Hydroxyprogesterone caproate: Difference between revisions
Anusha Vege (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| (20 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{ | |authorTag={{AJ}} | ||
|genericName=Hydroxyprogesterone | |genericName=Hydroxyprogesterone caproate | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=progestin | |drugClass=[[progestin]] and [[endocrine|endocrine-metabolic]] agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=indicated to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth | |indication=indicated to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]] | ||
|adverseReactions=injection site reactions | |adverseReactions=[[injection site reactions]], [[pain]], [[swelling]], [[pruritus]], [[nodule]], [[urticaria]], [[nausea]], and [[diarrhea]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=Used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. The effectiveness of | |fdaLIADAdult=* Used to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]]. The effectiveness of Hydroxyprogesterone caproate is based on improvement in the proportion of women who [[delivery|delivered]] <37 weeks of [[gestation]]. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in [[neonatal]] [[mortality]] and [[morbidity]]. | ||
Limitation of use: While there are many risk factors for preterm birth, safety and efficacy of | * Limitation of use: While there are many risk factors for [[preterm birth]], [[safety]] and [[efficacy]] of Hydroxyprogesterone caproate has been demonstrated only in women with a prior spontaneous singleton [[preterm birth]]. It is not intended for use in women with multiple [[gestations]] or other risk factors for [[preterm birth]]. | ||
==== | ====Dosing Information==== | ||
Administer intramuscularly at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider | |||
Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of gestation | * Administer [[intramuscularly]] at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider | ||
Continue administration once weekly until week 37 (through 36 weeks, 6 days) of gestation or delivery, whichever occurs first. | * Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]] | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in adult patients. | Continue administration once weekly until week 37 (through 36 weeks, 6 days) of [[gestation]] or [[delivery]], whichever occurs first. | ||
|offLabelAdultNoGuideSupport=Amenorrhea | |||
Endometrial carcinoma | =====Preparation and Administration===== | ||
Estrogen measurement, Endogenous; Diagnosis | |||
|fdaLIADPed= | * Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Hydroxyprogesterone caproate is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy. | ||
* Instructions for administration: | |||
:* Clean the vial top with an alcohol swab before use. | |||
:* Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle. | |||
:* Change the needle to a 21 gauge 1 1/2 inch needle. | |||
:* After preparing the skin, inject in the upper outer quadrant of the gluteus maximus. The solution is viscous and oily. Slow injection (over one minute or longer) is recommended. | |||
:* Applying pressure to the injection site may minimize bruising and swelling. | |||
Discard any unused product 5 weeks after first use. | |||
|offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in adult patients. | |||
|offLabelAdultNoGuideSupport=* [[Amenorrhea]] | |||
* [[Endometrial carcinoma]] | |||
* [[Estrogen]] measurement, [[Endogenous]]; [[Diagnosis]] | |||
|fdaLIADPed=There is limited information regarding <i>FDA-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | ||
|contraindications=Current or history of thrombosis or thromboembolic disorders | |contraindications=* Do not use Hydroxyprogesterone caproate in women with any of the following conditions: | ||
* Known or suspected breast cancer, other hormone-sensitive cancer, or history of these conditions | :* Current or history of [[thrombosis]] or [[thromboembolic disorders]] | ||
* Undiagnosed abnormal vaginal bleeding unrelated to pregnancy | :* Known or suspected [[breast cancer]], other [[breast cancer|hormone-sensitive cancer]], or history of these conditions | ||
* Cholestatic jaundice of pregnancy | :* Undiagnosed abnormal [[vaginal bleeding]] unrelated to [[pregnancy]] | ||
* Liver tumors, benign or malignant, or active liver disease | :* [[Cholestatic jaundice of pregnancy]] | ||
* Uncontrolled hypertension | :* [[Liver Tumors|Liver tumors]], [[Hepatocellular carcinoma|benign]] or [[Hepatocellular carcinoma|malignant]], or [[liver disease|active liver disease]] | ||
:* [[hypertension|Uncontrolled hypertension]] | |||
|warnings=====Thromboembolic Disorders==== | |warnings=====Thromboembolic Disorders==== | ||
Discontinue | * Discontinue Hydroxyprogesterone caproate if an [[arterial]] or [[deep vein thrombosis|deep venous thrombotic]] or [[thromboembolic event]] occurs. | ||
====Allergic Reactions==== | ====Allergic Reactions==== | ||
Allergic reactions, including urticaria, pruritus and angioedema, have been reported with use of | * [[Allergic reactions]], including [[urticaria]],[[ pruritus]] and [[angioedema]], have been reported with use of Hydroxyprogesterone caproate or with other products containing castor oil. Consider discontinuing the drug if such reactions occur. | ||
====Decrease in Glucose Tolerance==== | ====Decrease in Glucose Tolerance==== | ||
A decrease in glucose tolerance has been observed in some patients on progestin treatment. The mechanism of this decrease is not known. Carefully monitor prediabetic and diabetic women while they are receiving | * A decrease in [[glucose tolerance]] has been observed in some patients on [[progestin]] treatment. The mechanism of this decrease is not known. Carefully monitor [[diabetic|prediabetic]] and [[diabetic]] women while they are receiving Hydroxyprogesterone caproate. | ||
====Fluid Retention==== | ====Fluid Retention==== | ||
Because progestational drugs may cause some degree of fluid retention, carefully monitor women with conditions that might be influenced by this effect (e.g., preeclampsia, epilepsy, migraine, asthma, cardiac or renal dysfunction). | * Because progestational drugs may cause some degree of [[fluid retention]], carefully monitor women with conditions that might be influenced by this effect (e.g., [[preeclampsia]], [[epilepsy]], [[migraine]], [[asthma]], [[cardiac]] or [[renal dysfunction]]). | ||
====Depression==== | ====Depression==== | ||
Monitor women who have a history of clinical depression and discontinue | * Monitor women who have a history of clinical [[depression]] and discontinue Hydroxyprogesterone caproate if clinical [[depression]] recurs. | ||
====Jaundice==== | ====Jaundice==== | ||
Carefully monitor women who develop jaundice while receiving | * Carefully monitor women who develop [[jaundice]] while receiving Hydroxyprogesterone caproate and consider whether the benefit of use warrants continuation. | ||
====Hypertension==== | ====Hypertension==== | ||
Carefully monitor women who develop hypertension while receiving | * Carefully monitor women who develop [[hypertension]] while receiving Hydroxyprogesterone caproate and consider whether the benefit of use warrants continuation. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because [[clinical trials]] are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
* In a vehicle ([[placebo]])-controlled [[clinical trial]] of 463 [[pregnant]] women at risk for spontaneous [[preterm delivery]] based on [[obstetrical]] history, 310 received 250 mg of Hydroxyprogesterone caproate and 153 received a vehicle formulation containing no drug by a weekly [[intramuscular injection]] beginning at 16 to 20 weeks of [[gestation]] and continuing until 37 weeks of [[gestation]] or [[delivery]], whichever occurred first. | |||
* Certain [[pregnancy]]-related [[fetal]] and maternal complications or events were numerically increased in the Hydroxyprogesterone caproate-treated subjects as compared to control subjects, including [[miscarriage]] and [[stillbirth]], admission for [[preterm labor]], [[preeclampsia]] or [[gestational hypertension]], [[gestational diabetes]], and [[oligohydramnios]]. | |||
[[File:Hydroxprogesterone complication.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
1 N = Total number of subjects enrolled prior to 20 weeks 0 days | |||
2 N = Total number of subjects at risk ≥ 20 weeks | |||
[[File:table2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
| | |||
| | |||
====Common Adverse Reactions==== | |||
* The most common adverse reaction was [[Injection site reaction|injection site pain]], which was reported after at least one injection by 34.8% of the Hydroxyprogesterone caproate group and 32.7% of the control group. Table 3 lists adverse reactions that occurred in ≥2% of subjects and at a higher rate in the Hydroxyprogesterone caproate group than in the control group. | |||
| | |||
[[File:table3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* In the [[clinical trial]], 2.2% of subjects receiving Hydroxyprogesterone caproate were reported as discontinuing therapy due to adverse reactions compared to 2.6% of control subjects. * The most common adverse reactions that led to discontinuation in both groups were [[urticaria]] and [[Injection site reaction|injection site pain]]/[[swelling]] (1% each). | |||
* [[Pulmonary embolus]] in one subject and [[Injection site reaction|injection site]] [[cellulitis]] in another subject were reported as serious adverse reactions in Hydroxyprogesterone caproate-treated subjects. | |||
|postmarketing=* The following adverse reactions have been identified during postapproval use of Hydroxyprogesterone caproate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
* Body as a whole: | |||
:* Local [[injection site reactions]] (including [[erythema]], [[urticaria]], [[rash]], [[irritation]], [[hypersensitivity]], warmth); [[fatigue]]; [[fever]]; [[hot flashes]]/[[flushes]] | |||
* Digestive disorders: | |||
:* [[Vomiting]] | |||
* Infections: | |||
:* [[Urinary tract infection]] | |||
* Nervous system disorders: | |||
:* [[Headache]], [[dizziness]] | |||
* Pregnancy, puerperium and perinatal conditions: | |||
:* [[Cervical incompetence]], [[premature rupture of membranes]] | |||
* Reproductive system and breast disorders: [[Cervical dilation]], [[Cervical|shortened cervix]] | |||
* Respiratory disorders: | |||
:* [[Dyspnea]], [[chest discomfort]] | |||
* Skin: | |||
:* [[Rash]] | |||
|drugInteractions=* [[Cytochrome P450]] (CYP) enzymes: An in vitro inhibition study using human [[liver]] [[microsomes]] and CYP isoform-selective substrates indicated that hydroxyprogesterone caproate increased the metabolic rate of [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] by approximately 80%, 150%, and 80%, respectively. However, in another in vitro study using human [[hepatocytes]] under conditions where the prototypical inducers or inhibitors caused the anticipated increases or decreases in CYP enzyme activities, hydroxyprogesterone caproate did not induce or inhibit [[CYP1A2]], [[CYP2A6]], or [[CYP2B6]] activity. Overall, the findings indicate that hydroxyprogesterone caproate has minimal potential for [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] related drug-drug interactions at the clinically relevant concentrations. | |||
* In vitro data indicated that therapeutic concentration of hydroxyprogesterone caproate is not likely to inhibit the activity of [[CYP2C8]], [[CYP2C9]], [[CYP2C19]], [[CYP2D6]], [[CYP2E1]], and [[CYP3A4]]. | |||
| | |FDAPregCat=B | ||
|useInPregnancyFDA=* There are no adequate and well-controlled studies of Hydroxyprogesterone caproate use in women during the [[first trimester of pregnancy]]. Data from a vehicle ([[placebo]])-controlled [[clinical trial]] of 310 [[pregnant]] women who received Hydroxyprogesterone caproate at weekly doses of 250 mg by [[intramuscular injection]] in their [[pregnancy|second and third trimesters]]1, as well as long-term (2-5 years) follow-up safety data on 194 of their infants 2, did not demonstrate any [[teratogenic]] risks to infants from in utero exposure to Hydroxyprogesterone caproate. | |||
* Reproduction studies have been performed in mice and rats at doses up to 95 and 5, respectively, times the human dose and have revealed no evidence of impaired [[fertility]] or harm to the [[fetus]] due to Hydroxyprogesterone caproate. | |||
The | * Hydroxyprogesterone caproate administration produced embryolethality in rhesus monkeys but not in cynomolgus monkeys exposed to 1 and 10 times the human dose equivalent every 7 days between days 20 and 146 of [[gestation]]. There were no [[teratogenic]] effects in either species. | ||
|useInLaborDelivery=* Hydroxyprogesterone caproate is not intended for use to stop active [[preterm labor]]. The effect of Hydroxyprogesterone caproate in active [[labor]] is unknown. | |||
|useInNursing=* Discontinue Hydroxyprogesterone caproate at 37 weeks of gestation or upon [[delivery]]. Detectable amounts of progestins have been identified in the milk of mothers receiving progestin treatment. Many studies have found no adverse effects of progestins on breastfeeding performance, or on the health, growth, or development of the [[infant]] | |||
|useInPed=* Hydroxyprogesterone caproate is not indicated for use in children. Safety and effectiveness in [[pediatric]] patients less than 16 years of age have not been established. A small number of women under age 18 years were studied; safety and efficacy are expected to be the same in women aged 16 years and above as for users 18 years and older. | |||
|useInGeri=* Hydroxyprogesterone caproate is not intended for use in [[postmenopausal]] women. Safety and effectiveness in [[postmenopausal]] women have not been established. | |||
|useInRenalImpair=No studies have been conducted to examine the [[pharmacokinetics]] of Hydroxyprogesterone caproate in patients with [[renal impairment]]. | |||
|useInHepaticImpair=* No studies have been conducted to examine the pharmacokinetics of Hydroxyprogesterone caproate in patients with hepatic impairment. Hydroxyprogesterone caproate is extensively metabolized and [[hepatic impairment]] may reduce the elimination of Hydroxyprogesterone caproate. | |||
|administration=* Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Hydroxyprogesterone caproate is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy. | |||
====Instructions for administration==== | |||
* Clean the vial top with an [[alcohol]] swab before use. | |||
* Draw up 1 mL of drug into a 3 mL [[syringe]] with an 18 gauge needle. | |||
* Change the needle to a 21 gauge 1 1/2 inch needle. | |||
* After preparing the [[skin]], inject in the upper outer quadrant of the [[gluteus maximus]]. | |||
* The solution is viscous and oily. Slow injection (over one minute or longer) is recommended. | |||
* Applying pressure to the [[injection site]] may minimize [[bruising]] and [[swelling]]. | |||
* Discard any unused product 5 weeks after first use. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 477198932 | |||
| IUPAC_name = [(8''R'',9''S'',10''R'',13''S'',14''S'',17''R'')-17-acetyl-10,13-dimethyl-<br>3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-<br>1H-cyclopenta[a]phenanthren-17-yl] hexanoate | |||
| image = Hydroxyprogesterone caproate.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 630-56-8 | |||
| ATC_prefix = | |||
| ATC_suffix = | |||
| PubChem = 169870 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 148552 | |||
<!--Chemical data--> | |||
| C=27 | H=40 | O=4 | |||
| molecular_weight = 428.6041 g/mol | |||
| smiles = O=C4\C=C2/[C@]([C@H]1CC[C@@]3([C@@](OC(=O)CCCCC)(C(=O)C)CC[C@H]3[C@@H]1CC2)C)(C)CC4 | |||
| InChI = 1/C27H40O4/c1-5-6-7-8-24(30)31-27(18(2)28)16-13-23-21-10-9-19-17-20(29)11-14-25(19,3)22(21)12-15-26(23,27)4/h17,21-23H,5-16H2,1-4H3/t21-,22+,23+,25+,26+,27+/m1/s1 | |||
| InChIKey = DOMWKUIIPQCAJU-LJHIYBGHBI | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C27H40O4/c1-5-6-7-8-24(30)31-27(18(2)28)16-13-23-21-10-9-19-17-20(29)11-14-25(19,3)22(21)12-15-26(23,27)4/h17,21-23H,5-16H2,1-4H3/t21-,22+,23+,25+,26+,27+/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = DOMWKUIIPQCAJU-LJHIYBGHSA-N | |||
}} | |||
|mechAction=* Hydroxyprogesterone caproate is a synthetic [[progestin]]. The mechanism by which hydroxyprogesterone caproate reduces the risk recurrent preterm birth is not known. | |||
|structure=* The active pharmaceutical ingredient in Hydroxyprogesterone caproate is hydroxyprogesterone caproate. | |||
* The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60. Hydroxyprogesterone caproate exists as white to practically white crystals or powder with a melting point of 120°-124°C. | |||
The structural formula is: | |||
[[File:Hydroxprogesterone structure.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|PD=No specific pharmacodynamic studies were conducted with Hydroxyprogesterone caproate. | |||
|PK======Absorption:===== | |||
* Peak serum levels of hydroxyprogesterone caproate appeared after 3-7 days in non-pregnant female subjects following a single [[intramuscular injection]] of 1000 mg hydroxyprogesterone caproate. Based on [[pharmacokinetic]] analysis of five non-pregnant female subjects who received a single [[intramuscular]] administration of 1000 mg hydroxyprogesterone caproate, the mean (±SD) Cmax is estimated to be 27.8 (±5.3) ng/mL, and the [[Tmax]] is estimated to be 4.6 (±1.7) days. The elimination half-life of hydroxyprogesterone caproate was 7.8 (±3.0) days. Once-weekly intramuscular administration of 1000 mg hydroxyprogesterone caproate to non-pregnant women resulted in trough concentration of 60.0 (±14) ng/mL after 13 weeks. The [[pharmacokinetics]] of the 250 mg dose of hydroxyprogesterone caproate has not been evaluated. | |||
=====Distribution:===== | |||
* Hydroxyprogesterone caproate binds extensively to [[plasma proteins]] including albumin and [[corticosteroid]] binding [[globulins]]. | |||
=====Metabolism:===== | |||
* In vitro studies have shown that hydroxyprogesterone caproate can be [[metabolized]] by human [[hepatocytes]], both by phase I and phase II reactions. Hydroxyprogesterone caproate undergoes extensive reduction, [[hydroxylation]] and [[conjugation]]. The conjugated [[metabolites]] include [[sulfated]], [[glucuronidated]] and [[acetylated]] products. In vitro data indicate that the [[metabolism]] of hydroxyprogesterone caproate is predominantly mediated by [[CYP3A4]] and [[CYP3A5]]. The in vitro data indicate that the caproate group is retained during [[metabolism]] of hydroxyprogesterone caproate. | |||
=====Excretion:===== | |||
* Both [[conjugated]] metabolites and free [[steroids]] are excreted in the [[urine]] and [[feces]], with the [[conjugated]] [[metabolites]] being prominent. Following intramuscular administration to pregnant women at 10-12 weeks gestation, approximately 50% of a dose was recovered in the [[feces]] and approximately 30% recovered in the [[urine]]. | |||
|clinicalStudies======Clinical Trial to Evaluate Reduction of Risk of Preterm Birth===== | |||
* In a multicenter, randomized, double-blind, vehicle (placebo)-controlled clinical trial, the safety and effectiveness of Hydroxyprogesterone caproate for the reduction of the risk of spontaneous [[preterm birth]] was studied in women with a singleton [[pregnancy]] (age 16 to 43 years) who had a documented history of singleton [[preterm birth|spontaneous preterm birth]] (defined as [[delivery]] at less than 37 weeks of [[gestation]] following spontaneous [[preterm labor]] or [[premature rupture of membranes]]).1 At the time of randomization (between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]]), an [[ultrasound]] examination had confirmed [[gestational]] age and no known [[fetal]] [[anomaly]]. Women were excluded for prior [[progesterone]] treatment or [[heparin]] therapy during the current [[pregnancy]], a history of [[thromboembolic disease]], or maternal/obstetrical complications (such as current or planned [[cerclage]], hypertension requiring medication, or a [[seizure]] disorder). | |||
* A total of 463 [[pregnant]] women were randomized to receive either Hydroxyprogesterone caproate (N=310) or vehicle (N=153) at a dose of 250 mg administered weekly by [[intramuscular]] injection starting between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]], and continuing until 37 weeks of [[gestation]] or [[delivery]]. Demographics of the Hydroxyprogesterone caproate-treated women were similar to those in the control group, and included: 59.0% Black, 25.5% Caucasian, 13.9% Hispanic and 0.6% Asian. The mean body mass index was 26.9 kg/m2. | |||
* The proportions of women in each treatment arm who delivered at <37 (the primary study endpoint), <35, and <32 weeks of [[gestation]] are displayed in Table 4. | |||
[[File:table4.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:* Four Hydroxyprogesterone caproate-treated subjects were lost to follow-up. They were counted as deliveries at their [[gestational]] ages at time of last contact (184, 220, 343 and 364 weeks). | |||
:* Adjusted for interim analysis. | |||
* Compared to controls, treatment with Hydroxyprogesterone caproate reduced the proportion of women who [[delivery|delivered]] [[preterm]] at <37 weeks. The proportions of women [[delivery|delivering]] at <35 and < 32 weeks also were lower among women treated with Hydroxyprogesterone caproate. The upper bounds of the confidence intervals for the treatment difference at < 35 and <32 weeks were close to zero. Inclusion of zero in a confidence interval would indicate the treatment difference is not statistically significant. Compared to the other [[gestational]] ages evaluated, the number of [[preterm]] births at <32 weeks was limited. | |||
* After adjusting for time in the study, 7.5% of Hydroxyprogesterone caproate-treated subjects delivered prior to 25 weeks compared to 4.7% of control subjects; see Figure 1. | |||
[[File:Hydroxprogesterone graph.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* The rates of [[fetal]] and [[neonatal]] deaths in each treatment arm are displayed in Table 5. Due to the higher rate of [[miscarriages]] and [[stillbirths]] in the Hydroxyprogesterone caproate arm, there was no overall survival difference demonstrated in this [[clinical trial]]. | |||
[[File:table5.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:* A Four of the 310 Hydroxyprogesterone caproate-treated subjects were lost to follow-up and [[stillbirth]] or [[neonatal]] status could not be determined | |||
:* Percentages are based on the number of enrolled subjects and not adjusted for time on drug | |||
:* Percentage adjusted for the number of at risk subjects (n=209 for Hydroxyprogesterone caproate, n=107 for control) enrolled at <20 weeks [[gestation]]. | |||
* A composite [[neonatal]] [[morbidity]]/[[mortality]] index evaluated adverse outcomes in livebirths. It was based on the number of [[neonates]] who died or experienced [[respiratory distress syndrome]], [[bronchopulmonary dysplasia]], grade 3 or 4 [[intraventricular hemorrhage]], proven [[sepsis]], or [[necrotizing enterocolitis]]. Although the proportion of neonates who experienced 1 or more events was numerically lower in the Hydroxyprogesterone caproate arm (11.9% vs. 17.2%), the number of adverse outcomes was limited and the difference between arms was not statistically significant. | |||
=====Infant Follow-Up Safety Study===== | |||
* [[Infants]] born to women enrolled in this study, and who survived to be discharged from the nursery, were eligible for participation in a follow-up safety study. Of 348 eligible offspring, 79.9% enrolled: 194 children of Hydroxyprogesterone caproate-treated women and 84 children of control subjects. The primary endpoint was the score on the Ages & Stages Questionnaire (ASQ), which evaluates communication, gross motor, fine motor, problem solving, and personal/social parameters. The proportion of children whose scores met the screening threshold for [[developmental]] delay in each developmental domain was similar for each treatment group. | |||
|howSupplied=Hydroxyprogesterone caproate (NDC 64011-243-01) is supplied as 5 mL of a [[sterile]] solution in a multidose glass vial. | |||
Each 5 mL vial contains hydroxyprogesterone caproate USP, 250 mg/mL (25% w/v), in [[castor oil]] USP (28.6% v/v) and [[benzyl benzoate]] USP (46% v/v) with the preservative [[benzyl alcohol]] NF (2% v/v). | |||
Single unit carton: Contains one 5 mL multidose vial of Hydroxyprogesterone caproate (250 mg/mL) containing 1250 mg of hydroxyprogesterone caproate. | |||
|storage=* Store at controlled room temperature [15°-30°C (59°-86°F)]. Use within 5 weeks after first use. | |||
* Caution: Protect vial from light. Store vial in its box. Store upright. | |||
|packLabel=[[File:Hydroxprogesterone drug label.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Carton Label | Carton Label | ||
| Line 314: | Line 286: | ||
Chesterfield, MO 63005 | Chesterfield, MO 63005 | ||
|alcohol=Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Hylutin® | |||
* Prodrox® | |||
* Makena® | |||
}} | }} | ||
Latest revision as of 15:02, 2 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hydroxyprogesterone caproate is a progestin and endocrine-metabolic agent that is FDA approved for the treatment of indicated to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. Common adverse reactions include injection site reactions, pain, swelling, pruritus, nodule, urticaria, nausea, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. The effectiveness of Hydroxyprogesterone caproate is based on improvement in the proportion of women who delivered <37 weeks of gestation. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in neonatal mortality and morbidity.
- Limitation of use: While there are many risk factors for preterm birth, safety and efficacy of Hydroxyprogesterone caproate has been demonstrated only in women with a prior spontaneous singleton preterm birth. It is not intended for use in women with multiple gestations or other risk factors for preterm birth.
Dosing Information
- Administer intramuscularly at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider
- Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of gestation
Continue administration once weekly until week 37 (through 36 weeks, 6 days) of gestation or delivery, whichever occurs first.
Preparation and Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Hydroxyprogesterone caproate is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy.
- Instructions for administration:
- Clean the vial top with an alcohol swab before use.
- Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle.
- Change the needle to a 21 gauge 1 1/2 inch needle.
- After preparing the skin, inject in the upper outer quadrant of the gluteus maximus. The solution is viscous and oily. Slow injection (over one minute or longer) is recommended.
- Applying pressure to the injection site may minimize bruising and swelling.
Discard any unused product 5 weeks after first use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyprogesterone caproate in adult patients.
Non–Guideline-Supported Use
- Amenorrhea
- Endometrial carcinoma
- Estrogen measurement, Endogenous; Diagnosis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Label Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Contraindications
- Do not use Hydroxyprogesterone caproate in women with any of the following conditions:
- Current or history of thrombosis or thromboembolic disorders
- Known or suspected breast cancer, other hormone-sensitive cancer, or history of these conditions
- Undiagnosed abnormal vaginal bleeding unrelated to pregnancy
- Cholestatic jaundice of pregnancy
- Liver tumors, benign or malignant, or active liver disease
- Uncontrolled hypertension
Warnings
Thromboembolic Disorders
- Discontinue Hydroxyprogesterone caproate if an arterial or deep venous thrombotic or thromboembolic event occurs.
Allergic Reactions
- Allergic reactions, including urticaria,pruritus and angioedema, have been reported with use of Hydroxyprogesterone caproate or with other products containing castor oil. Consider discontinuing the drug if such reactions occur.
Decrease in Glucose Tolerance
- A decrease in glucose tolerance has been observed in some patients on progestin treatment. The mechanism of this decrease is not known. Carefully monitor prediabetic and diabetic women while they are receiving Hydroxyprogesterone caproate.
Fluid Retention
- Because progestational drugs may cause some degree of fluid retention, carefully monitor women with conditions that might be influenced by this effect (e.g., preeclampsia, epilepsy, migraine, asthma, cardiac or renal dysfunction).
Depression
- Monitor women who have a history of clinical depression and discontinue Hydroxyprogesterone caproate if clinical depression recurs.
Jaundice
- Carefully monitor women who develop jaundice while receiving Hydroxyprogesterone caproate and consider whether the benefit of use warrants continuation.
Hypertension
- Carefully monitor women who develop hypertension while receiving Hydroxyprogesterone caproate and consider whether the benefit of use warrants continuation.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In a vehicle (placebo)-controlled clinical trial of 463 pregnant women at risk for spontaneous preterm delivery based on obstetrical history, 310 received 250 mg of Hydroxyprogesterone caproate and 153 received a vehicle formulation containing no drug by a weekly intramuscular injection beginning at 16 to 20 weeks of gestation and continuing until 37 weeks of gestation or delivery, whichever occurred first.
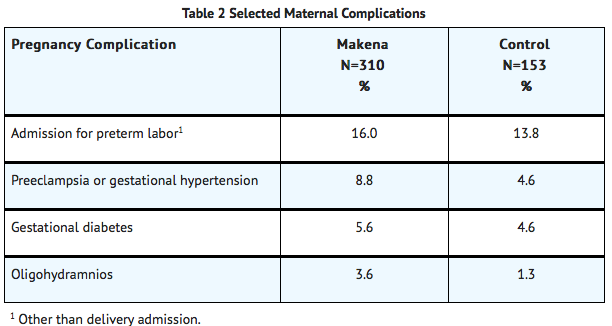
- Certain pregnancy-related fetal and maternal complications or events were numerically increased in the Hydroxyprogesterone caproate-treated subjects as compared to control subjects, including miscarriage and stillbirth, admission for preterm labor, preeclampsia or gestational hypertension, gestational diabetes, and oligohydramnios.

1 N = Total number of subjects enrolled prior to 20 weeks 0 days
2 N = Total number of subjects at risk ≥ 20 weeks
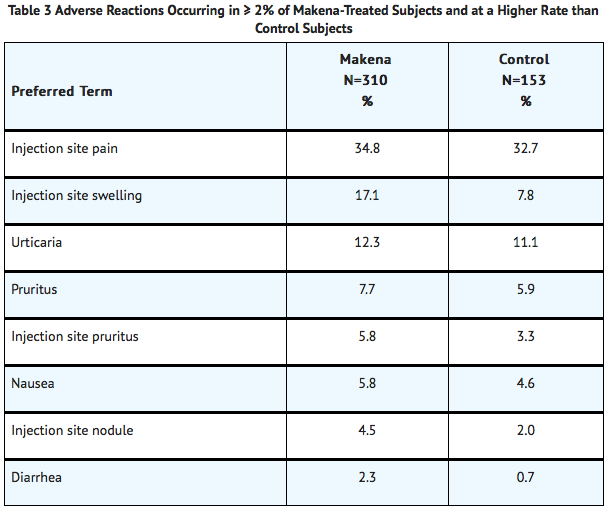
Common Adverse Reactions
- The most common adverse reaction was injection site pain, which was reported after at least one injection by 34.8% of the Hydroxyprogesterone caproate group and 32.7% of the control group. Table 3 lists adverse reactions that occurred in ≥2% of subjects and at a higher rate in the Hydroxyprogesterone caproate group than in the control group.
- In the clinical trial, 2.2% of subjects receiving Hydroxyprogesterone caproate were reported as discontinuing therapy due to adverse reactions compared to 2.6% of control subjects. * The most common adverse reactions that led to discontinuation in both groups were urticaria and injection site pain/swelling (1% each).
- Pulmonary embolus in one subject and injection site cellulitis in another subject were reported as serious adverse reactions in Hydroxyprogesterone caproate-treated subjects.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Hydroxyprogesterone caproate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Body as a whole:
- Local injection site reactions (including erythema, urticaria, rash, irritation, hypersensitivity, warmth); fatigue; fever; hot flashes/flushes
- Digestive disorders:
- Infections:
- Nervous system disorders:
- Pregnancy, puerperium and perinatal conditions:
- Reproductive system and breast disorders: Cervical dilation, shortened cervix
- Respiratory disorders:
- Skin:
Drug Interactions
- Cytochrome P450 (CYP) enzymes: An in vitro inhibition study using human liver microsomes and CYP isoform-selective substrates indicated that hydroxyprogesterone caproate increased the metabolic rate of CYP1A2, CYP2A6, and CYP2B6 by approximately 80%, 150%, and 80%, respectively. However, in another in vitro study using human hepatocytes under conditions where the prototypical inducers or inhibitors caused the anticipated increases or decreases in CYP enzyme activities, hydroxyprogesterone caproate did not induce or inhibit CYP1A2, CYP2A6, or CYP2B6 activity. Overall, the findings indicate that hydroxyprogesterone caproate has minimal potential for CYP1A2, CYP2A6, and CYP2B6 related drug-drug interactions at the clinically relevant concentrations.
- In vitro data indicated that therapeutic concentration of hydroxyprogesterone caproate is not likely to inhibit the activity of CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Hydroxyprogesterone caproate use in women during the first trimester of pregnancy. Data from a vehicle (placebo)-controlled clinical trial of 310 pregnant women who received Hydroxyprogesterone caproate at weekly doses of 250 mg by intramuscular injection in their second and third trimesters1, as well as long-term (2-5 years) follow-up safety data on 194 of their infants 2, did not demonstrate any teratogenic risks to infants from in utero exposure to Hydroxyprogesterone caproate.
- Reproduction studies have been performed in mice and rats at doses up to 95 and 5, respectively, times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Hydroxyprogesterone caproate.
- Hydroxyprogesterone caproate administration produced embryolethality in rhesus monkeys but not in cynomolgus monkeys exposed to 1 and 10 times the human dose equivalent every 7 days between days 20 and 146 of gestation. There were no teratogenic effects in either species.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydroxyprogesterone caproate in women who are pregnant.
Labor and Delivery
- Hydroxyprogesterone caproate is not intended for use to stop active preterm labor. The effect of Hydroxyprogesterone caproate in active labor is unknown.
Nursing Mothers
- Discontinue Hydroxyprogesterone caproate at 37 weeks of gestation or upon delivery. Detectable amounts of progestins have been identified in the milk of mothers receiving progestin treatment. Many studies have found no adverse effects of progestins on breastfeeding performance, or on the health, growth, or development of the infant
Pediatric Use
- Hydroxyprogesterone caproate is not indicated for use in children. Safety and effectiveness in pediatric patients less than 16 years of age have not been established. A small number of women under age 18 years were studied; safety and efficacy are expected to be the same in women aged 16 years and above as for users 18 years and older.
Geriatic Use
- Hydroxyprogesterone caproate is not intended for use in postmenopausal women. Safety and effectiveness in postmenopausal women have not been established.
Gender
There is no FDA guidance on the use of Hydroxyprogesterone caproate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydroxyprogesterone caproate with respect to specific racial populations.
Renal Impairment
No studies have been conducted to examine the pharmacokinetics of Hydroxyprogesterone caproate in patients with renal impairment.
Hepatic Impairment
- No studies have been conducted to examine the pharmacokinetics of Hydroxyprogesterone caproate in patients with hepatic impairment. Hydroxyprogesterone caproate is extensively metabolized and hepatic impairment may reduce the elimination of Hydroxyprogesterone caproate.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydroxyprogesterone caproate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydroxyprogesterone caproate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Hydroxyprogesterone caproate is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy.
Instructions for administration
- Clean the vial top with an alcohol swab before use.
- Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle.
- Change the needle to a 21 gauge 1 1/2 inch needle.
- After preparing the skin, inject in the upper outer quadrant of the gluteus maximus.
- The solution is viscous and oily. Slow injection (over one minute or longer) is recommended.
- Applying pressure to the injection site may minimize bruising and swelling.
- Discard any unused product 5 weeks after first use.
Monitoring
There is limited information regarding Hydroxyprogesterone caproate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hydroxyprogesterone caproate and IV administrations.
Overdosage
There is limited information regarding Hydroxyprogesterone caproate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Hydroxyprogesterone caproate
| |
| Systematic (IUPAC) name | |
| [(8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl- 3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro- 1H-cyclopenta[a]phenanthren-17-yl] hexanoate | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 428.6041 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Hydroxyprogesterone caproate is a synthetic progestin. The mechanism by which hydroxyprogesterone caproate reduces the risk recurrent preterm birth is not known.
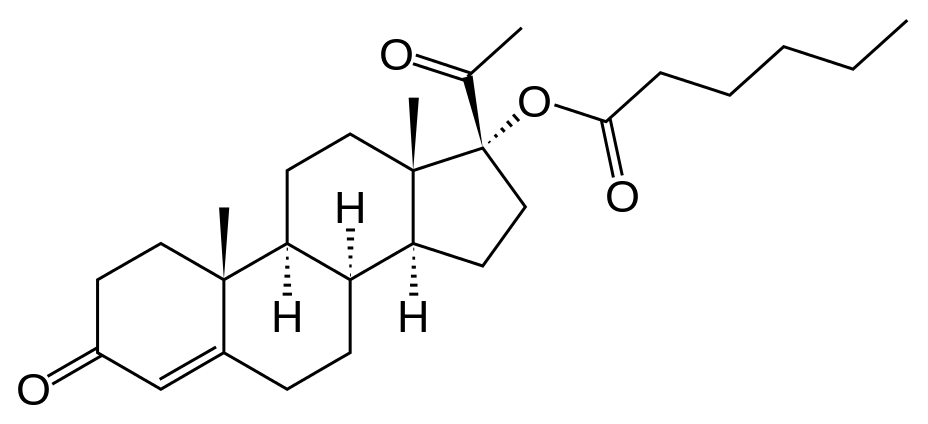
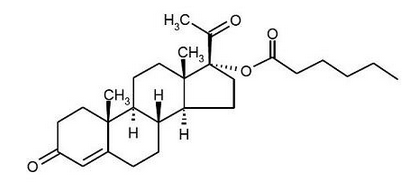
Structure
- The active pharmaceutical ingredient in Hydroxyprogesterone caproate is hydroxyprogesterone caproate.
- The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60. Hydroxyprogesterone caproate exists as white to practically white crystals or powder with a melting point of 120°-124°C.
The structural formula is:
Pharmacodynamics
No specific pharmacodynamic studies were conducted with Hydroxyprogesterone caproate.
Pharmacokinetics
Absorption:
- Peak serum levels of hydroxyprogesterone caproate appeared after 3-7 days in non-pregnant female subjects following a single intramuscular injection of 1000 mg hydroxyprogesterone caproate. Based on pharmacokinetic analysis of five non-pregnant female subjects who received a single intramuscular administration of 1000 mg hydroxyprogesterone caproate, the mean (±SD) Cmax is estimated to be 27.8 (±5.3) ng/mL, and the Tmax is estimated to be 4.6 (±1.7) days. The elimination half-life of hydroxyprogesterone caproate was 7.8 (±3.0) days. Once-weekly intramuscular administration of 1000 mg hydroxyprogesterone caproate to non-pregnant women resulted in trough concentration of 60.0 (±14) ng/mL after 13 weeks. The pharmacokinetics of the 250 mg dose of hydroxyprogesterone caproate has not been evaluated.
Distribution:
- Hydroxyprogesterone caproate binds extensively to plasma proteins including albumin and corticosteroid binding globulins.
Metabolism:
- In vitro studies have shown that hydroxyprogesterone caproate can be metabolized by human hepatocytes, both by phase I and phase II reactions. Hydroxyprogesterone caproate undergoes extensive reduction, hydroxylation and conjugation. The conjugated metabolites include sulfated, glucuronidated and acetylated products. In vitro data indicate that the metabolism of hydroxyprogesterone caproate is predominantly mediated by CYP3A4 and CYP3A5. The in vitro data indicate that the caproate group is retained during metabolism of hydroxyprogesterone caproate.
Excretion:
- Both conjugated metabolites and free steroids are excreted in the urine and feces, with the conjugated metabolites being prominent. Following intramuscular administration to pregnant women at 10-12 weeks gestation, approximately 50% of a dose was recovered in the feces and approximately 30% recovered in the urine.
Nonclinical Toxicology
There is limited information regarding Hydroxyprogesterone caproate Nonclinical Toxicology in the drug label.
Clinical Studies
Clinical Trial to Evaluate Reduction of Risk of Preterm Birth
- In a multicenter, randomized, double-blind, vehicle (placebo)-controlled clinical trial, the safety and effectiveness of Hydroxyprogesterone caproate for the reduction of the risk of spontaneous preterm birth was studied in women with a singleton pregnancy (age 16 to 43 years) who had a documented history of singleton spontaneous preterm birth (defined as delivery at less than 37 weeks of gestation following spontaneous preterm labor or premature rupture of membranes).1 At the time of randomization (between 16 weeks, 0 days and 20 weeks, 6 days of gestation), an ultrasound examination had confirmed gestational age and no known fetal anomaly. Women were excluded for prior progesterone treatment or heparin therapy during the current pregnancy, a history of thromboembolic disease, or maternal/obstetrical complications (such as current or planned cerclage, hypertension requiring medication, or a seizure disorder).
- A total of 463 pregnant women were randomized to receive either Hydroxyprogesterone caproate (N=310) or vehicle (N=153) at a dose of 250 mg administered weekly by intramuscular injection starting between 16 weeks, 0 days and 20 weeks, 6 days of gestation, and continuing until 37 weeks of gestation or delivery. Demographics of the Hydroxyprogesterone caproate-treated women were similar to those in the control group, and included: 59.0% Black, 25.5% Caucasian, 13.9% Hispanic and 0.6% Asian. The mean body mass index was 26.9 kg/m2.
- The proportions of women in each treatment arm who delivered at <37 (the primary study endpoint), <35, and <32 weeks of gestation are displayed in Table 4.

- Four Hydroxyprogesterone caproate-treated subjects were lost to follow-up. They were counted as deliveries at their gestational ages at time of last contact (184, 220, 343 and 364 weeks).
- Adjusted for interim analysis.
- Compared to controls, treatment with Hydroxyprogesterone caproate reduced the proportion of women who delivered preterm at <37 weeks. The proportions of women delivering at <35 and < 32 weeks also were lower among women treated with Hydroxyprogesterone caproate. The upper bounds of the confidence intervals for the treatment difference at < 35 and <32 weeks were close to zero. Inclusion of zero in a confidence interval would indicate the treatment difference is not statistically significant. Compared to the other gestational ages evaluated, the number of preterm births at <32 weeks was limited.
- After adjusting for time in the study, 7.5% of Hydroxyprogesterone caproate-treated subjects delivered prior to 25 weeks compared to 4.7% of control subjects; see Figure 1.

- The rates of fetal and neonatal deaths in each treatment arm are displayed in Table 5. Due to the higher rate of miscarriages and stillbirths in the Hydroxyprogesterone caproate arm, there was no overall survival difference demonstrated in this clinical trial.
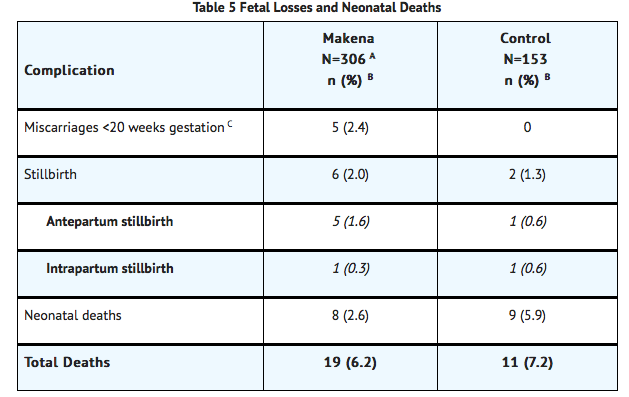
- A Four of the 310 Hydroxyprogesterone caproate-treated subjects were lost to follow-up and stillbirth or neonatal status could not be determined
- Percentages are based on the number of enrolled subjects and not adjusted for time on drug
- Percentage adjusted for the number of at risk subjects (n=209 for Hydroxyprogesterone caproate, n=107 for control) enrolled at <20 weeks gestation.
- A composite neonatal morbidity/mortality index evaluated adverse outcomes in livebirths. It was based on the number of neonates who died or experienced respiratory distress syndrome, bronchopulmonary dysplasia, grade 3 or 4 intraventricular hemorrhage, proven sepsis, or necrotizing enterocolitis. Although the proportion of neonates who experienced 1 or more events was numerically lower in the Hydroxyprogesterone caproate arm (11.9% vs. 17.2%), the number of adverse outcomes was limited and the difference between arms was not statistically significant.
Infant Follow-Up Safety Study
- Infants born to women enrolled in this study, and who survived to be discharged from the nursery, were eligible for participation in a follow-up safety study. Of 348 eligible offspring, 79.9% enrolled: 194 children of Hydroxyprogesterone caproate-treated women and 84 children of control subjects. The primary endpoint was the score on the Ages & Stages Questionnaire (ASQ), which evaluates communication, gross motor, fine motor, problem solving, and personal/social parameters. The proportion of children whose scores met the screening threshold for developmental delay in each developmental domain was similar for each treatment group.
How Supplied
Hydroxyprogesterone caproate (NDC 64011-243-01) is supplied as 5 mL of a sterile solution in a multidose glass vial.
Each 5 mL vial contains hydroxyprogesterone caproate USP, 250 mg/mL (25% w/v), in castor oil USP (28.6% v/v) and benzyl benzoate USP (46% v/v) with the preservative benzyl alcohol NF (2% v/v).
Single unit carton: Contains one 5 mL multidose vial of Hydroxyprogesterone caproate (250 mg/mL) containing 1250 mg of hydroxyprogesterone caproate.
Storage
- Store at controlled room temperature [15°-30°C (59°-86°F)]. Use within 5 weeks after first use.
- Caution: Protect vial from light. Store vial in its box. Store upright.
Images
Drug Images
{{#ask: Page Name::Hydroxyprogesterone caproate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

Carton Label
NDC 64011-243-01
Makena® hydroxyprogesterone caproate injection
1250 mg/5 mL (250 mg/mL)
FOR INTRAMUSCULAR USE
5 mL multidose vial Rx ONLY
Store at controlled room temperature 15°-30°C (59°-86°F).
Caution: Protect from light. Store vial in its box. Store upright. Use within 5 weeks after first use.
Each mL contains: hydroxyprogesterone caproate 250 mg, benzyl benzoate 46%, castor oil 28.6%, and benzyl alcohol 2%, as preservative.
See package insert for full prescribing and patient information.
Lumara Health
P8009 6/14
Marketed by Lumara Health™ Chesterfield, MO 63005 {{#ask: Label Page::Hydroxyprogesterone caproate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Hydroxyprogesterone caproate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Hylutin®
- Prodrox®
- Makena®
Look-Alike Drug Names
There is limited information regarding Hydroxyprogesterone caproate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.