Glycogen storage disease type VI: Difference between revisions
| (45 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
'''For the main page on glycogen storage disease, please click [[Glycogen storage disease|here]]'''<br> | |||
{{SI}} | |||
{{SI}} | |||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} {{MKK}}, {{Anmol}} | ||
{{SK}} | {{SK}}Her's disease; phosphorylase deficiency glycogen-storage disease of liver; glycogen storage disease type 6; GSD IV; GSD type 6 | ||
==Overview== | ==Overview== | ||
Glycogen storage type disease VI is caused by the deficiency of [[Phosphorylase B kinase|phosphorylase B kinase]]. In 1959, Dr. Hers first discovered glycogen storage type VI disease in the patients with [[liver]] [[phosphorylase]] deficiency. Glycogen storage type disease VI is an [[autosomal recessive]] disease and some forms are [[X-linked recessive]]. Glycogen storage disease type VI is classified according to the pattern of inheritance associated with the [[enzyme]] deficiency into 2 subtypes, [[autosomal recessive]] [[liver]] [[phosphorylase kinase]] deficiency and [[x-linked recessive]] [[liver]] [[phosphorylase kinase]] deficiency. Glycogen storage disease type VI presents at the age of 1-5 years. A positive history of the protuberant abdomen, [[growth retardation]] and the slight delay in motor milestones is suggestive of glycogen storage disease type VI and some children have the history of mild fasting [[hypoglycemia]] and [[hypotonia]].Patient with the glycogen storage disease type VI presents with the symptoms of [[hypoglycemia]] on fasting, such as [[faintness]], [[weakness]], and [[nervousness]]. On physical examination, the increased liver span is present. The mainstay of treatment is the dietary therapy which includes frequent meals, high [[carbohydrate]] diet, high [[protein]] diet and supplementation of [[unsaturated fats]]. | |||
==Historical Perspective== | ==Historical Perspective== | ||
*In 1959, Dr.Hers | *In 1959, Dr. Hers first discovered glycogen storage type disease VI in the patients with [[liver]] [[phosphorylase]] deficiency.<ref name="pmid13646331">{{cite journal |vauthors=HERS HG |title=[Enzymatic studies of hepatic fragments; application to the classification of glycogenoses] |language=French |journal=Rev Int Hepatol |volume=9 |issue=1 |pages=35–55 |year=1959 |pmid=13646331 |doi= |url=}}</ref> | ||
*In 1960, Stetten & Stetten described glycogen storage type disease VI disease after its initial discovery.<ref name="pmid13834511">{{cite journal |vauthors=STETTEN D, STETTEN MR |title=Glycogen metabolism |journal=Physiol. Rev. |volume=40 |issue= |pages=505–37 |year=1960 |pmid=13834511 |doi=10.1152/physrev.1960.40.3.505 |url=}}</ref> | |||
*In 1960 | *In 1987, [[Gene mutation|gene mutations]] encoding [[liver]] [[phosphorylase]] present on [[chromosome]] 14q21-q22 became known to be associated with the [[pathogenesis]] of glycogen storage type disease VI disease.<ref name="pmid2883891">{{cite journal |vauthors=Newgard CB, Fletterick RJ, Anderson LA, Lebo RV |title=The polymorphic locus for glycogen storage disease VI (liver glycogen phosphorylase) maps to chromosome 14 |journal=Am. J. Hum. Genet. |volume=40 |issue=4 |pages=351–64 |year=1987 |pmid=2883891 |pmc=1684093 |doi= |url=}}</ref> | ||
*In 1987, Gene mutations encoding liver phosphorylase present on chromosome 14q21-q22 | |||
==Classification== | ==Classification== | ||
Glycogen storage disease type VI is classified according to the pattern of inheritance associated with the [[enzyme]] deficiency into 2 subtypes:<ref name="pmid7959740">{{cite journal |vauthors=Hendrickx J, Coucke P, Hors-Cayla MC, Smit GP, Shin YS, Deutsch J, Smeitink J, Berger R, Lee P, Fernandes J |title=Localization of a new type of X-linked liver glycogenosis to the chromosomal region Xp22 containing the liver alpha-subunit of phosphorylase kinase (PHKA2) |journal=Genomics |volume=21 |issue=3 |pages=620–5 |year=1994 |pmid=7959740 |doi= |url=}}</ref><ref name="pmid9529348">{{cite journal |vauthors=Burwinkel B, Bakker HD, Herschkovitz E, Moses SW, Shin YS, Kilimann MW |title=Mutations in the liver glycogen phosphorylase gene (PYGL) underlying glycogenosis type VI |journal=Am. J. Hum. Genet. |volume=62 |issue=4 |pages=785–91 |year=1998 |pmid=9529348 |pmc=1377030 |doi= |url=}}</ref> | |||
Glycogen storage disease type VI is classified according to the | *[[Autosomal recessive]] [[liver]] [[phosphorylase kinase]] deficiency | ||
*Autosomal recessive liver phosphorylase kinase deficiency | *[[X-linked recessive]] [[liver]] [[phosphorylase kinase]] deficiency | ||
*X-linked liver phosphorylase kinase deficiency | |||
*It is further classified into two types: | *It is further classified into two types: | ||
**X-linked liver phosphorylase kinase deficiency classical type | **[[X-linked recessive]] [[liver]] [[phosphorylase kinase]] deficiency classical type (type I) | ||
**X-linked liver phosphorylase kinase deficiency | **[[X-linked recessive]] [[liver]] [[phosphorylase kinase]] deficiency variant type (type II) | ||
<br> | |||
{{Family tree/start}} | |||
{{Family tree| | | | | | | | | A01 | | | | | | | | A01=Glycogen storage disease type VI}} | |||
{{Family tree| | | | | |,|-|-|-|^|-|-|-|.| | | | | }} | |||
{{Family tree| | | | | B01 | | | | | | B02 | | | | B01=[[Autosomal recessive]]<br>[[liver]] [[phosphorylase kinase]] deficiency|B02=[[X-linked recessive]]<br>[[liver]] [[phosphorylase kinase]] deficiency}} | |||
{{Family tree| | | | | | | | | | |,|-|-|^|-|-|.| | | }} | |||
{{Family tree| | | | | | | | | | C01 | | | | C02 |C01=[[X-linked recessive]]<br>[[liver]] [[phosphorylase kinase]] deficiency<br>classical type (type I)|C02=[[X-linked recessive]]<br>[[liver]] [[phosphorylase kinase]] deficiency<br>variant type (type II) }} | |||
{{Family tree/end}} | |||
<br> | |||
==Pathophysiology== | |||
===Pathogenesis=== | |||
*Glycogen storage disease type VI is caused by the deficiency of liver [[Phosphorylase B kinase|phosphorylase B kinase]]. | |||
*The [[Rate-determining step|rate-limiting]] [[enzyme]] of glycogen breakdown is [[phosphorylase]], which is activated by a series of enzymes, including [[Adenylate cyclase|adenyl cyclase]], phosphorylase b kinase, and [[cAMP-dependent protein kinase]]. | |||
*[[Enzyme]] deficiency leads to the impaired breakdown of [[glycogen]] into [[glucose]]. | |||
*In most patients, the [[enzyme]] deficiency is incomplete, and [[gluconeogenesis]] remains intact. | |||
*Due to impaired functioning of [[liver]] [[phosphorylase]], there is difficulty to maintain [[glucose]] level at fasting, leading to [[hypoglycemia]] at fasting. | |||
*There is also associated [[hyperketosis]] and increased levels of urinary [[ketones]] and serum [[ketone]] bodies. | |||
*There is mild-moderate [[hyperlipidemia]] and increased levels of [[transaminases]].<ref name="urlGlycogen Storage Disease Type VI - GeneReviews® - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK5941/ |title=Glycogen Storage Disease Type VI - GeneReviews® - NCBI Bookshelf |format= |work= |accessdate=}}</ref><ref name="urlType VI Glycogen Storage Disease | Association for Glycogen Storage Disease">{{cite web |url=http://www.agsdus.org/type-vi.php |title=Type VI Glycogen Storage Disease | Association for Glycogen Storage Disease |format= |work= |accessdate=}}</ref> | |||
== | ===Metabolic Pathway=== | ||
[[File:GSD TYPE VI.png|center|800px|frame| Metabolic pathways showing defects in glycogen storage disease VI, (ɔ) Image courtesy of WikiDoc.org, by '''"[[User:Anmol Pitliya|Dr. Anmol Pitliya]]"''']] | |||
==Causes== | ==Causes== | ||
*The most common cause of | *The most common cause of glycogen storage disease type VI is the deficiency of liver [[Phosphorylase B kinase|phosphorylase]] b kinase, an [[enzyme]] that activates [[phosphorylase]] by [[phosphorylation]].<ref name="pmid12930917">{{cite journal |vauthors=Burwinkel B, Rootwelt T, Kvittingen EA, Chakraborty PK, Kilimann MW |title=Severe phenotype of phosphorylase kinase-deficient liver glycogenosis with mutations in the PHKG2 gene |journal=Pediatr. Res. |volume=54 |issue=6 |pages=834–9 |year=2003 |pmid=12930917 |doi=10.1203/01.PDR.0000088069.09275.10 |url=}}</ref> | ||
*Phosphorylase b kinase has 4 subunits, each is encoded by different genes present on different chromosomes. | *Phosphorylase b kinase has 4 subunits, each is encoded by different [[genes]] present on different [[chromosomes]]. | ||
*Mutations in three subunits (PHKA2, PHKB, and PHKG2) is most commonly seen in patients with phosphorylase b kinase deficiency. | *[[Mutations]] in three subunits (PHKA2, PHKB, and PHKG2) is most commonly seen in patients with [[phosphorylase]] b kinase deficiency. | ||
*If there is the mutation in PHKG2, then the patient has significant liver fibrosis and cirrhosis.<ref name="pmid24326380">{{cite journal |vauthors=Albash B, Imtiaz F, Al-Zaidan H, Al-Manea H, Banemai M, Allam R, Al-Suheel A, Al-Owain M |title=Novel PHKG2 mutation causing GSD IX with prominent liver disease: report of three cases and review of literature |journal=Eur. J. Pediatr. |volume=173 |issue=5 |pages=647–53 |year=2014 |pmid=24326380 |doi=10.1007/s00431-013-2223-0 |url=}}</ref> | *If there is the mutation only in PHKG2, then the patient has significant [[liver]] [[fibrosis]] and [[cirrhosis]].<ref name="pmid24326380">{{cite journal |vauthors=Albash B, Imtiaz F, Al-Zaidan H, Al-Manea H, Banemai M, Allam R, Al-Suheel A, Al-Owain M |title=Novel PHKG2 mutation causing GSD IX with prominent liver disease: report of three cases and review of literature |journal=Eur. J. Pediatr. |volume=173 |issue=5 |pages=647–53 |year=2014 |pmid=24326380 |doi=10.1007/s00431-013-2223-0 |url=}}</ref><ref name="pmid95360912">{{cite journal |vauthors=Chang S, Rosenberg MJ, Morton H, Francomano CA, Biesecker LG |title=Identification of a mutation in liver glycogen phosphorylase in glycogen storage disease type VI |journal=Hum. Mol. Genet. |volume=7 |issue=5 |pages=865–70 |year=1998 |pmid=9536091 |doi= |url=}}</ref> | ||
==Differentiating Glycogen storage disease type VI from Other Diseases== | ==Differentiating Glycogen storage disease type VI from Other Diseases== | ||
Glycogen storage disease type VI | <small> | ||
{| | |||
! colspan="15" style="background:#4479BA; color: #FFFFFF;" align="center" + | Differentiating Glycogen Storage Diseases | |||
|- | |||
! colspan="3" rowspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |Glycogen storage disease | |||
! rowspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |Enzyme deficiency | |||
! colspan="3" style="background:#4479BA; color: #FFFFFF;" align="center" + |Genetics | |||
! colspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |History and symptoms | |||
! colspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |Physical examination | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Laboratory findings | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Imaging | |||
! rowspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |Other features | |||
|- | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Gene mutation | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Inheritance | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Chromosome | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Hypoglycemia | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Muscle weakness | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Hypotonia | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Hepatomegaly | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Elevated CK | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Cardiomegaly | |||
|- | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type I|'''Glycogen storage disease type I''']]<ref name="pmid10322403">{{cite journal| author=Mansfield BC| title=Molecular Genetics of Type 1 Glycogen Storage Diseases. | journal=Trends Endocrinol Metab | year= 1999 | volume= 10 | issue= 3 | pages= 104-113 | pmid=10322403 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10322403 }} </ref><ref name="pmid17552001">{{cite journal| author=Ozen H| title=Glycogen storage diseases: new perspectives. | journal=World J Gastroenterol | year= 2007 | volume= 13 | issue= 18 | pages= 2541-53 | pmid=17552001 | doi= | pmc=4146814 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17552001 }} </ref><ref name="pmid21599942">{{cite journal| author=Froissart R, Piraud M, Boudjemline AM, Vianey-Saban C, Petit F, Hubert-Buron A et al.| title=Glucose-6-phosphatase deficiency. | journal=Orphanet J Rare Dis | year= 2011 | volume= 6 | issue= | pages= 27 | pmid=21599942 | doi=10.1186/1750-1172-6-27 | pmc=3118311 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21599942 }} </ref><ref name="KishnaniAustin2014">{{cite journal|last1=Kishnani|first1=Priya S.|last2=Austin|first2=Stephanie L.|last3=Abdenur|first3=Jose E.|last4=Arn|first4=Pamela|last5=Bali|first5=Deeksha S.|last6=Boney|first6=Anne|last7=Chung|first7=Wendy K.|last8=Dagli|first8=Aditi I.|last9=Dale|first9=David|last10=Koeberl|first10=Dwight|last11=Somers|first11=Michael J.|last12=Burns Wechsler|first12=Stephanie|last13=Weinstein|first13=David A.|last14=Wolfsdorf|first14=Joseph I.|last15=Watson|first15=Michael S.|title=Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics|journal=Genetics in Medicine|year=2014|issn=1098-3600|doi=10.1038/gim.2014.128}}</ref><ref name="pmid12373567">{{cite journal |vauthors=Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP |title=Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I) |journal=Eur. J. Pediatr. |volume=161 Suppl 1 |issue= |pages=S20–34 |year=2002 |pmid=12373567 |doi=10.1007/s00431-002-0999-4 |url=}}</ref><ref>Bali DS, Chen YT, Austin S, et al. Glycogen Storage Disease Type I. 2006 Apr 19 [Updated 2016 Aug 25]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1312/</ref> | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Von Gierke's disease|'''Von Gierke's disease''']] | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type Ia''' | |||
| style="background:#F5F5F5;" align="center" + |[[Glucose-6-phosphatase]] | |||
| style="background:#F5F5F5;" align="center" + |[[G6PC]] [[gene mutation]] | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |17q21 | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | - | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | - | |||
| rowspan="2" style="background:#F5F5F5;" + | | |||
* [[Lactic acidosis]] | |||
* [[Hyperlipidemia]] | |||
* [[Hyperuricemia]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type Ib''' | |||
| style="background:#F5F5F5;" align="center" + | [[Microsomal]] [[glucose-6-phosphate]] [[Membrane transport protein|transporter]] | |||
| style="background:#F5F5F5;" align="center" + | [[SLC37A4]] [[gene mutation]] | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |11q23 | |||
|- | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type II|'''Glycogen storage disease type II''']]<ref>Leslie N, Bailey L. Pompe Disease. 2007 Aug 31 [Updated 2017 May 11]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1261/</ref><ref name="pmid17915568">{{cite journal| author=Di Rocco M, Buzzi D, Tarò M| title=Glycogen storage disease type II: clinical overview. | journal=Acta Myol | year= 2007 | volume= 26 | issue= 1 | pages= 42-4 | pmid=17915568 | doi= | pmc=2949314 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17915568 }} </ref><ref name="pmid16737883">{{cite journal| author=Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D et al.| title=A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. | journal=J Pediatr | year= 2006 | volume= 148 | issue= 5 | pages= 671-676 | pmid=16737883 | doi=10.1016/j.jpeds.2005.11.033 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16737883 }} </ref><ref name="pmid12897283">{{cite journal| author=van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT et al.| title=The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. | journal=Pediatrics | year= 2003 | volume= 112 | issue= 2 | pages= 332-40 | pmid=12897283 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12897283 }} </ref><ref name="pmid10931430">{{cite journal| author=Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F| title=Identification of two subtypes of infantile acid maltase deficiency. | journal=J Pediatr | year= 2000 | volume= 137 | issue= 2 | pages= 283-5 | pmid=10931430 | doi=10.1067/mpd.2000.107112 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10931430 }} </ref><ref name="pmid2111708">{{cite journal| author=Martiniuk F, Mehler M, Tzall S, Meredith G, Hirschhorn R| title=Sequence of the cDNA and 5'-flanking region for human acid alpha-glucosidase, detection of an intron in the 5' untranslated leader sequence, definition of 18-bp polymorphisms, and differences with previous cDNA and amino acid sequences. | journal=DNA Cell Biol | year= 1990 | volume= 9 | issue= 2 | pages= 85-94 | pmid=2111708 | doi=10.1089/dna.1990.9.85 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2111708 }} </ref><ref name="pmid3049072">{{cite journal| author=Hoefsloot LH, Hoogeveen-Westerveld M, Kroos MA, van Beeumen J, Reuser AJ, Oostra BA| title=Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. | journal=EMBO J | year= 1988 | volume= 7 | issue= 6 | pages= 1697-704 | pmid=3049072 | doi= | pmc=457155 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3049072 }} </ref><ref name="pmid2268276">{{cite journal| author=Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, Oostra BA| title=Characterization of the human lysosomal alpha-glucosidase gene. | journal=Biochem J | year= 1990 | volume= 272 | issue= 2 | pages= 493-7 | pmid=2268276 | doi= | pmc=1149727 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2268276 }} </ref><ref name="pmid8786092">{{cite journal| author=Kuo WL, Hirschhorn R, Huie ML, Hirschhorn K| title=Localization and ordering of acid alpha-glucosidase (GAA) and thymidine kinase (TK1) by fluorescence in situ hybridization. | journal=Hum Genet | year= 1996 | volume= 97 | issue= 3 | pages= 404-6 | pmid=8786092 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8786092 }} </ref> | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Pompe disease|'''Pompe disease''']] | |||
| style="background:#DCDCDC;" align="center" + |'''Infantile onset''' | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |[[Acid alpha-glucosidase]] | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |GAA gene | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |17q25 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" + | | |||
* Elevated [[LDH]] | |||
* Elevated [[liver]] aminotransferases | |||
* Elevated urinary glc4 | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Late onset''' | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | +/- | |||
|- | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type III|'''Glycogen storage disease type III''']]<ref name="pmid8755644">{{cite journal| author=Shen J, Bao Y, Liu HM, Lee P, Leonard JV, Chen YT| title=Mutations in exon 3 of the glycogen debranching enzyme gene are associated with glycogen storage disease type III that is differentially expressed in liver and muscle. | journal=J Clin Invest | year= 1996 | volume= 98 | issue= 2 | pages= 352-7 | pmid=8755644 | doi=10.1172/JCI118799 | pmc=507437 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8755644 }} </ref><ref name="pmid2295969">{{cite journal| author=Ding JH, de Barsy T, Brown BI, Coleman RA, Chen YT| title=Immunoblot analyses of glycogen debranching enzyme in different subtypes of glycogen storage disease type III. | journal=J Pediatr | year= 1990 | volume= 116 | issue= 1 | pages= 95-100 | pmid=2295969 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2295969 }} </ref><ref name="pmid19834502">{{cite journal| author=Aoyama Y, Ozer I, Demirkol M, Ebara T, Murase T, Podskarbi T et al.| title=Molecular features of 23 patients with glycogen storage disease type III in Turkey: a novel mutation p.R1147G associated with isolated glucosidase deficiency, along with 9 AGL mutations. | journal=J Hum Genet | year= 2009 | volume= 54 | issue= 11 | pages= 681-6 | pmid=19834502 | doi=10.1038/jhg.2009.100 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19834502 }} </ref><ref name="KishnaniAustin2010">{{cite journal|last1=Kishnani|first1=Priya S|last2=Austin|first2=Stephanie L|last3=Arn|first3=Pamela|last4=Bali|first4=Deeksha S|last5=Boney|first5=Anne|last6=Case|first6=Laura E|last7=Chung|first7=Wendy K|last8=Desai|first8=Dev M|last9=El-Gharbawy|first9=Areeg|last10=Haller|first10=Ronald|last11=Smit|first11=G Peter A|last12=Smith|first12=Alastair D|last13=Hobson-Webb|first13=Lisa D|last14=Wechsler|first14=Stephanie Burns|last15=Weinstein|first15=David A|last16=Watson|first16=Michael S|title=Glycogen Storage Disease Type III diagnosis and management guidelines|journal=Genetics in Medicine|volume=12|issue=7|year=2010|pages=446–463|issn=1098-3600|doi=10.1097/GIM.0b013e3181e655b6}}</ref><ref>Dagli A, Sentner CP, Weinstein DA. Glycogen Storage Disease Type III. 2010 Mar 9 [Updated 2016 Dec 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26372/</ref><ref name="pmid12618563">{{cite journal| author=Wolfsdorf JI, Weinstein DA| title=Glycogen storage diseases. | journal=Rev Endocr Metab Disord | year= 2003 | volume= 4 | issue= 1 | pages= 95-102 | pmid=12618563 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12618563 }} </ref> | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Cori disease|'''Cori disease''']] | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type IIIa''' | |||
| style="background:#F5F5F5;" align="center" + |[[Debranching enzyme]] (deficiency in muscle and liver) | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |AGL [[gene mutation]] | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |1p21 | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" + | | |||
* [[Ketosis]] | |||
* [[Hyperlipidemia]] | |||
* Elevated liver aminotransferases | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type IIIb''' | |||
| style="background:#F5F5F5;" align="center" + |[[Debranching enzyme]] (deficiency in liver only) | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type IV|'''Glycogen storage disease type IV''']]<ref name="pmid15452297">{{cite journal| author=Bruno C, van Diggelen OP, Cassandrini D, Gimpelev M, Giuffrè B, Donati MA et al.| title=Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV). | journal=Neurology | year= 2004 | volume= 63 | issue= 6 | pages= 1053-8 | pmid=15452297 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15452297 }} </ref><ref name="pmid17915577">{{cite journal| author=Bruno C, Cassandrini D, Assereto S, Akman HO, Minetti C, Di Mauro S| title=Neuromuscular forms of glycogen branching enzyme deficiency. | journal=Acta Myol | year= 2007 | volume= 26 | issue= 1 | pages= 75-8 | pmid=17915577 | doi= | pmc=2949312 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17915577 }} </ref><ref name="pmid5229990">{{cite journal| author=Brown BI, Brown DH| title=Lack of an alpha-1,4-glucan: alpha-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. | journal=Proc Natl Acad Sci U S A | year= 1966 | volume= 56 | issue= 2 | pages= 725-9 | pmid=5229990 | doi= | pmc=224432 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5229990 }} </ref><ref name="pmid8830177">{{cite journal| author=McConkie-Rosell A, Wilson C, Piccoli DA, Boyle J, DeClue T, Kishnani P et al.| title=Clinical and laboratory findings in four patients with the non-progressive hepatic form of type IV glycogen storage disease. | journal=J Inherit Metab Dis | year= 1996 | volume= 19 | issue= 1 | pages= 51-8 | pmid=8830177 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8830177 }} </ref><ref>Magoulas PL, El-Hattab AW. Glycogen Storage Disease Type IV. 2013 Jan 3. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK115333/</ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |[[Andersen's disease|'''Andersen's disease''']] | |||
| style="background:#F5F5F5;" align="center" + |Branching enzyme | |||
| style="background:#F5F5F5;" align="center" + | GBE1 gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |3p12 | |||
| style="background:#F5F5F5;" align="center" + | +/- | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type V|'''Glycogen storage disease type V''']]<ref name="pmid24540673">{{cite journal| author=McARDLE B| title=Myopathy due to a defect in muscle glycogen breakdown. | journal=Clin Sci | year= 1951 | volume= 10 | issue= 1 | pages= 13-35 | pmid=24540673 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24540673 }} </ref><ref name="pmid14442994">{{cite journal| author=SCHMID R, MAHLER R| title=Chronic progressive myopathy with myoglobinuria: demonstration of a glycogenolytic defect in the muscle. | journal=J Clin Invest | year= 1959 | volume= 38 | issue= | pages= 2044-58 | pmid=14442994 | doi=10.1172/JCI103983 | pmc=441792 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14442994 }} </ref><ref name="pmid16590445">{{cite journal| author=Mommaerts WF, Illingworth B, Pearson CM, Guillory RJ, Seraydarian K| title=A FUNCTIONAL DISORDER OF MUSCLE ASSOCIATED WITH THE ABSENCE OF PHOSPHORYLASE. | journal=Proc Natl Acad Sci U S A | year= 1959 | volume= 45 | issue= 6 | pages= 791-7 | pmid=16590445 | doi= | pmc=222638 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16590445 }} </ref><ref name="pmid13733779">{{cite journal| author=PEARSON CM, RIMER DG, MOMMAERTS WF| title=A metabolic myopathy due to absence of muscle phosphorylase. | journal=Am J Med | year= 1961 | volume= 30 | issue= | pages= 502-17 | pmid=13733779 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=13733779 }} </ref><ref name="pmid4502558">{{cite journal| author=Grünfeld JP, Ganeval D, Chanard J, Fardeau M, Dreyfus JC| title=Acute renal failure in McArdle's disease. Report of two cases. | journal=N Engl J Med | year= 1972 | volume= 286 | issue= 23 | pages= 1237-41 | pmid=4502558 | doi=10.1056/NEJM197206082862304 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4502558 }} </ref><ref name="pmid3476861">{{cite journal| author=Schmidt B, Servidei S, Gabbai AA, Silva AC, de Sousa Bulle de Oliveira A, DiMauro S| title=McArdle's disease in two generations: autosomal recessive transmission with manifesting heterozygote. | journal=Neurology | year= 1987 | volume= 37 | issue= 9 | pages= 1558-61 | pmid=3476861 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3476861 }} </ref><ref>Martín MA, Lucía A, Arenas J, et al. Glycogen Storage Disease Type V. 2006 Apr 19 [Updated 2014 Jun 26]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1344/</ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |[[McArdle disease|'''McArdle disease''']] | |||
| style="background:#F5F5F5;" align="center" + |Muscle [[glycogen phosphorylase]] | |||
| style="background:#F5F5F5;" align="center" + |PYGM gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |11q13 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* [[Myoglobinuria]], may result in [[renal failure]] | |||
|- | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type VI|'''Glycogen storage disease type VI''']]<ref name="pmid5904467">{{cite journal| author=Wallis PG, Sidbury JB, Harris RC| title=Hepatic phosphorylase defect. Studies on peripheral blood. | journal=Am J Dis Child | year= 1966 | volume= 111 | issue= 3 | pages= 278-82 | pmid=5904467 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5904467 }} </ref><ref name="pmid25266922">{{cite journal| author=Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J et al.| title=The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. | journal=Mol Genet Metab | year= 2014 | volume= 113 | issue= 3 | pages= 171-6 | pmid=25266922 | doi=10.1016/j.ymgme.2014.09.005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25266922 }} </ref><ref name="pmid9529348">{{cite journal| author=Burwinkel B, Bakker HD, Herschkovitz E, Moses SW, Shin YS, Kilimann MW| title=Mutations in the liver glycogen phosphorylase gene (PYGL) underlying glycogenosis type VI. | journal=Am J Hum Genet | year= 1998 | volume= 62 | issue= 4 | pages= 785-91 | pmid=9529348 | doi= | pmc=1377030 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9529348 }} </ref><ref name="pmid9536091">{{cite journal| author=Chang S, Rosenberg MJ, Morton H, Francomano CA, Biesecker LG| title=Identification of a mutation in liver glycogen phosphorylase in glycogen storage disease type VI. | journal=Hum Mol Genet | year= 1998 | volume= 7 | issue= 5 | pages= 865-70 | pmid=9536091 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9536091 }} </ref><ref>Dagli AI, Weinstein DA. Glycogen Storage Disease Type VI. 2009 Apr 23 [Updated 2011 May 17]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK5941/</ref> | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + |[[Hers' disease|'''Hers' disease''']] | |||
| style="background:#DCDCDC;" align="center" + |'''Autosomal''' | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + |Liver [[glycogen phosphorylase]] | |||
| style="background:#F5F5F5;" align="center" + | PYGL gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |14q22 | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | +/- | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | +/- | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | + | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | - | |||
| rowspan="2" style="background:#F5F5F5;" align="center" + | - | |||
| rowspan="2" style="background:#F5F5F5;" + | | |||
* [[Hyperlipidemia]] | |||
* Elevated liver aminotransferases | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''X-linked''' | |||
| style="background:#F5F5F5;" align="center" + | PYGL gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[X-linked recessive]] | |||
| style="background:#F5F5F5;" align="center" + |X | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |[[Glycogen storage disease type VII|'''Glycogen storage disease type VII''']]<ref name="pmid7550225">{{cite journal| author=Raben N, Sherman JB| title=Mutations in muscle phosphofructokinase gene. | journal=Hum Mutat | year= 1995 | volume= 6 | issue= 1 | pages= 1-6 | pmid=7550225 | doi=10.1002/humu.1380060102 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7550225 }} </ref><ref name="pmid14339001">{{cite journal| author=TARUI S, OKUNO G, IKURA Y, TANAKA T, SUDA M, NISHIKAWA M| title=PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS. | journal=Biochem Biophys Res Commun | year= 1965 | volume= 19 | issue= | pages= 517-23 | pmid=14339001 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14339001 }} </ref><ref name="pmid4228297">{{cite journal| author=Layzer RB, Rowland LP, Ranney HM| title=Muscle phosphofructokinase deficiency. | journal=Arch Neurol | year= 1967 | volume= 17 | issue= 5 | pages= 512-23 | pmid=4228297 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4228297 }} </ref><ref name="pmid4228753">{{cite journal| author=Satoyoshi E, Kowa H| title=A myopathy due to glycolytic abnormality. | journal=Arch Neurol | year= 1967 | volume= 17 | issue= 3 | pages= 248-56 | pmid=4228753 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4228753 }} </ref><ref name="pmid4258222">{{cite journal| author=Waterbury L, Frenkel EP| title=Hereditary nonspherocytic hemolysis with erythrocyte phosphofructokinase deficiency. | journal=Blood | year= 1972 | volume= 39 | issue= 3 | pages= 415-25 | pmid=4258222 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4258222 }} </ref><ref name="pmid6444532">{{cite journal| author=Vora S, Corash L, Engel WK, Durham S, Seaman C, Piomelli S| title=The molecular mechanism of the inherited phosphofructokinase deficiency associated with hemolysis and myopathy. | journal=Blood | year= 1980 | volume= 55 | issue= 4 | pages= 629-35 | pmid=6444532 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6444532 }} </ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |[[Tarui's disease|'''Tarui's disease''']] | |||
| style="background:#F5F5F5;" align="center" + |Muscle [[phosphofructokinase]] | |||
| style="background:#F5F5F5;" align="center" + |PFKM gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |12q13 | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" + | | |||
* [[Reticulocyte|Reticulocytosis]] | |||
* [[Hyperuricemia]] | |||
* [[Myoglobinuria]] | |||
* [[Hemolytic anemia]] | |||
|- | |||
| colspan="2" rowspan="2" style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type IX'''<ref name="pmid17689125">{{cite journal| author=Beauchamp NJ, Dalton A, Ramaswami U, Niinikoski H, Mention K, Kenny P et al.| title=Glycogen storage disease type IX: High variability in clinical phenotype. | journal=Mol Genet Metab | year= 2007 | volume= 92 | issue= 1-2 | pages= 88-99 | pmid=17689125 | doi=10.1016/j.ymgme.2007.06.007 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17689125 }} </ref><ref name="pmid25266922">{{cite journal| author=Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J et al.| title=The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. | journal=Mol Genet Metab | year= 2014 | volume= 113 | issue= 3 | pages= 171-6 | pmid=25266922 | doi=10.1016/j.ymgme.2014.09.005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25266922 }} </ref><ref>Goldstein J, Austin S, Kishnani P, et al. Phosphorylase Kinase Deficiency. 2011 May 31. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK55061/</ref> | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type IXa'''<ref name="pmid3859203">{{cite journal| author=Keating JP, Brown BI, White NH, DiMauro S| title=X-linked glycogen storage disease. A cause of hypotonia, hyperuricemia, and growth retardation. | journal=Am J Dis Child | year= 1985 | volume= 139 | issue= 6 | pages= 609-13 | pmid=3859203 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3859203 }} </ref><ref name="pmid7959740">{{cite journal| author=Hendrickx J, Coucke P, Hors-Cayla MC, Smit GP, Shin YS, Deutsch J et al.| title=Localization of a new type of X-linked liver glycogenosis to the chromosomal region Xp22 containing the liver alpha-subunit of phosphorylase kinase (PHKA2). | journal=Genomics | year= 1994 | volume= 21 | issue= 3 | pages= 620-5 | pmid=7959740 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7959740 }} </ref><ref name="pmid4518931">{{cite journal| author=Schimke RN, Zakheim RM, Corder RC, Hug G| title=Glycogen storage disease type IX: benign glycogenosis of liver and hepatic phosphorylase kinase deficiency. | journal=J Pediatr | year= 1973 | volume= 83 | issue= 6 | pages= 1031-4 | pmid=4518931 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4518931 }} </ref><ref name="pmid2303074">{{cite journal| author=Willems PJ, Gerver WJ, Berger R, Fernandes J| title=The natural history of liver glycogenosis due to phosphorylase kinase deficiency: a longitudinal study of 41 patients. | journal=Eur J Pediatr | year= 1990 | volume= 149 | issue= 4 | pages= 268-71 | pmid=2303074 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2303074 }} </ref><ref name="pmid9835437">{{cite journal| author=Hendrickx J, Bosshard NU, Willems P, Gitzelmann R| title=Clinical, biochemical and molecular findings in a patient with X-linked liver glycogenosis followed for 40 years. | journal=Eur J Pediatr | year= 1998 | volume= 157 | issue= 11 | pages= 919-23 | pmid=9835437 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9835437 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |Phosphorylase b kinase (deficiency in liver only) | |||
| style="background:#F5F5F5;" align="center" + |[[PHKA2]] gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[X-linked recessive]] | |||
| style="background:#F5F5F5;" align="center" + |Xp22 | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* [[Hyperlipidemia]] | |||
* Elevated liver aminotransferases | |||
* [[Hyperuricemia]] | |||
* Fasting [[ketosis]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''GSD type IXb'''<ref name="pmid6938920">{{cite journal| author=Bashan N, Iancu TC, Lerner A, Fraser D, Potashnik R, Moses SW| title=Glycogenosis due to liver and muscle phosphorylase kinase deficiency. | journal=Pediatr Res | year= 1981 | volume= 15 | issue= 4 Pt 1 | pages= 299-303 | pmid=6938920 | doi=10.1203/00006450-198104000-00002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6938920 }} </ref><ref name="pmid6422139">{{cite journal| author=Gray RG, Kumar D, Whitfield AE| title=Glycogen phosphorylase b kinase deficiency in three siblings. | journal=J Inherit Metab Dis | year= 1983 | volume= 6 | issue= 3 | pages= 107 | pmid=6422139 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6422139 }} </ref><ref name="pmid9215682">{{cite journal| author=Burwinkel B, Maichele AJ, Aagenaes O, Bakker HD, Lerner A, Shin YS et al.| title=Autosomal glycogenosis of liver and muscle due to phosphorylase kinase deficiency is caused by mutations in the phosphorylase kinase beta subunit (PHKB). | journal=Hum Mol Genet | year= 1997 | volume= 6 | issue= 7 | pages= 1109-15 | pmid=9215682 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9215682 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |Phosphorylase b kinase (deficiency in liver and muscle) | |||
| style="background:#F5F5F5;" align="center" + |[[PHKB]] gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |16q12 | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* [[Hyperlipidemia]] | |||
* Elevated liver aminotransferases | |||
|- | |||
| colspan="3" style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type X'''<ref name="pmid10545043">{{cite journal| author=Hadjigeorgiou GM, Kawashima N, Bruno C, Andreu AL, Sue CM, Rigden DJ et al.| title=Manifesting heterozygotes in a Japanese family with a novel mutation in the muscle-specific phosphoglycerate mutase (PGAM-M) gene. | journal=Neuromuscul Disord | year= 1999 | volume= 9 | issue= 6-7 | pages= 399-402 | pmid=10545043 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10545043 }} </ref><ref name="pmid8447317">{{cite journal| author=Tsujino S, Shanske S, Sakoda S, Fenichel G, DiMauro S| title=The molecular genetic basis of muscle phosphoglycerate mutase (PGAM) deficiency. | journal=Am J Hum Genet | year= 1993 | volume= 52 | issue= 3 | pages= 472-7 | pmid=8447317 | doi= | pmc=1682163 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8447317 }} </ref><ref name="pmid2987758">{{cite journal| author=Kissel JT, Beam W, Bresolin N, Gibbons G, DiMauro S, Mendell JR| title=Physiologic assessment of phosphoglycerate mutase deficiency: incremental exercise test. | journal=Neurology | year= 1985 | volume= 35 | issue= 6 | pages= 828-33 | pmid=2987758 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2987758 }} </ref><ref name="pmid6262916">{{cite journal| author=DiMauro S, Miranda AF, Khan S, Gitlin K, Friedman R| title=Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy. | journal=Science | year= 1981 | volume= 212 | issue= 4500 | pages= 1277-9 | pmid=6262916 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6262916 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |[[Phosphoglycerate mutase]] | |||
| style="background:#F5F5F5;" align="center" + |[[PGAM2]] gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |7p13 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* [[Myoglobinuria]] | |||
* [[Gout]] (tophy) | |||
* Severe [[coronary]] [[arteriosclerosis]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type XI'''<ref name="pmid3789777">{{cite journal| author=Yoshikuni K, Tagami H, Yamada M, Sudo K, Kanno T| title=Erythematosquamous skin lesions in hereditary lactate dehydrogenase M-subunit deficiency. | journal=Arch Dermatol | year= 1986 | volume= 122 | issue= 12 | pages= 1420-4 | pmid=3789777 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3789777 }} </ref><ref name="pmid3383424">{{cite journal| author=Kanno T, Sudo K, Maekawa M, Nishimura Y, Ukita M, Fukutake K| title=Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy. | journal=Clin Chim Acta | year= 1988 | volume= 173 | issue= 1 | pages= 89-98 | pmid=3383424 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3383424 }} </ref><ref name="pmid3092644">{{cite journal| author=Maekawa M, Sudo K, Kanno T| title=Immunochemical studies on lactate dehydrogenase A subunit deficiencies. | journal=Am J Hum Genet | year= 1986 | volume= 39 | issue= 2 | pages= 232-8 | pmid=3092644 | doi= | pmc=1683931 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3092644 }} </ref><ref name="pmid1999544">{{cite journal| author=Takayasu S, Fujiwara S, Waki T| title=Hereditary lactate dehydrogenase M-subunit deficiency: lactate dehydrogenase activity in skin lesions and in hair follicles. | journal=J Am Acad Dermatol | year= 1991 | volume= 24 | issue= 2 Pt 2 | pages= 339-42 | pmid=1999544 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1999544 }} </ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |'''Lactate dehydrogenase A deficiency''' | |||
| style="background:#F5F5F5;" align="center" + |[[Lactate dehydrogenase A]] | |||
| style="background:#F5F5F5;" align="center" + |LDHA gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |11p15 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* Muscle [[stiffness]] | |||
* [[Lactic acidosis]] | |||
* [[Myoglobinuria]] | |||
* Easy [[fatigue]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type XII'''<ref name="pmid2825199">{{cite journal| author=Kishi H, Mukai T, Hirono A, Fujii H, Miwa S, Hori K| title=Human aldolase A deficiency associated with a hemolytic anemia: thermolabile aldolase due to a single base mutation. | journal=Proc Natl Acad Sci U S A | year= 1987 | volume= 84 | issue= 23 | pages= 8623-7 | pmid=2825199 | doi= | pmc=299598 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2825199 }} </ref><ref name="pmid4788792">{{cite journal| author=Beutler E, Scott S, Bishop A, Margolis N, Matsumoto F, Kuhl W| title=Red cell aldolase deficiency and hemolytic anemia: a new syndrome. | journal=Trans Assoc Am Physicians | year= 1973 | volume= 86 | issue= | pages= 154-66 | pmid=4788792 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4788792 }} </ref><ref name="pmid8598869">{{cite journal| author=Kreuder J, Borkhardt A, Repp R, Pekrun A, Göttsche B, Gottschalk U et al.| title=Brief report: inherited metabolic myopathy and hemolysis due to a mutation in aldolase A. | journal=N Engl J Med | year= 1996 | volume= 334 | issue= 17 | pages= 1100-4 | pmid=8598869 | doi=10.1056/NEJM199604253341705 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8598869 }} </ref><ref name="pmid3688035">{{cite journal| author=Hurst JA, Baraitser M, Winter RM| title=A syndrome of mental retardation, short stature, hemolytic anemia, delayed puberty, and abnormal facial appearance: similarities to a report of aldolase A deficiency. | journal=Am J Med Genet | year= 1987 | volume= 28 | issue= 4 | pages= 965-70 | pmid=3688035 | doi=10.1002/ajmg.1320280423 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3688035 }} </ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |'''Aldolase A deficiency''' | |||
| style="background:#F5F5F5;" align="center" + |[[Aldolase A]] | |||
| style="background:#F5F5F5;" align="center" + |ALDOA gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |16p11 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* [[Hemolytic anemia]] | |||
* [[Splenomegaly]] | |||
|- | |||
| colspan="3" style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type XIII<ref name="pmid11506403">{{cite journal| author=Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A, Jann S et al.| title=Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. | journal=Ann Neurol | year= 2001 | volume= 50 | issue= 2 | pages= 202-7 | pmid=11506403 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11506403 }} </ref>''' | |||
| style="background:#F5F5F5;" align="center" + |Beta-enolase | |||
| style="background:#F5F5F5;" align="center" + | ENO3 gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |17p13 | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
|- | |||
| colspan="3" style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type XIV'''<ref name="pmid24499211">{{cite journal| author=Tegtmeyer LC, Rust S, van Scherpenzeel M, Ng BG, Losfeld ME, Timal S et al.| title=Multiple phenotypes in phosphoglucomutase 1 deficiency. | journal=N Engl J Med | year= 2014 | volume= 370 | issue= 6 | pages= 533-42 | pmid=24499211 | doi=10.1056/NEJMoa1206605 | pmc=4373661 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24499211 }} </ref><ref name="pmid19625727">{{cite journal| author=Stojkovic T, Vissing J, Petit F, Piraud M, Orngreen MC, Andersen G et al.| title=Muscle glycogenosis due to phosphoglucomutase 1 deficiency. | journal=N Engl J Med | year= 2009 | volume= 361 | issue= 4 | pages= 425-7 | pmid=19625727 | doi=10.1056/NEJMc0901158 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19625727 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |[[Phosphoglucomutase]] type 2 | |||
| style="background:#F5F5F5;" align="center" + |[[PGM1]] gene mutation | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |1p31 | |||
| style="background:#F5F5F5;" align="center" + | +/- | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* Elevated liver aminotransferases | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Glycogen storage disease type 0'''<ref name="pmid9691087">{{cite journal| author=Orho M, Bosshard NU, Buist NR, Gitzelmann R, Aynsley-Green A, Blümel P et al.| title=Mutations in the liver glycogen synthase gene in children with hypoglycemia due to glycogen storage disease type 0. | journal=J Clin Invest | year= 1998 | volume= 102 | issue= 3 | pages= 507-15 | pmid=9691087 | doi=10.1172/JCI2890 | pmc=508911 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9691087 }} </ref><ref name="pmid12794686">{{cite journal| author=Laberge AM, Mitchell GA, van de Werve G, Lambert M| title=Long-term follow-up of a new case of liver glycogen synthase deficiency. | journal=Am J Med Genet A | year= 2003 | volume= 120A | issue= 1 | pages= 19-22 | pmid=12794686 | doi=10.1002/ajmg.a.20110 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12794686 }} </ref><ref name="pmid8831078">{{cite journal| author=Gitzelmann R, Spycher MA, Feil G, Müller J, Seilnacht B, Stahl M et al.| title=Liver glycogen synthase deficiency: a rarely diagnosed entity. | journal=Eur J Pediatr | year= 1996 | volume= 155 | issue= 7 | pages= 561-7 | pmid=8831078 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8831078 }} </ref><ref name="pmid11483824">{{cite journal| author=Rutledge SL, Atchison J, Bosshard NU, Steinmann B| title=Case report: liver glycogen synthase deficiency--a cause of ketotic hypoglycemia. | journal=Pediatrics | year= 2001 | volume= 108 | issue= 2 | pages= 495-7 | pmid=11483824 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11483824 }} </ref> | |||
| colspan="2" style="background:#DCDCDC;" align="center" + |'''Lewis' disease''' | |||
| style="background:#F5F5F5;" align="center" + |Hepatic [[glycogen synthase]] | |||
| style="background:#F5F5F5;" align="center" + |GYS2 gene mutation (liver) | |||
| style="background:#F5F5F5;" align="center" + |[[Autosomal recessive]] | |||
| style="background:#F5F5F5;" align="center" + |12p12 | |||
| style="background:#F5F5F5;" align="center" + | + | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" align="center" + | - | |||
| style="background:#F5F5F5;" + | | |||
* Fasting [[hypoglycemia]] and [[ketosis]] | |||
* Postprandial [[hyperglycemia]] and [[Lactic acidosis (patient information)|lactic acidosis]] | |||
|} | |||
</small> | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
*The prevalence of | *The prevalence of glycogen storage type disease VI is approximately 1 per 100,000 individuals worldwide. | ||
*In the Mennonite population, the prevalence of | *In the Mennonite population, the prevalence of glycogen storage type VI is 1 per 1000 individuals due to defect in founder variant.<ref name="pmid9536091">{{cite journal |vauthors=Chang S, Rosenberg MJ, Morton H, Francomano CA, Biesecker LG |title=Identification of a mutation in liver glycogen phosphorylase in glycogen storage disease type VI |journal=Hum. Mol. Genet. |volume=7 |issue=5 |pages=865–70 |year=1998 |pmid=9536091 |doi= |url=}}</ref> | ||
* | *Glycogen storage type disease VI usually develops in early [[childhood]]. | ||
* | *Glycogen storage type disease VI affects individuals of the Mennonite religious group. | ||
*One of the forms of liver phosphorylase kinase deficiency is X-linked recessive | *One of the forms of liver [[Phosphorylase B kinase|phosphorylase B kinase]] deficiency is [[X-linked recessive]] present in affected males, although asymptomatic [[Male|males]] and heterozygous (carrier) [[females]] presents with mild symptoms. All other types of glycogen storage type disease VI is [[autosomal recessive]] affects men and women equally. | ||
==Risk Factors== | ==Risk Factors== | ||
The most potent risk factor in the development of glycogen storage disease type VI is a family member with glycogen storage disease type VI. | |||
The most potent risk factor in the development of | |||
==Screening== | ==Screening== | ||
*Glycogen storage disease type VI is an [[autosomal recessive]] disease. | *Glycogen storage disease type VI is an [[autosomal recessive]] disease and some forms are [[X-linked recessive]]. | ||
*[[Carrier]] [[Screening (medicine)|screening]] of at-risk relatives may be done. | *[[Carrier]] [[Screening (medicine)|screening]] of at-risk relatives may be done. | ||
*[[Screening (medicine)|Screening]] requires prior PYGL | *[[Screening (medicine)|Screening]] requires prior PYGL identification of variants in the family. | ||
*Prenatal diagnosis and preimplantation genetic diagnosis (PGD) for at-risk pregnancies | *[[Prenatal diagnosis]] and [[preimplantation genetic diagnosis]] (PGD) for at-risk [[pregnancies]] may be done.<ref name="urlGlycogen storage disease type VI - Genetics Home Reference">{{cite web |url=https://ghr.nlm.nih.gov/condition/glycogen-storage-disease-type-vi |title=Glycogen storage disease type VI - Genetics Home Reference |format= |work= |accessdate=}}</ref> | ||
==Natural History, Complications, and Prognosis== | ==Natural History, Complications, and Prognosis== | ||
If left untreated, | *If left untreated, 1% of patients with glycogen storage disease type VI may progress to [[hepatocellular carcinoma]].<ref name="pmid25266922">{{cite journal |vauthors=Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J, Raiman J, Schulze A, Siriwardena K, Mercimek-Mahmutoglu S |title=The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada |journal=Mol. Genet. Metab. |volume=113 |issue=3 |pages=171–6 |year=2014 |pmid=25266922 |doi=10.1016/j.ymgme.2014.09.005 |url=}}</ref> | ||
*Other complications include [[cardiomyopathy]]. | |||
*Prognosis is generally excellent if symptoms are controlled with [[diet]]. | |||
Prognosis is generally excellent | |||
==Diagnosis== | ==Diagnosis== | ||
===Diagnostic | ===Diagnostic Study of Choice=== | ||
There are no established criteria for the diagnosis of | There are no established criteria for the [[diagnosis]] of glycogen storage disease type VI. | ||
===History and Symptoms=== | ===History and Symptoms=== | ||
*Glycogen storage disease type VI presents at the age of 1-5 years. | *Glycogen storage disease type VI presents at the age of 1-5 years. | ||
*A positive history of the protuberant abdomen, growth retardation and the slight delay in motor milestones is suggestive of | *A positive history of the protuberant abdomen, [[growth retardation]] and the slight delay in motor milestones is suggestive of glycogen storage disease type VI. | ||
*Some children have the history of mild fasting hypoglycemia and hypotonia. | *Some children have the history of mild fasting [[hypoglycemia]] and [[hypotonia]]. | ||
Common symptoms of | |||
* | * Common symptoms of glycogen storage disease type VI include: | ||
**Symptoms of [[hypoglycemia]] on fasting such as [[faintness]], [[weakness]], and [[nervousness]]. | |||
===Physical Examination=== | ===Physical Examination=== | ||
Patients with | *Patients with glycogen storage disease type VI usually appear normal. | ||
*Physical examination of patients with glycogen storage disease type VI is usually remarkable for [[hepatomegaly]]. | |||
*In a young child, [[Delayed milestone|delay in motor milestones]], mild [[hypotonia]] and [[muscle weakness]]. | |||
===Laboratory Findings=== | ===Laboratory Findings=== | ||
*Laboratory findings consistent with the diagnosis of | *Laboratory findings consistent with the diagnosis of glycogen storage disease type VI include: | ||
**Serum triglycerides, cholesterol, and liver transaminases are slightly increased. | **Serum [[triglycerides]], [[cholesterol]], and liver [[transaminases]] are slightly increased. | ||
**Creatine kinase is normal. | **[[Creatine kinase]] is normal. | ||
**Uric acid and lactic acid is normal. | **[[Uric acid]] and [[lactic acid]] is normal. | ||
**Glucose does not increase following glucagon administration confirms hypoglycemia. | **[[Glucose]] does not increase following [[glucagon]] administration confirms [[hypoglycemia]]. | ||
'''Fasting test''': | '''Fasting test''': | ||
*The blood glucose level is assessed after 3-5 hour of fasting, mild hypoglycemia is noticed. | *The [[blood glucose]] level is assessed after 3-5 hour of fasting, mild [[hypoglycemia]] is noticed. | ||
*The urine ketones and serum ketone bodies (eg, acetoacetate, beta-hydroxybutyrate) after few hours of fasting is raised. | *The urine [[Ketone|ketones]] and serum [[ketone bodies]] (eg, [[acetoacetate]], [[beta-hydroxybutyrate]]) after few hours of fasting is raised. | ||
===Electrocardiogram=== | ===Electrocardiogram=== | ||
There are no ECG findings associated with | There are no ECG findings associated with glycogen storage disease type VI. | ||
===X-ray=== | ===X-ray=== | ||
There are no X-ray findings associated with glycogen storage type VI disease. | |||
=== Ultrasound=== | === Ultrasound=== | ||
Ultrasound may be helpful in the diagnosis of | Ultrasound may be helpful in the diagnosis of glycogen storage disease type VI. Findings on an ultrasound suggestive of glycogen storage disease type VI include [[hepatomegaly]]. | ||
===CT scan=== | ===CT scan=== | ||
CT scan may be helpful in the diagnosis of | CT scan may be helpful in the diagnosis of glycogen storage disease type VI. Findings on CT scan suggestive of glycogen storage disease type VI is [[hepatomegaly]]. | ||
===MRI=== | ===MRI=== | ||
MRI may be helpful in the diagnosis of | MRI may be helpful in the diagnosis of glycogen storage disease type VI. Findings on MRI suggestive of glycogen storage disease type VI is [[hepatomegaly]]. | ||
===Other Imaging Findings=== | ===Other Imaging Findings=== | ||
*Other imaging studies can be in glycogen storage disease type VI is [[Bone scan]] | |||
===Other Diagnostic Studies=== | ===Other Diagnostic Studies=== | ||
Findings suggestive of | ==== Molecular genetic testing ==== | ||
*Glycogen distended liver cells are seen. | Molecular genetic testing is done under the following conditions : | ||
*Glycogen content is increased to four times in liver cells than muscle cells. | *Children with [[hepatomegaly]] and ketotic [[hypoglycemia]]. | ||
*The accumulated glycogen ( | *Children with unexplained [[hepatomegaly]] with a mild-moderate elevation of [[transaminase]]. | ||
* | [[Liver biopsy]] is reserved for those in whom the diagnosis cannot be confirmed by molecular [[Genetics|genetic]] techniques. | ||
==== Enzyme activity assay ==== | |||
*Assay of hepatic [[glycogen]] [[phosphorylase]] enzyme activity can be performed on [[red blood cells]], [[leukocytes]], and [[liver cells]]. | |||
==== '''Liver biopsy''' ==== | |||
The liver biopsy is helpful in the diagnosis of glycogen storage disease type VI. | |||
Findings suggestive of glycogen storage disease type VI include:<ref name="urlglycogen storage disease type 6 - Humpath.com - Human pathology">{{cite web |url=https://www.humpath.com/spip.php?article15594 |title=glycogen storage disease type 6 - Humpath.com - Human pathology |format= |work= |accessdate=}}</ref> | |||
*[[Glycogen]] distended [[liver cells]] are seen. | |||
*[[Glycogen]] content is increased to four times in liver cells than [[muscle cells]]. | |||
*The accumulated [[glycogen]] ( alpha particles, rosette form) looks frayed or burst. | |||
*[[Interlobular bile ducts|Interlobular]] [[fibrous]] [[septa]] and low-grade [[inflammatory]] changes are seen. | |||
==Treatment== | ==Treatment== | ||
===Medical Therapy=== | ===Medical Therapy=== | ||
*The mainstay of treatment is dietary therapy.<ref name="pmid21634085">{{cite journal |vauthors=Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, Goldstein J, Austin S, Kishnani P, Bali D |title= |journal= |volume= |issue= |pages= |year= |pmid=21634085 |doi= |url=}}</ref> | *The mainstay of treatment is dietary therapy.<ref name="pmid21634085">{{cite journal |vauthors=Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, Goldstein J, Austin S, Kishnani P, Bali D |title= |journal= |volume= |issue= |pages= |year= |pmid=21634085 |doi= |url=}}</ref><ref name="urlHers Disease - NORD (National Organization for Rare Disorders)">{{cite web |url=https://rarediseases.org/rare-diseases/hers-disease/ |title=Hers Disease - NORD (National Organization for Rare Disorders) |format= |work= |accessdate=}}</ref> | ||
*Most of the patient has better growth with therapy but some doesn't require therapy. | *Most of the patient has better growth with therapy but some doesn't require therapy. | ||
*Dietary therapy includes frequent meals, high carbohydrate diet, high protein diet and supplementation of unsaturated fats. | *Dietary therapy includes frequent meals, high [[carbohydrate]] diet, high [[protein]] diet and supplementation of [[unsaturated fats]]. | ||
*Therapy depends on the symptoms of the patient: | *Therapy depends on the symptoms of the patient: | ||
**Before starting therapy, it is always better to measure blood glucose level. | **Before starting therapy, it is always better to measure [[blood glucose]] level. | ||
**For the hypoglycemic patient, frequent small meals and uncooked cornstarch 1.5-2 g/kg TID normalize blood glucose concentration and avoid ketosis. | **For the [[hypoglycemic]] patient, frequent small meals and uncooked [[cornstarch]] 1.5-2 g/kg TID normalize [[blood glucose]] concentration and avoid [[ketosis]]. | ||
**For children and adults with no hypoglycemic episodes, a bedtime dose of cornstarch 1.5-2 g/kg is given to normalize blood glucose. | **For children and adults with no [[hypoglycemic]] episodes, a bedtime dose of [[cornstarch]] 1.5-2 g/kg is given to normalize [[blood glucose]]. | ||
**For infants(<6 months), cornstarch causes gastrointestinal distress, it should be avoided. | **For infants (<6 months), [[cornstarch]] causes [[gastrointestinal]] distress, it should be avoided. | ||
===Surgery=== | ===Surgery=== | ||
Surgical intervention is not recommended for the management of | Surgical intervention is not recommended for the management of glycogen storage disease type VI. | ||
===Primary Prevention=== | ===Primary Prevention=== | ||
Effective measures for primary prevention of glycogen storage disease type VI include:<ref name="pmid7957405">{{cite journal |vauthors=Nakai A, Shigematsu Y, Takano T, Kikawa Y, Sudo M |title=Uncooked cornstarch treatment for hepatic phosphorylase kinase deficiency |journal=Eur. J. Pediatr. |volume=153 |issue=8 |pages=581–3 |year=1994 |pmid=7957405 |doi= |url=}}</ref> | Effective measures for primary prevention of glycogen storage disease type VI include:<ref name="pmid7957405">{{cite journal |vauthors=Nakai A, Shigematsu Y, Takano T, Kikawa Y, Sudo M |title=Uncooked cornstarch treatment for hepatic phosphorylase kinase deficiency |journal=Eur. J. Pediatr. |volume=153 |issue=8 |pages=581–3 |year=1994 |pmid=7957405 |doi= |url=}}</ref> | ||
* '''Genetic counseling:''' [[Genetic counseling]] should be offered to all parents with a child with GSD type VI. | * '''Genetic counseling:''' [[Genetic counseling]] should be offered to all parents with a child with GSD type VI. | ||
* '''Prenatal diagnosis:''' The preferred method for [[prenatal diagnosis]] is molecular testing when PGYL [[mutation]] is known. Mutation analysis is performed either on cultured chorionic villus samples or amniocytes. | * '''Prenatal diagnosis:''' The preferred method for [[prenatal diagnosis]] is molecular testing when PGYL [[mutation]] is known. Mutation analysis is performed either on cultured [[chorionic villus]] samples or amniocytes. | ||
* '''Screening:''' The [[Probands|proband's]] PGYL [[Mutation|mutations]] should be determined for diagnosis and direct further testing for family members. | * '''Screening:''' The [[Probands|proband's]] PGYL [[Mutation|mutations]] should be determined for diagnosis and direct further testing for family members. | ||
==Secondary Prevention== | ==Secondary Prevention== | ||
Effective measures for the secondary prevention of Her's disease include: | Effective measures for the secondary prevention of Her's disease include: | ||
*Osteoporosis | *Osteoporosis is common in glycogen storage disease type VI. Treatment includes: | ||
*Short stature and delayed puberty | ** Complex [[carbohydrates]] or [[cornstarch]] improves [[bone density]]. | ||
Surveillance | *[[Short stature]] and [[delayed puberty]] occurs due to chronic [[ketosis]], may improve with better metabolic control. | ||
*Routine monitoring of blood glucose concentration and blood ketones | === Surveillance: === | ||
*Monitoring of blood ketones | *Routine monitoring of [[blood glucose]] concentration and blood [[ketones]] is recommended especially for increased activity and illness. | ||
*Monitoring of blood glucose concentrations at 2 AM to 4 AM can | *Monitoring of blood [[ketones]] every morning and several times per month using a portable blood [[ketone]] meter is recommended. The goal is to maintain blood [[beta-hydroxybutyrate]] concentrations lower than 0.3 mmol/L. | ||
*Height and weight should be measured to monitor growth every year. | *Monitoring of [[blood glucose]] concentrations at 2 AM to 4 AM can predict the time of suboptimal control. | ||
*Liver ultrasound examinations are recommended starts at age five years should be done every year. | *[[Height]] and [[weight]] should be measured to monitor growth every year. | ||
*Bone density | *Liver [[ultrasound]] examinations are recommended starts at age five years should be done every year. | ||
Agents to avoid | *[[Bone density]] measurement are recommended after [[growth]] is complete. | ||
* | '''Agents to avoid''': | ||
*Glucagon administration as a rescue therapy for hypoglycemia | *To prevent excessive hepatic [[glycogen]] deposition, amounts of simple [[sugars]] should be limited. | ||
*Growth hormone | *[[Glucagon]] administration as a rescue therapy for [[hypoglycemia]]. | ||
*When hepatomegaly is present, contact sports are avoided. | *[[Growth hormone]] usually exacerbates [[ketosis]]. | ||
*When [[hepatomegaly]] is present, contact sports are avoided. | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Endocrinology]] | |||
[[Category:Hepatology]] | |||
[[Category:Gastroenterology]] | |||
[[Category:Pediatrics]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Genetic disorders]] | |||
[[Category:Metabolic disorders]] | |||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 14:23, 29 March 2018
For the main page on glycogen storage disease, please click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Manpreet Kaur, MD [2], Anmol Pitliya, M.B.B.S. M.D.[3]
Synonyms and keywords:Her's disease; phosphorylase deficiency glycogen-storage disease of liver; glycogen storage disease type 6; GSD IV; GSD type 6
Overview
Glycogen storage type disease VI is caused by the deficiency of phosphorylase B kinase. In 1959, Dr. Hers first discovered glycogen storage type VI disease in the patients with liver phosphorylase deficiency. Glycogen storage type disease VI is an autosomal recessive disease and some forms are X-linked recessive. Glycogen storage disease type VI is classified according to the pattern of inheritance associated with the enzyme deficiency into 2 subtypes, autosomal recessive liver phosphorylase kinase deficiency and x-linked recessive liver phosphorylase kinase deficiency. Glycogen storage disease type VI presents at the age of 1-5 years. A positive history of the protuberant abdomen, growth retardation and the slight delay in motor milestones is suggestive of glycogen storage disease type VI and some children have the history of mild fasting hypoglycemia and hypotonia.Patient with the glycogen storage disease type VI presents with the symptoms of hypoglycemia on fasting, such as faintness, weakness, and nervousness. On physical examination, the increased liver span is present. The mainstay of treatment is the dietary therapy which includes frequent meals, high carbohydrate diet, high protein diet and supplementation of unsaturated fats.
Historical Perspective
- In 1959, Dr. Hers first discovered glycogen storage type disease VI in the patients with liver phosphorylase deficiency.[1]
- In 1960, Stetten & Stetten described glycogen storage type disease VI disease after its initial discovery.[2]
- In 1987, gene mutations encoding liver phosphorylase present on chromosome 14q21-q22 became known to be associated with the pathogenesis of glycogen storage type disease VI disease.[3]
Classification
Glycogen storage disease type VI is classified according to the pattern of inheritance associated with the enzyme deficiency into 2 subtypes:[4][5]
- Autosomal recessive liver phosphorylase kinase deficiency
- X-linked recessive liver phosphorylase kinase deficiency
- It is further classified into two types:
- X-linked recessive liver phosphorylase kinase deficiency classical type (type I)
- X-linked recessive liver phosphorylase kinase deficiency variant type (type II)
| Glycogen storage disease type VI | |||||||||||||||||||||||||||||||||||||||
| Autosomal recessive liver phosphorylase kinase deficiency | X-linked recessive liver phosphorylase kinase deficiency | ||||||||||||||||||||||||||||||||||||||
| X-linked recessive liver phosphorylase kinase deficiency classical type (type I) | X-linked recessive liver phosphorylase kinase deficiency variant type (type II) | ||||||||||||||||||||||||||||||||||||||
Pathophysiology
Pathogenesis
- Glycogen storage disease type VI is caused by the deficiency of liver phosphorylase B kinase.
- The rate-limiting enzyme of glycogen breakdown is phosphorylase, which is activated by a series of enzymes, including adenyl cyclase, phosphorylase b kinase, and cAMP-dependent protein kinase.
- Enzyme deficiency leads to the impaired breakdown of glycogen into glucose.
- In most patients, the enzyme deficiency is incomplete, and gluconeogenesis remains intact.
- Due to impaired functioning of liver phosphorylase, there is difficulty to maintain glucose level at fasting, leading to hypoglycemia at fasting.
- There is also associated hyperketosis and increased levels of urinary ketones and serum ketone bodies.
- There is mild-moderate hyperlipidemia and increased levels of transaminases.[6][7]
Metabolic Pathway
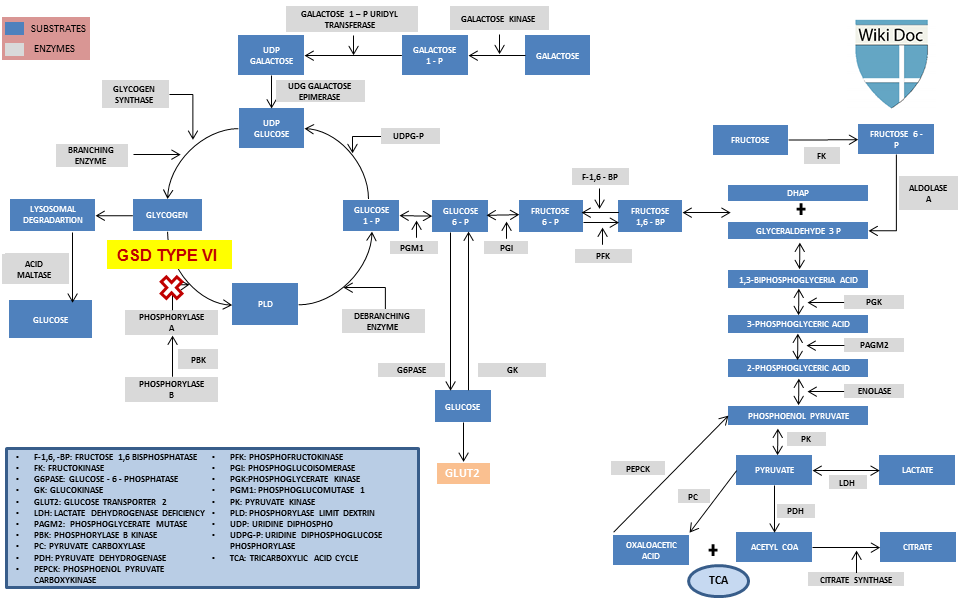
Causes
- The most common cause of glycogen storage disease type VI is the deficiency of liver phosphorylase b kinase, an enzyme that activates phosphorylase by phosphorylation.[8]
- Phosphorylase b kinase has 4 subunits, each is encoded by different genes present on different chromosomes.
- Mutations in three subunits (PHKA2, PHKB, and PHKG2) is most commonly seen in patients with phosphorylase b kinase deficiency.
- If there is the mutation only in PHKG2, then the patient has significant liver fibrosis and cirrhosis.[9][10]
Differentiating Glycogen storage disease type VI from Other Diseases
| Differentiating Glycogen Storage Diseases | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen storage disease | Enzyme deficiency | Genetics | History and symptoms | Physical examination | Laboratory findings | Imaging | Other features | |||||||
| Gene mutation | Inheritance | Chromosome | Hypoglycemia | Muscle weakness | Hypotonia | Hepatomegaly | Elevated CK | Cardiomegaly | ||||||
| Glycogen storage disease type I[11][12][13][14][15][16] | Von Gierke's disease | GSD type Ia | Glucose-6-phosphatase | G6PC gene mutation | Autosomal recessive | 17q21 | + | + | + | + | - | - | ||
| GSD type Ib | Microsomal glucose-6-phosphate transporter | SLC37A4 gene mutation | Autosomal recessive | 11q23 | ||||||||||
| Glycogen storage disease type II[17][18][19][20][21][22][23][24][25] | Pompe disease | Infantile onset | Acid alpha-glucosidase | GAA gene | Autosomal recessive | 17q25 | - | + | + | + | + | + | ||
| Late onset | Autosomal recessive | - | + | + | + | + | +/- | |||||||
| Glycogen storage disease type III[26][27][28][29][30][31] | Cori disease | GSD type IIIa | Debranching enzyme (deficiency in muscle and liver) | AGL gene mutation | Autosomal recessive | 1p21 | + | + | + | + | + | + |
| |
| GSD type IIIb | Debranching enzyme (deficiency in liver only) | Autosomal recessive | ||||||||||||
| Glycogen storage disease type IV[32][33][34][35][36] | Andersen's disease | Branching enzyme | GBE1 gene mutation | Autosomal recessive | 3p12 | +/- | + | + | + | + | + | - | ||
| Glycogen storage disease type V[37][38][39][40][41][42][43] | McArdle disease | Muscle glycogen phosphorylase | PYGM gene mutation | Autosomal recessive | 11q13 | - | + | - | - | + | - |
| ||
| Glycogen storage disease type VI[44][45][5][46][47] | Hers' disease | Autosomal | Liver glycogen phosphorylase | PYGL gene mutation | Autosomal recessive | 14q22 | +/- | + | +/- | + | - | - |
| |
| X-linked | PYGL gene mutation | X-linked recessive | X | |||||||||||
| Glycogen storage disease type VII[48][49][50][51][52][53] | Tarui's disease | Muscle phosphofructokinase | PFKM gene mutation | Autosomal recessive | 12q13 | + | + | - | - | + | + | |||
| Glycogen storage disease type IX[54][45][55] | GSD type IXa[56][4][57][58][59] | Phosphorylase b kinase (deficiency in liver only) | PHKA2 gene mutation | X-linked recessive | Xp22 | + | - | - | + | - | - |
| ||
| GSD type IXb[60][61][62] | Phosphorylase b kinase (deficiency in liver and muscle) | PHKB gene mutation | Autosomal recessive | 16q12 | + | - | - | + | - | - |
| |||
| Glycogen storage disease type X[63][64][65][66] | Phosphoglycerate mutase | PGAM2 gene mutation | Autosomal recessive | 7p13 | - | - | - | - | + | - |
| |||
| Glycogen storage disease type XI[67][68][69][70] | Lactate dehydrogenase A deficiency | Lactate dehydrogenase A | LDHA gene mutation | Autosomal recessive | 11p15 | - | - | - | - | + | - |
| ||
| Glycogen storage disease type XII[71][72][73][74] | Aldolase A deficiency | Aldolase A | ALDOA gene mutation | Autosomal recessive | 16p11 | - | + | - | + | - | - | |||
| Glycogen storage disease type XIII[75] | Beta-enolase | ENO3 gene mutation | Autosomal recessive | 17p13 | - | + | - | - | + | - | - | |||
| Glycogen storage disease type XIV[76][77] | Phosphoglucomutase type 2 | PGM1 gene mutation | Autosomal recessive | 1p31 | +/- | + | - | - | + | - |
| |||
| Glycogen storage disease type 0[78][79][80][81] | Lewis' disease | Hepatic glycogen synthase | GYS2 gene mutation (liver) | Autosomal recessive | 12p12 | + | - | - | - | - | - |
| ||
Epidemiology and Demographics
- The prevalence of glycogen storage type disease VI is approximately 1 per 100,000 individuals worldwide.
- In the Mennonite population, the prevalence of glycogen storage type VI is 1 per 1000 individuals due to defect in founder variant.[46]
- Glycogen storage type disease VI usually develops in early childhood.
- Glycogen storage type disease VI affects individuals of the Mennonite religious group.
- One of the forms of liver phosphorylase B kinase deficiency is X-linked recessive present in affected males, although asymptomatic males and heterozygous (carrier) females presents with mild symptoms. All other types of glycogen storage type disease VI is autosomal recessive affects men and women equally.
Risk Factors
The most potent risk factor in the development of glycogen storage disease type VI is a family member with glycogen storage disease type VI.
Screening
- Glycogen storage disease type VI is an autosomal recessive disease and some forms are X-linked recessive.
- Carrier screening of at-risk relatives may be done.
- Screening requires prior PYGL identification of variants in the family.
- Prenatal diagnosis and preimplantation genetic diagnosis (PGD) for at-risk pregnancies may be done.[82]
Natural History, Complications, and Prognosis
- If left untreated, 1% of patients with glycogen storage disease type VI may progress to hepatocellular carcinoma.[45]
- Other complications include cardiomyopathy.
- Prognosis is generally excellent if symptoms are controlled with diet.
Diagnosis
Diagnostic Study of Choice
There are no established criteria for the diagnosis of glycogen storage disease type VI.
History and Symptoms
- Glycogen storage disease type VI presents at the age of 1-5 years.
- A positive history of the protuberant abdomen, growth retardation and the slight delay in motor milestones is suggestive of glycogen storage disease type VI.
- Some children have the history of mild fasting hypoglycemia and hypotonia.
- Common symptoms of glycogen storage disease type VI include:
- Symptoms of hypoglycemia on fasting such as faintness, weakness, and nervousness.
Physical Examination
- Patients with glycogen storage disease type VI usually appear normal.
- Physical examination of patients with glycogen storage disease type VI is usually remarkable for hepatomegaly.
- In a young child, delay in motor milestones, mild hypotonia and muscle weakness.
Laboratory Findings
- Laboratory findings consistent with the diagnosis of glycogen storage disease type VI include:
- Serum triglycerides, cholesterol, and liver transaminases are slightly increased.
- Creatine kinase is normal.
- Uric acid and lactic acid is normal.
- Glucose does not increase following glucagon administration confirms hypoglycemia.
Fasting test:
- The blood glucose level is assessed after 3-5 hour of fasting, mild hypoglycemia is noticed.
- The urine ketones and serum ketone bodies (eg, acetoacetate, beta-hydroxybutyrate) after few hours of fasting is raised.
Electrocardiogram
There are no ECG findings associated with glycogen storage disease type VI.
X-ray
There are no X-ray findings associated with glycogen storage type VI disease.
Ultrasound
Ultrasound may be helpful in the diagnosis of glycogen storage disease type VI. Findings on an ultrasound suggestive of glycogen storage disease type VI include hepatomegaly.
CT scan
CT scan may be helpful in the diagnosis of glycogen storage disease type VI. Findings on CT scan suggestive of glycogen storage disease type VI is hepatomegaly.
MRI
MRI may be helpful in the diagnosis of glycogen storage disease type VI. Findings on MRI suggestive of glycogen storage disease type VI is hepatomegaly.
Other Imaging Findings
- Other imaging studies can be in glycogen storage disease type VI is Bone scan
Other Diagnostic Studies
Molecular genetic testing
Molecular genetic testing is done under the following conditions :
- Children with hepatomegaly and ketotic hypoglycemia.
- Children with unexplained hepatomegaly with a mild-moderate elevation of transaminase.
Liver biopsy is reserved for those in whom the diagnosis cannot be confirmed by molecular genetic techniques.
Enzyme activity assay
- Assay of hepatic glycogen phosphorylase enzyme activity can be performed on red blood cells, leukocytes, and liver cells.
Liver biopsy
The liver biopsy is helpful in the diagnosis of glycogen storage disease type VI.
Findings suggestive of glycogen storage disease type VI include:[83]
- Glycogen distended liver cells are seen.
- Glycogen content is increased to four times in liver cells than muscle cells.
- The accumulated glycogen ( alpha particles, rosette form) looks frayed or burst.
- Interlobular fibrous septa and low-grade inflammatory changes are seen.
Treatment
Medical Therapy
- The mainstay of treatment is dietary therapy.[84][85]
- Most of the patient has better growth with therapy but some doesn't require therapy.
- Dietary therapy includes frequent meals, high carbohydrate diet, high protein diet and supplementation of unsaturated fats.
- Therapy depends on the symptoms of the patient:
- Before starting therapy, it is always better to measure blood glucose level.
- For the hypoglycemic patient, frequent small meals and uncooked cornstarch 1.5-2 g/kg TID normalize blood glucose concentration and avoid ketosis.
- For children and adults with no hypoglycemic episodes, a bedtime dose of cornstarch 1.5-2 g/kg is given to normalize blood glucose.
- For infants (<6 months), cornstarch causes gastrointestinal distress, it should be avoided.
Surgery
Surgical intervention is not recommended for the management of glycogen storage disease type VI.
Primary Prevention
Effective measures for primary prevention of glycogen storage disease type VI include:[86]
- Genetic counseling: Genetic counseling should be offered to all parents with a child with GSD type VI.
- Prenatal diagnosis: The preferred method for prenatal diagnosis is molecular testing when PGYL mutation is known. Mutation analysis is performed either on cultured chorionic villus samples or amniocytes.
- Screening: The proband's PGYL mutations should be determined for diagnosis and direct further testing for family members.
Secondary Prevention
Effective measures for the secondary prevention of Her's disease include:
- Osteoporosis is common in glycogen storage disease type VI. Treatment includes:
- Complex carbohydrates or cornstarch improves bone density.
- Short stature and delayed puberty occurs due to chronic ketosis, may improve with better metabolic control.
Surveillance:
- Routine monitoring of blood glucose concentration and blood ketones is recommended especially for increased activity and illness.
- Monitoring of blood ketones every morning and several times per month using a portable blood ketone meter is recommended. The goal is to maintain blood beta-hydroxybutyrate concentrations lower than 0.3 mmol/L.
- Monitoring of blood glucose concentrations at 2 AM to 4 AM can predict the time of suboptimal control.
- Height and weight should be measured to monitor growth every year.
- Liver ultrasound examinations are recommended starts at age five years should be done every year.
- Bone density measurement are recommended after growth is complete.
Agents to avoid:
- To prevent excessive hepatic glycogen deposition, amounts of simple sugars should be limited.
- Glucagon administration as a rescue therapy for hypoglycemia.
- Growth hormone usually exacerbates ketosis.
- When hepatomegaly is present, contact sports are avoided.
References
- ↑ HERS HG (1959). "[Enzymatic studies of hepatic fragments; application to the classification of glycogenoses]". Rev Int Hepatol (in French). 9 (1): 35–55. PMID 13646331.
- ↑ STETTEN D, STETTEN MR (1960). "Glycogen metabolism". Physiol. Rev. 40: 505–37. doi:10.1152/physrev.1960.40.3.505. PMID 13834511.
- ↑ Newgard CB, Fletterick RJ, Anderson LA, Lebo RV (1987). "The polymorphic locus for glycogen storage disease VI (liver glycogen phosphorylase) maps to chromosome 14". Am. J. Hum. Genet. 40 (4): 351–64. PMC 1684093. PMID 2883891.
- ↑ 4.0 4.1 Hendrickx J, Coucke P, Hors-Cayla MC, Smit GP, Shin YS, Deutsch J, Smeitink J, Berger R, Lee P, Fernandes J (1994). "Localization of a new type of X-linked liver glycogenosis to the chromosomal region Xp22 containing the liver alpha-subunit of phosphorylase kinase (PHKA2)". Genomics. 21 (3): 620–5. PMID 7959740.
- ↑ 5.0 5.1 Burwinkel B, Bakker HD, Herschkovitz E, Moses SW, Shin YS, Kilimann MW (1998). "Mutations in the liver glycogen phosphorylase gene (PYGL) underlying glycogenosis type VI". Am. J. Hum. Genet. 62 (4): 785–91. PMC 1377030. PMID 9529348.
- ↑ "Glycogen Storage Disease Type VI - GeneReviews® - NCBI Bookshelf".
- ↑ "Type VI Glycogen Storage Disease | Association for Glycogen Storage Disease".
- ↑ Burwinkel B, Rootwelt T, Kvittingen EA, Chakraborty PK, Kilimann MW (2003). "Severe phenotype of phosphorylase kinase-deficient liver glycogenosis with mutations in the PHKG2 gene". Pediatr. Res. 54 (6): 834–9. doi:10.1203/01.PDR.0000088069.09275.10. PMID 12930917.
- ↑ Albash B, Imtiaz F, Al-Zaidan H, Al-Manea H, Banemai M, Allam R, Al-Suheel A, Al-Owain M (2014). "Novel PHKG2 mutation causing GSD IX with prominent liver disease: report of three cases and review of literature". Eur. J. Pediatr. 173 (5): 647–53. doi:10.1007/s00431-013-2223-0. PMID 24326380.
- ↑ Chang S, Rosenberg MJ, Morton H, Francomano CA, Biesecker LG (1998). "Identification of a mutation in liver glycogen phosphorylase in glycogen storage disease type VI". Hum. Mol. Genet. 7 (5): 865–70. PMID 9536091.
- ↑ Mansfield BC (1999). "Molecular Genetics of Type 1 Glycogen Storage Diseases". Trends Endocrinol Metab. 10 (3): 104–113. PMID 10322403.
- ↑ Ozen H (2007). "Glycogen storage diseases: new perspectives". World J Gastroenterol. 13 (18): 2541–53. PMC 4146814. PMID 17552001.
- ↑ Froissart R, Piraud M, Boudjemline AM, Vianey-Saban C, Petit F, Hubert-Buron A; et al. (2011). "Glucose-6-phosphatase deficiency". Orphanet J Rare Dis. 6: 27. doi:10.1186/1750-1172-6-27. PMC 3118311. PMID 21599942.
- ↑ Kishnani, Priya S.; Austin, Stephanie L.; Abdenur, Jose E.; Arn, Pamela; Bali, Deeksha S.; Boney, Anne; Chung, Wendy K.; Dagli, Aditi I.; Dale, David; Koeberl, Dwight; Somers, Michael J.; Burns Wechsler, Stephanie; Weinstein, David A.; Wolfsdorf, Joseph I.; Watson, Michael S. (2014). "Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics". Genetics in Medicine. doi:10.1038/gim.2014.128. ISSN 1098-3600.
- ↑ Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP (2002). "Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I)". Eur. J. Pediatr. 161 Suppl 1: S20–34. doi:10.1007/s00431-002-0999-4. PMID 12373567.
- ↑ Bali DS, Chen YT, Austin S, et al. Glycogen Storage Disease Type I. 2006 Apr 19 [Updated 2016 Aug 25]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1312/
- ↑ Leslie N, Bailey L. Pompe Disease. 2007 Aug 31 [Updated 2017 May 11]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1261/
- ↑ Di Rocco M, Buzzi D, Tarò M (2007). "Glycogen storage disease type II: clinical overview". Acta Myol. 26 (1): 42–4. PMC 2949314. PMID 17915568.
- ↑ Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D; et al. (2006). "A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease". J Pediatr. 148 (5): 671–676. doi:10.1016/j.jpeds.2005.11.033. PMID 16737883.
- ↑ van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT; et al. (2003). "The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature". Pediatrics. 112 (2): 332–40. PMID 12897283.
- ↑ Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F (2000). "Identification of two subtypes of infantile acid maltase deficiency". J Pediatr. 137 (2): 283–5. doi:10.1067/mpd.2000.107112. PMID 10931430.
- ↑ Martiniuk F, Mehler M, Tzall S, Meredith G, Hirschhorn R (1990). "Sequence of the cDNA and 5'-flanking region for human acid alpha-glucosidase, detection of an intron in the 5' untranslated leader sequence, definition of 18-bp polymorphisms, and differences with previous cDNA and amino acid sequences". DNA Cell Biol. 9 (2): 85–94. doi:10.1089/dna.1990.9.85. PMID 2111708.
- ↑ Hoefsloot LH, Hoogeveen-Westerveld M, Kroos MA, van Beeumen J, Reuser AJ, Oostra BA (1988). "Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex". EMBO J. 7 (6): 1697–704. PMC 457155. PMID 3049072.
- ↑ Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, Oostra BA (1990). "Characterization of the human lysosomal alpha-glucosidase gene". Biochem J. 272 (2): 493–7. PMC 1149727. PMID 2268276.
- ↑ Kuo WL, Hirschhorn R, Huie ML, Hirschhorn K (1996). "Localization and ordering of acid alpha-glucosidase (GAA) and thymidine kinase (TK1) by fluorescence in situ hybridization". Hum Genet. 97 (3): 404–6. PMID 8786092.
- ↑ Shen J, Bao Y, Liu HM, Lee P, Leonard JV, Chen YT (1996). "Mutations in exon 3 of the glycogen debranching enzyme gene are associated with glycogen storage disease type III that is differentially expressed in liver and muscle". J Clin Invest. 98 (2): 352–7. doi:10.1172/JCI118799. PMC 507437. PMID 8755644.
- ↑ Ding JH, de Barsy T, Brown BI, Coleman RA, Chen YT (1990). "Immunoblot analyses of glycogen debranching enzyme in different subtypes of glycogen storage disease type III". J Pediatr. 116 (1): 95–100. PMID 2295969.
- ↑ Aoyama Y, Ozer I, Demirkol M, Ebara T, Murase T, Podskarbi T; et al. (2009). "Molecular features of 23 patients with glycogen storage disease type III in Turkey: a novel mutation p.R1147G associated with isolated glucosidase deficiency, along with 9 AGL mutations". J Hum Genet. 54 (11): 681–6. doi:10.1038/jhg.2009.100. PMID 19834502.
- ↑ Kishnani, Priya S; Austin, Stephanie L; Arn, Pamela; Bali, Deeksha S; Boney, Anne; Case, Laura E; Chung, Wendy K; Desai, Dev M; El-Gharbawy, Areeg; Haller, Ronald; Smit, G Peter A; Smith, Alastair D; Hobson-Webb, Lisa D; Wechsler, Stephanie Burns; Weinstein, David A; Watson, Michael S (2010). "Glycogen Storage Disease Type III diagnosis and management guidelines". Genetics in Medicine. 12 (7): 446–463. doi:10.1097/GIM.0b013e3181e655b6. ISSN 1098-3600.
- ↑ Dagli A, Sentner CP, Weinstein DA. Glycogen Storage Disease Type III. 2010 Mar 9 [Updated 2016 Dec 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26372/
- ↑ Wolfsdorf JI, Weinstein DA (2003). "Glycogen storage diseases". Rev Endocr Metab Disord. 4 (1): 95–102. PMID 12618563.
- ↑ Bruno C, van Diggelen OP, Cassandrini D, Gimpelev M, Giuffrè B, Donati MA; et al. (2004). "Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV)". Neurology. 63 (6): 1053–8. PMID 15452297.
- ↑ Bruno C, Cassandrini D, Assereto S, Akman HO, Minetti C, Di Mauro S (2007). "Neuromuscular forms of glycogen branching enzyme deficiency". Acta Myol. 26 (1): 75–8. PMC 2949312. PMID 17915577.
- ↑ Brown BI, Brown DH (1966). "Lack of an alpha-1,4-glucan: alpha-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis". Proc Natl Acad Sci U S A. 56 (2): 725–9. PMC 224432. PMID 5229990.
- ↑ McConkie-Rosell A, Wilson C, Piccoli DA, Boyle J, DeClue T, Kishnani P; et al. (1996). "Clinical and laboratory findings in four patients with the non-progressive hepatic form of type IV glycogen storage disease". J Inherit Metab Dis. 19 (1): 51–8. PMID 8830177.
- ↑ Magoulas PL, El-Hattab AW. Glycogen Storage Disease Type IV. 2013 Jan 3. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK115333/
- ↑ McARDLE B (1951). "Myopathy due to a defect in muscle glycogen breakdown". Clin Sci. 10 (1): 13–35. PMID 24540673.
- ↑ SCHMID R, MAHLER R (1959). "Chronic progressive myopathy with myoglobinuria: demonstration of a glycogenolytic defect in the muscle". J Clin Invest. 38: 2044–58. doi:10.1172/JCI103983. PMC 441792. PMID 14442994.
- ↑ Mommaerts WF, Illingworth B, Pearson CM, Guillory RJ, Seraydarian K (1959). "A FUNCTIONAL DISORDER OF MUSCLE ASSOCIATED WITH THE ABSENCE OF PHOSPHORYLASE". Proc Natl Acad Sci U S A. 45 (6): 791–7. PMC 222638. PMID 16590445.
- ↑ PEARSON CM, RIMER DG, MOMMAERTS WF (1961). "A metabolic myopathy due to absence of muscle phosphorylase". Am J Med. 30: 502–17. PMID 13733779.
- ↑ Grünfeld JP, Ganeval D, Chanard J, Fardeau M, Dreyfus JC (1972). "Acute renal failure in McArdle's disease. Report of two cases". N Engl J Med. 286 (23): 1237–41. doi:10.1056/NEJM197206082862304. PMID 4502558.
- ↑ Schmidt B, Servidei S, Gabbai AA, Silva AC, de Sousa Bulle de Oliveira A, DiMauro S (1987). "McArdle's disease in two generations: autosomal recessive transmission with manifesting heterozygote". Neurology. 37 (9): 1558–61. PMID 3476861.
- ↑ Martín MA, Lucía A, Arenas J, et al. Glycogen Storage Disease Type V. 2006 Apr 19 [Updated 2014 Jun 26]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1344/
- ↑ Wallis PG, Sidbury JB, Harris RC (1966). "Hepatic phosphorylase defect. Studies on peripheral blood". Am J Dis Child. 111 (3): 278–82. PMID 5904467.
- ↑ 45.0 45.1 45.2 Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J; et al. (2014). "The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada". Mol Genet Metab. 113 (3): 171–6. doi:10.1016/j.ymgme.2014.09.005. PMID 25266922.
- ↑ 46.0 46.1 Chang S, Rosenberg MJ, Morton H, Francomano CA, Biesecker LG (1998). "Identification of a mutation in liver glycogen phosphorylase in glycogen storage disease type VI". Hum Mol Genet. 7 (5): 865–70. PMID 9536091.
- ↑ Dagli AI, Weinstein DA. Glycogen Storage Disease Type VI. 2009 Apr 23 [Updated 2011 May 17]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK5941/
- ↑ Raben N, Sherman JB (1995). "Mutations in muscle phosphofructokinase gene". Hum Mutat. 6 (1): 1–6. doi:10.1002/humu.1380060102. PMID 7550225.
- ↑ TARUI S, OKUNO G, IKURA Y, TANAKA T, SUDA M, NISHIKAWA M (1965). "PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS". Biochem Biophys Res Commun. 19: 517–23. PMID 14339001.
- ↑ Layzer RB, Rowland LP, Ranney HM (1967). "Muscle phosphofructokinase deficiency". Arch Neurol. 17 (5): 512–23. PMID 4228297.
- ↑ Satoyoshi E, Kowa H (1967). "A myopathy due to glycolytic abnormality". Arch Neurol. 17 (3): 248–56. PMID 4228753.
- ↑ Waterbury L, Frenkel EP (1972). "Hereditary nonspherocytic hemolysis with erythrocyte phosphofructokinase deficiency". Blood. 39 (3): 415–25. PMID 4258222.
- ↑ Vora S, Corash L, Engel WK, Durham S, Seaman C, Piomelli S (1980). "The molecular mechanism of the inherited phosphofructokinase deficiency associated with hemolysis and myopathy". Blood. 55 (4): 629–35. PMID 6444532.
- ↑ Beauchamp NJ, Dalton A, Ramaswami U, Niinikoski H, Mention K, Kenny P; et al. (2007). "Glycogen storage disease type IX: High variability in clinical phenotype". Mol Genet Metab. 92 (1–2): 88–99. doi:10.1016/j.ymgme.2007.06.007. PMID 17689125.
- ↑ Goldstein J, Austin S, Kishnani P, et al. Phosphorylase Kinase Deficiency. 2011 May 31. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK55061/
- ↑ Keating JP, Brown BI, White NH, DiMauro S (1985). "X-linked glycogen storage disease. A cause of hypotonia, hyperuricemia, and growth retardation". Am J Dis Child. 139 (6): 609–13. PMID 3859203.
- ↑ Schimke RN, Zakheim RM, Corder RC, Hug G (1973). "Glycogen storage disease type IX: benign glycogenosis of liver and hepatic phosphorylase kinase deficiency". J Pediatr. 83 (6): 1031–4. PMID 4518931.
- ↑ Willems PJ, Gerver WJ, Berger R, Fernandes J (1990). "The natural history of liver glycogenosis due to phosphorylase kinase deficiency: a longitudinal study of 41 patients". Eur J Pediatr. 149 (4): 268–71. PMID 2303074.
- ↑ Hendrickx J, Bosshard NU, Willems P, Gitzelmann R (1998). "Clinical, biochemical and molecular findings in a patient with X-linked liver glycogenosis followed for 40 years". Eur J Pediatr. 157 (11): 919–23. PMID 9835437.
- ↑ Bashan N, Iancu TC, Lerner A, Fraser D, Potashnik R, Moses SW (1981). "Glycogenosis due to liver and muscle phosphorylase kinase deficiency". Pediatr Res. 15 (4 Pt 1): 299–303. doi:10.1203/00006450-198104000-00002. PMID 6938920.
- ↑ Gray RG, Kumar D, Whitfield AE (1983). "Glycogen phosphorylase b kinase deficiency in three siblings". J Inherit Metab Dis. 6 (3): 107. PMID 6422139.
- ↑ Burwinkel B, Maichele AJ, Aagenaes O, Bakker HD, Lerner A, Shin YS; et al. (1997). "Autosomal glycogenosis of liver and muscle due to phosphorylase kinase deficiency is caused by mutations in the phosphorylase kinase beta subunit (PHKB)". Hum Mol Genet. 6 (7): 1109–15. PMID 9215682.
- ↑ Hadjigeorgiou GM, Kawashima N, Bruno C, Andreu AL, Sue CM, Rigden DJ; et al. (1999). "Manifesting heterozygotes in a Japanese family with a novel mutation in the muscle-specific phosphoglycerate mutase (PGAM-M) gene". Neuromuscul Disord. 9 (6–7): 399–402. PMID 10545043.
- ↑ Tsujino S, Shanske S, Sakoda S, Fenichel G, DiMauro S (1993). "The molecular genetic basis of muscle phosphoglycerate mutase (PGAM) deficiency". Am J Hum Genet. 52 (3): 472–7. PMC 1682163. PMID 8447317.
- ↑ Kissel JT, Beam W, Bresolin N, Gibbons G, DiMauro S, Mendell JR (1985). "Physiologic assessment of phosphoglycerate mutase deficiency: incremental exercise test". Neurology. 35 (6): 828–33. PMID 2987758.
- ↑ DiMauro S, Miranda AF, Khan S, Gitlin K, Friedman R (1981). "Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy". Science. 212 (4500): 1277–9. PMID 6262916.
- ↑ Yoshikuni K, Tagami H, Yamada M, Sudo K, Kanno T (1986). "Erythematosquamous skin lesions in hereditary lactate dehydrogenase M-subunit deficiency". Arch Dermatol. 122 (12): 1420–4. PMID 3789777.
- ↑ Kanno T, Sudo K, Maekawa M, Nishimura Y, Ukita M, Fukutake K (1988). "Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy". Clin Chim Acta. 173 (1): 89–98. PMID 3383424.
- ↑ Maekawa M, Sudo K, Kanno T (1986). "Immunochemical studies on lactate dehydrogenase A subunit deficiencies". Am J Hum Genet. 39 (2): 232–8. PMC 1683931. PMID 3092644.
- ↑ Takayasu S, Fujiwara S, Waki T (1991). "Hereditary lactate dehydrogenase M-subunit deficiency: lactate dehydrogenase activity in skin lesions and in hair follicles". J Am Acad Dermatol. 24 (2 Pt 2): 339–42. PMID 1999544.
- ↑ Kishi H, Mukai T, Hirono A, Fujii H, Miwa S, Hori K (1987). "Human aldolase A deficiency associated with a hemolytic anemia: thermolabile aldolase due to a single base mutation". Proc Natl Acad Sci U S A. 84 (23): 8623–7. PMC 299598. PMID 2825199.
- ↑ Beutler E, Scott S, Bishop A, Margolis N, Matsumoto F, Kuhl W (1973). "Red cell aldolase deficiency and hemolytic anemia: a new syndrome". Trans Assoc Am Physicians. 86: 154–66. PMID 4788792.
- ↑ Kreuder J, Borkhardt A, Repp R, Pekrun A, Göttsche B, Gottschalk U; et al. (1996). "Brief report: inherited metabolic myopathy and hemolysis due to a mutation in aldolase A." N Engl J Med. 334 (17): 1100–4. doi:10.1056/NEJM199604253341705. PMID 8598869.
- ↑ Hurst JA, Baraitser M, Winter RM (1987). "A syndrome of mental retardation, short stature, hemolytic anemia, delayed puberty, and abnormal facial appearance: similarities to a report of aldolase A deficiency". Am J Med Genet. 28 (4): 965–70. doi:10.1002/ajmg.1320280423. PMID 3688035.
- ↑ Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A, Jann S; et al. (2001). "Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis". Ann Neurol. 50 (2): 202–7. PMID 11506403.
- ↑ Tegtmeyer LC, Rust S, van Scherpenzeel M, Ng BG, Losfeld ME, Timal S; et al. (2014). "Multiple phenotypes in phosphoglucomutase 1 deficiency". N Engl J Med. 370 (6): 533–42. doi:10.1056/NEJMoa1206605. PMC 4373661. PMID 24499211.
- ↑ Stojkovic T, Vissing J, Petit F, Piraud M, Orngreen MC, Andersen G; et al. (2009). "Muscle glycogenosis due to phosphoglucomutase 1 deficiency". N Engl J Med. 361 (4): 425–7. doi:10.1056/NEJMc0901158. PMID 19625727.
- ↑ Orho M, Bosshard NU, Buist NR, Gitzelmann R, Aynsley-Green A, Blümel P; et al. (1998). "Mutations in the liver glycogen synthase gene in children with hypoglycemia due to glycogen storage disease type 0". J Clin Invest. 102 (3): 507–15. doi:10.1172/JCI2890. PMC 508911. PMID 9691087.
- ↑ Laberge AM, Mitchell GA, van de Werve G, Lambert M (2003). "Long-term follow-up of a new case of liver glycogen synthase deficiency". Am J Med Genet A. 120A (1): 19–22. doi:10.1002/ajmg.a.20110. PMID 12794686.
- ↑ Gitzelmann R, Spycher MA, Feil G, Müller J, Seilnacht B, Stahl M; et al. (1996). "Liver glycogen synthase deficiency: a rarely diagnosed entity". Eur J Pediatr. 155 (7): 561–7. PMID 8831078.
- ↑ Rutledge SL, Atchison J, Bosshard NU, Steinmann B (2001). "Case report: liver glycogen synthase deficiency--a cause of ketotic hypoglycemia". Pediatrics. 108 (2): 495–7. PMID 11483824.
- ↑ "Glycogen storage disease type VI - Genetics Home Reference".
- ↑ "glycogen storage disease type 6 - Humpath.com - Human pathology".
- ↑ Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean L, Stephens K, Amemiya A, Goldstein J, Austin S, Kishnani P, Bali D. PMID 21634085. Vancouver style error: initials (help); Missing or empty
|title=(help) - ↑ "Hers Disease - NORD (National Organization for Rare Disorders)".
- ↑ Nakai A, Shigematsu Y, Takano T, Kikawa Y, Sudo M (1994). "Uncooked cornstarch treatment for hepatic phosphorylase kinase deficiency". Eur. J. Pediatr. 153 (8): 581–3. PMID 7957405.