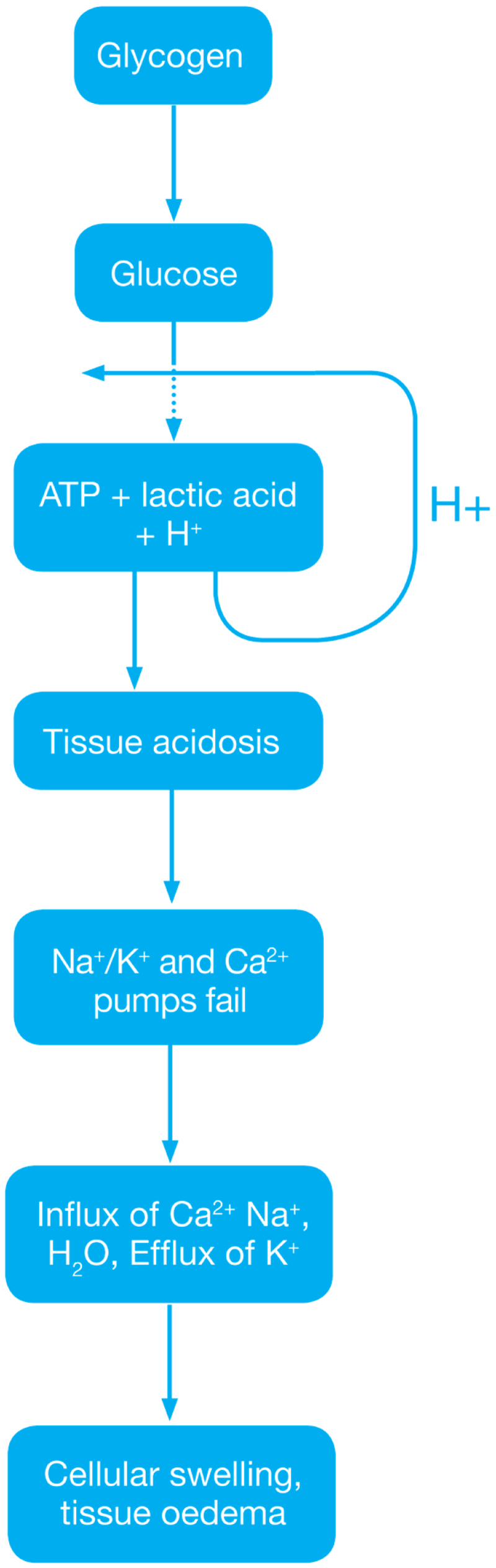
Reperfusion injury ( Ischemic Phase) During this phase mainly the dysregulation of metabolic pathways occurs and in the reperfusion phase there will be a generation of free radicals.
- Ischemia when the blood supply to the tissues decreases with respect to the demand required to function properly. This results in deficiency in oxygen, glucose and various other substrates required for cellular metabolism. As previously dais the derangement or dysregulation of metabolic function begins in this phase[1]. Due to less oxygen supply cellular metabolism shifts to anaerobic glycolysis causing the glycogen to breakdown resulting in the production of 2 ATP and a lactic acid. This decrease in tissue PH starts further inhibits the ATP generation by negative feed back mechanism. ATP gets broken down into ADP, AMP and IMP. This finally gets converted to adenosine, inosine, hypoxanthine and xanthine.
- Lack of ATP at the cellular level causes impairment in the function of ionic pumps - Na+/K+ and Ca2+ pumps. As a result cytosolic sodium rises which in turn withdraws water to maintain the osmotic equilibrium consequently resulting in the cellular swelling[2]. To maintain ionic balance potassium ion escape from the cell. Calcium is released from the mitochondria to the cytoplasm and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent cytosolic proteases. These converts the enzyme xanthine dehydrogenase to xanthine oxidase. Phospholipases activated during ischemia promotes membrane degradation and increases level of free fatty acids
- Ischemia also induces expression of a large number of genes and transcription factors, which play a major role in the damage to the tissues[3][4].
- Transcription factors
- Activating protein-1 (AP-1)
- Hypoxia-inducible factor-1 (HIF-1) which in turn activates transcription of VEGF, Erythropoietin and Glucose transporter-1
- Nuclear factor-kappa b (NF-kb)
- Activation of NF-kb occurs during both the ischemic and reperfusion phases
- Transcription factors
Reperfusion Phase
Reactive oxygen species
The ROS play major role in the tissue damage related to ischemia reperfusion injury. Once the ischemic tissue is reperfused the molecular oxygen catalyzes the conversion of hypoxanthine to uric acid and liberating the superoxide anion (O2-). This superoxide gets further converted to (H2O2) and the hydroxyl radical (OH•). This OH ion causes the peroxidation lipids in the cell membranes resulting in the production and release of proinflammatory eicosanoids and ultimately cell death[5]. Reperfusion Injury

During the Ischemia-reperfusion injury ROS also activate endothelial cells, which further produces numerous adhesion molecules.[6]
- E-selectin
- VCAM-1 (vascular cell adhesion molecule-1)
- ICAM-1 (intercellular adhesion molecule-1)
- EMLMl Am -1 ( endothelial-leukocyte adhesion molecule)
- PAi-1 (plasminogen activator inhibitor-1 ), and
- Interleukin-8 (il-8)
Eicosanoids
ROS causes lipid peroxidation of cell membranes resulting in the release of:
- Arachidonic acid (substrate for prostaglandins)
- Prostaglandins usually have a vasodilatory effect hat provides protective effect during Ischemia reperfusion injury. But they have a short life so their fast depletion leads to vasoconstriction ultimately leading to reduced blood flow and exacerbation of ischemia[7].
- Thromboxane
- Plasma thromboxane A2 level rises within minutes after reperfusion, resulting in vasoconstriction and platelet aggregation. This usually coincide with rapid rise in pulmonary artery pressure and a subsequent increase in pulmonary microvascular permeability[8].
- Leukotrienes
- Leukotrienes are also synthesized from arachidonic acid. Leukotrienes acts directly in the endothelial cells, smooth muscle and indirectly on the neutrophils. The leukotrienes C4, D4, and E4 alters the endothelial cytoskeleton, resulting in increased vascular permeability and smooth muscle contraction, and finally leading to vasoconstriction[9][10].
Nitric oxide
L-arginine is the substrate for the synthesis of Nitric oxide with the help of nitric oxide synthase enzyme. The nitric oxide synthase enzyme is usually of 3 types[11]
- CNOS- Constitutive nitric oxide synthase enzyme
- INO S- Inducible nitric oxide synthase enzyme
- ENO S- Endothelial nitric oxide synthase enzyme
In the first 15 minutes of ischemia NO level rises due to transient ENOS activation. As said this elevation is transient so ultimately after a few minutes there will be a general decline in endothelial function resulting in the fall of NO production. The reduction in ENOS levels during ischemia reperfusion injury are also predisposed to vasoconstriction, the response mainly seen in IRI[12].
Endothelin
These are peptide vasoconstrictors mainly produced from the endothelium. They mainly mediate vasoconstriction through Ca2+-mediated vasoconstriction. Endothelin -1 levels increase during ischemia reperfusion injury in both the phases of ischemia as well as reperfusion, that mainly help in capillary vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting vasoconstriction and increasing vascular permeability[13].
Cytokines
Ischemia and reperfusion phase of ischemia reperfusion injury induces expression of numerous cytokines mainly:
- TNF-a
- Elevated levels detected during cerebral and skeletal IRI[14]. it can also induce the generation of ROS and enhance the susceptibility of vascular endothelium to neutrophil-mediated injury by increasing the expression of ICAM-1 which helps in binding of neutrophils to the endothelium[15].
- IL-1, IL-6, IL-8
- IL-6 is a proinflammatory cytokine produces in large amounts in hypo perfused tissues.
- IL-8 is a neutrophil chemotactic and activating factor and mainly results in the diapedesis of activated neutrophils through the endothelium.
- PAF
- It enhances the binding of neutrophils to the endothelial cells[16][17].
These cytokines mainly generate systemic inflammatory response ultimately leads to multi organ failure.
Neutrophils and endothelial interactions
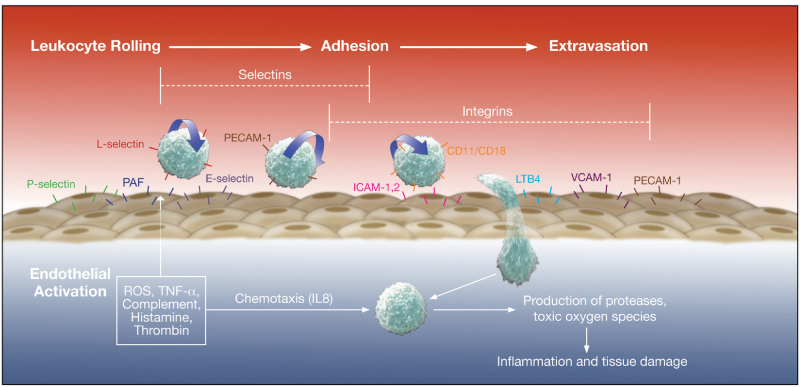
Neutrophils plays Important role in the tissue damage[18]. Activated neutrophils secrete proteases, metalloproteinase, that results in the degradation of basement membrane and contributes to tissue damage. Selectins[19] are expressed on the surface of leucocytes, endothelial cells and platelets. Selectins[20] play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent adhesion and extravasation. L-selectin are present on surface of neutrophils and help in the reversible attachment of neutrophils to endothelial cells. Antibody-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration.
Complement activation
Contributes in the pathogenesis of IRI. Reperfusion is usually associated with depletion of complement proteins, factor B that will indicates the turning on of alternate complement pathway[21]. The C5b-9[22] also gets deposited into the endothelial cell after ischemia leading to osmotic lysis.
Main organs affected in reperfusion injury
- Central Nervous System
- Reperfusion injury is a major pathophysiological mechanism involved in ischemia related injury to the central nervous system consequently resulting in the patients landing up with complications of a stroke, TIA, and other neurological problems. A lot of studies regarding this are still under the pipeline.
- Cardiovascular system
- In the cardiovascular system, the most common complications studied are arrhythmias, and myocardial stunning and myocardial cells death also. According to various studies done so far, Impaired microvascular function is the main reason behind the myocardial stunning.
References
- ↑ Allen DG, Xiao XH (September 2000). "Activity of the Na+/H+ exchanger contributes to cardiac damage following ischaemia and reperfusion". Clin. Exp. Pharmacol. Physiol. 27 (9): 727–33. doi:10.1046/j.1440-1681.2000.03329.x. PMID 10972541.
- ↑ Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L (December 2002). "Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice". Physiol. Genomics. 11 (3): 263–72. doi:10.1152/physiolgenomics.00110.2002. PMID 12399448.
- ↑ Safronova O, Morita I (May 2010). "Transcriptome remodeling in hypoxic inflammation". J. Dent. Res. 89 (5): 430–44. doi:10.1177/0022034510366813. PMID 20348484.
- ↑ Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ (2001). "Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events in hemorrhagic shock". Arch Orthop Trauma Surg. 121 (4): 219–22. doi:10.1007/s004020000211. PMID 11317684.
- ↑ Yokoyama K, Kimura M, Nakamura K, Nakamura K, Itoman M (April 1999). "Time course of post-ischemic superoxide generation in venous effluent from reperfused rabbit hindlimbs". J Reconstr Microsurg. 15 (3): 215–21. doi:10.1055/s-2007-1000094. PMID 10226957.
- ↑ Pacher P, Nivorozhkin A, Szabó C (March 2006). "Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol". Pharmacol. Rev. 58 (1): 87–114. doi:10.1124/pr.58.1.6. PMC 2233605. PMID 16507884.
- ↑ Neumann UP, Kaisers U, Langrehr JM, Glanemann M, Müller AR, Lang M, Jörres A, Settmacher U, Bechstein WO, Neuhaus P (February 2000). "Administration of prostacyclin after liver transplantation: a placebo controlled randomized trial". Clin Transplant. 14 (1): 70–4. doi:10.1034/j.1399-0012.2000.140113.x. PMID 10693639.
- ↑ Katircioğlu SF, Küçükaksu DS, Bozdayi M, Taşdemir O, Bayazit K (August 1995). "Beneficial effects of prostacyclin treatment on reperfusion of the myocardium". Cardiovasc Surg. 3 (4): 405–8. doi:10.1016/0967-2109(95)94159-t. PMID 7582995.
- ↑ Rowlands TE, Gough MJ, Homer-Vanniasinkam S (November 1999). "Do prostaglandins have a salutary role in skeletal muscle ischaemia-reperfusion injury?". Eur J Vasc Endovasc Surg. 18 (5): 439–44. doi:10.1053/ejvs.1999.0929. PMID 10610833.
- ↑ Mangino MJ, Murphy MK, Anderson CB (April 1994). "Effects of the arachidonate 5-lipoxygenase synthesis inhibitor A-64077 in intestinal ischemia-reperfusion injury". J. Pharmacol. Exp. Ther. 269 (1): 75–81. PMID 8169854.
- ↑ Khanna A, Cowled PA, Fitridge RA (September 2005). "Nitric oxide and skeletal muscle reperfusion injury: current controversies (research review)". J. Surg. Res. 128 (1): 98–107. doi:10.1016/j.jss.2005.04.020. PMID 15961106.
- ↑ Cowled PA, Khanna A, Laws PE, Field JB, Varelias A, Fitridge RA (August 2007). "Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury". J. Surg. Res. 141 (2): 267–76. doi:10.1016/j.jss.2006.11.021. PMID 17559881.
- ↑ Kiriş I, Narin C, Gülmen S, Yilmaz N, Sütçü R, Kapucuoğlu N (2009). "Endothelin receptor antagonism by tezosentan attenuates lung injury induced by aortic ischemia-reperfusion". Ann Vasc Surg. 23 (3): 382–91. doi:10.1016/j.avsg.2008.10.003. PMID 19135850.
- ↑ Lutz J, Thürmel K, Heemann U (May 2010). "Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation". J Inflamm (Lond). 7: 27. doi:10.1186/1476-9255-7-27. PMC 2894818. PMID 20509932.
- ↑ Tassiopoulos AK, Carlin RE, Gao Y, Pedoto A, Finck CM, Landas SK, Tice DG, Marx W, Hakim TS, McGraw DJ (October 1997). "Role of nitric oxide and tumor necrosis factor on lung injury caused by ischemia/reperfusion of the lower extremities". J. Vasc. Surg. 26 (4): 647–56. doi:10.1016/s0741-5214(97)70065-x. PMID 9357467.
- ↑ Durán WN, Milazzo VJ, Sabido F, Hobson RW (January 1996). "Platelet-activating factor modulates leukocyte adhesion to endothelium in ischemia-reperfusion". Microvasc. Res. 51 (1): 108–115. doi:10.1006/mvre.1996.0011. PMID 8812764.
- ↑ Börjesson A, Wang X, Sun Z, Inghammar M, Truedsson L, Andersson R (March 2002). "Early treatment with lexipafant, a platelet-activating factor-receptor antagonist, is not sufficient to prevent pulmonary endothelial damage after intestinal ischaemia and reperfusion in rats". Dig Liver Dis. 34 (3): 190–6. doi:10.1016/s1590-8658(02)80192-x. PMID 11990391.
- ↑ Martinez-Mier G, Toledo-Pereyra LH, McDuffie JE, Warner RL, Ward PA (2001). "Neutrophil depletion and chemokine response after liver ischemia and reperfusion". J Invest Surg. 14 (2): 99–107. doi:10.1080/08941930152024228. PMID 11396626.
- ↑ Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES (December 2000). "Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke". Stroke. 31 (12): 3047–53. PMID 11108771.
- ↑ Calvey CR, Toledo-Pereyra LH (2007). "Selectin inhibitors and their proposed role in ischemia and reperfusion". J Invest Surg. 20 (2): 71–85. doi:10.1080/08941930701250212. PMID 17454392.
- ↑ Rubin BB, Smith A, Liauw S, Isenman D, Romaschin AD, Walker PM (August 1990). "Complement activation and white cell sequestration in postischemic skeletal muscle". Am. J. Physiol. 259 (2 Pt 2): H525–31. doi:10.1152/ajpheart.1990.259.2.H525. PMID 2167024.
- ↑ Harkin DW, Marron CD, Rother RP, Romaschin A, Rubin BB, Lindsay TF (October 2005). "C5 complement inhibition attenuates shock and acute lung injury in an experimental model of ruptured abdominal aortic aneurysm". Br J Surg. 92 (10): 1227–34. doi:10.1002/bjs.4938. PMID 16078298.