Epstein Barr virus
| Epstein-Barr Virus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 Two Epstein-Barr virions
| ||||||||||
| Virus classification | ||||||||||
|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [4]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [5] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Related Key Words and Synonyms: EBV
Overview
The Epstein-Barr Virus (EBV), also called Human herpesvirus 4 (HHV-4), is a virus of the herpes family (which includes Herpes simplex virus and Cytomegalovirus), and is one of the most common viruses in humans. Most people become infected with EBV, which is often asymptomatic but commonly causes infectious mononucleosis.
EBV is named after Michael Epstein and Yvonne Barr, who together with Bert Achong, discovered the virus in 1964.[1]
Epidemiology and Demographics
The virus occurs worldwide, and most people become infected with EBV sometime during their lives. In the United States, as many as 95% of adults between 35 and 40 years of age have been infected. Infants become susceptible to EBV as soon as maternal antibody protection (present at birth) disappears. Many children become infected with EBV, and these infections usually cause no symptoms or are indistinguishable from the other mild, brief illnesses of childhood. In the United States and in other developed countries, many persons are not infected with EBV in their childhood years. When infection with EBV occurs during adolescence or young adulthood, it causes infectious mononucleosis 35% to 50% of the time.
Biology
On infecting the B-lymphocyte, the linear virus genome circularizes and the virus subsequently persists within the cell as an episome.
The virus can execute many distinct programs of gene expression which can be broadly categorized as being lytic cycle or latent cycle.
The lytic cycle or productive infection results in staged expression of a host of viral proteins with the ultimate objective of producing infectious virions. Formally, this phase of infection does not inevitably lead to lysis of the host cell as EBV virions are produced by budding from the infected cell.
The latent cycle (lysogenic) programs are those that do not result in production of virions. A very limited, distinct set of viral proteins are produced during latent cycle infection. These include Epstein-Barr nuclear antigen (EBNA)-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, EBNA-leader protein (EBNA-LP) and latent membrane proteins (LMP)-1, LMP-2A and LMP-2B and the Epstein-Barr encoded RNAs (EBERs). In addition, EBV codes for at least twenty microRNAs which are expressed in latently infected cells.[2]
From studies of EBV gene expression in cultured Burkitt's lymphoma cell lines, at least three programs exist:
- EBNA1 only (group I)
- EBNA1 + EBNA2 (group II)
- Latent cycle proteins (group III).
It is also postulated that a program in which all viral protein expression is shut off exists.
When EBV infects B-lymphocytes in vitro, lymphoblastoid cell lines eventually emerge that are capable of indefinite growth. The growth transformation of these cell lines is the consequence of viral protein expression.
EBNA-2, EBNA-3C and LMP-1 are essential for transformation while EBNA-LP and the EBERs are not. The EBNA-1 protein is essential for maintenance of the virus genome.[3]
It is postulated that following natural infection with EBV, the virus executes some or all of its repertoire of gene expression programs to establish a persistent infection. Given the initial absence of host immunity, the lytic cycle produces large amounts of virus to infect other (presumably) B-lymphocytes within the host.
The latent programs reprogram and subvert infected B-lymphocytes to proliferate and bring infected cells to the sites at which the virus presumably persists. Eventually, when host immunity develops, the virus persists by turning off most (or possibly all) of its genes, only occasionally reactivating to produce fresh virions. A balance is eventually struck between occasional viral reactivation and host immune surveillance removing cells that activate viral gene expression.
The site of persistence of EBV may be bone marrow. EBV-positive patients who have had their own bone marrow replaced with bone marrow from an EBV-negative donor are found to be EBV-negative after transplantation.[4]
EBV latent antigens
All EBV nuclear proteins are produced by alternative splicing of a transcript starting at either the Cp or Wp promoters at the left end of the genome (in the conventional nomenclature). The genes are ordered EBNA-LP/EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 within the genome.
The initiation codon of the EBNA-LP coding region is created by an alternate splice of the nuclear protein transcript. In the absence of this initiation codon, EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 will be expressed depending on which of these genes is alternatively spliced into the transcript.
- EBNA-1
EBNA-1 protein binds to a replication origin (oriP) within the viral genome and mediates replication and partitioning of the episome during division of the host cell. It is the only viral protein expressed during group I latency. EBNA-1 possesses a glycine-alanine repeat that impairs antigen processing and MHC class I-restricted antigen presentation thereby inhibiting the CD8-restricted cytotoxic T-cell response against virus infected cells.[5]
EBNA-1 was initially identified as the target antigen of sera from rheumatoid arthritis patients (rheumatoid arthritis-associated nuclear antigen; RANA).
- EBNA-2
EBNA-2 is the main viral transactivator, switching transcription from the Wp promoters used during initially after infection to the Cp promoter. Together with EBNA-3C, it also activates the LMP-1 promoter. It is known to bind the host RBP-Jκ protein that is a key player in the Notch pathway. EBNA-2 is essential for EBV-mediated growth transformation.
- EBNA-3A/EBNA-3B/EBNA-3C
These genes also bind the host RBP-Jκ protein.
- EBNA-3C
EBNA-3C is also a ubiquitin-ligase and has been shown to target cell cycle regulators like pRb[6][7]
- LMP-1
LMP-1 is a six-span transmembrane protein that is also essential for EBV-mediated growth transformation. LMP-1 mediates signaling through the Tumor necrosis factor-alpha/CD40 pathway.
- LMP-2A/LMP-2B
LMP-2A/LMP-2B are transmembrane proteins that act to block tyrosine kinase signaling. It is believed that they act to inhibit activation of the viral lytic cycle. It's unknown whether LMP-2B is required for EBV-mediated growth transformation, while different groups have reported that LMP-2A alternatively is, or is not needed for transformation.
- EBER-1/EBER-2
EBER-1/EBER-2 are small nuclear RNAs of an unknown role. They are not required for EBV-mediated growth transformation.
- miRNAs
EBV microRNAs are encoded by two transcripts, one set in the BART gene and one set near the BHRF1 cluster. The three BHRF1 miRNAS are expressed during type III latency while the large cluster of BART miRNAs (up to 20 miRNAs) are expressed during type II latency. The functions of these miRNAs are currently unknown.
EBV surface receptors
The Epstein-Barr Virus surface glycoprotein H (gH) is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. [8]
In laboratory and animal trials in 2000, it was shown that both antagonism of RA-mediated growth inhibition and promotion of LCL proliferation were efficiently reversed by the glucocorticoid receptor (GR) antagonist RU486.[6]
Pathophysiology & Etiology
Epstein-Barr virus, frequently referred to as EBV, is a member of the herpesvirus family and one of the most common human viruses.
Most individuals exposed to people with infectious mononucleosis have previously been infected with EBV and are not at risk for infectious mononucleosis. In addition, transmission of EBV requires intimate contact with the saliva (found in the mouth) of an infected person.
Transmission
Transmission of this virus through the air or blood does not normally occur. The incubation period, or the time from infection to appearance of symptoms, ranges from 4 to 6 weeks. Persons with infectious mononucleosis may be able to spread the infection to others for a period of weeks. However, no special precautions or isolation procedures are recommended, since the virus is also found frequently in the saliva of healthy people. In fact, many healthy people can carry and spread the virus intermittently for life. These people are usually the primary reservoir for person-to-person transmission. For this reason, transmission of the virus is almost impossible to prevent.
Infectious mononucleosis
Epstein-Barr can cause infectious mononucleosis, also known as 'glandular fever', 'Mono' and 'Pfeiffer's disease'. Infectious mononucleosis is caused when a person is first exposed to the virus during or after adolescence. Though once deemed "The Kissing Disease," recent research has shown that transmission of Mono not only occurs from exchanging saliva, but also from contact with the airborne virus. It is predominantly found in the developing world, and most children in the developing world are found to have already been infected by around 18 months of age. EBV antibody tests turn up almost universally positive. In the United States roughly half of five-year-olds have been infected[9], and up to 95% of adults between 35 and 40 years of age.[10]
EBV-associated malignancies
The strongest evidence linking EBV and cancer formation is found in Burkitt's lymphoma and nasopharyngeal carcinoma. It has been postulated to be a trigger for a subset of chronic fatigue syndrome patients[11] as well as multiple sclerosis and other autoimmune diseases.[12]
Burkitt's lymphoma is a type of Non-Hodgkin's lymphoma [7] and is most common in equatorial Africa and is co-existent with the presence of malaria. Malaria infection causes reduced immune surveillance of EBV immortalized B cells, so allowing their proliferation. This proliferation increases the chance of a mutation to occur. Repeated mutations can lead to the B cells escaping the body's cell-cycle control, so allowing the cells to proliferate unchecked, resulting in the formation of Burkitt's lymphoma. Burkitt's lymphoma commonly affects the jaw bone, forming a huge tumor mass. It responds quickly to chemotherapy treatment, namely cyclophosphamide, but recurrence is common.
Other B cell lymphomas arise in immunocompromised patients such as those with AIDS or who have undergone organ transplantation with associated immunosuppression (Post-Transplant Lymphoproliferative Disorder (PTLPD)). Smooth muscle tumors are also associated with the virus in malignant patients.[13]
Nasopharyngeal carcinoma is a cancer found in the upper respiratory tract, most commonly in the nasopharynx, and is linked to the EBV virus. It is found predominantly in Southern China and Africa, due to both genetic and environmental factors. It is much more common in people of Chinese ancestry (genetic), but is also linked to the Chinese diet of a high amount of smoked fish, which contain nitrosamines, well known carcinogens (environmental).[14]
Chronic fatigue syndrome
In the late 1980s and early 1990s, EBV became the favored explanation for chronic fatigue syndrome. It was noted that people with chronic exhaustion had EBV, although it was also noted EBV was present in almost everyone. In a four year study, the Centers for Disease Control and Prevention found that the virus did not adhere to Koch's Postulates and therefore had no definitive association between CFS and EBV but it is still being studied by researchers.
Diseases associated with EBV
- Infectious mononucleosis
- Stevens-Johnson syndrome
- Hepatitis
- Herpes
- Alice in Wonderland syndrome
- Several Non-Hodgkin's lymphomas, including primary cerebral lymphoma
- Hodgkin's disease
- Post-transplant lymphoproliferative disorder
- Herpangina
- Multiple Sclerosis (higher risk in patients infected as teenagers than as children)
- Hairy leukoplakia
- Common variable immunodeficiency (CVID)
- Kikuchi's disease
- Nasopharyngeal cancer
- Subepithelial Infiltrates
- Smooth muscle tumors [15]
Diagnosis
In most cases of infectious mononucleosis, the clinical diagnosis can be made from the characteristic triad of fever, pharyngitis, and lymphadenopathy lasting for 1 to 4 weeks. Serologic test results include a normal to moderately elevated white blood cell count, an increased total number of lymphocytes, greater than 10% atypical lymphocytes, and a positive reaction to a "mono spot" test. In patients with symptoms compatible with infectious mononucleosis, a positive Paul-Bunnell heterophile antibody test result is diagnostic, and no further testing is necessary. Moderate-to-high levels of heterophile antibodies are seen during the first month of illness and decrease rapidly after week 4. False-positive results may be found in a small number of patients, and false-negative results may be obtained in 10% to 15% of patients, primarily in children younger than 10 years of age. True outbreaks of infectious mononucleosis are extremely rare. A substantial number of pseudo-outbreaks have been linked to laboratory error, as reported in CDC's Morbidity and Mortality Weekly Report, vol. 40, no. 32, on August 16, 1991.
When "mono spot" or heterophile test results are negative, additional laboratory testing may be needed to differentiate EBV infections from a mononucleosis-like illness induced by cytomegalovirus, adenovirus, or Toxoplasma gondii. Direct detection of EBV in blood or lymphoid tissues is a research tool and is not available for routine diagnosis. Instead, serologic testing is the method of choice for diagnosing primary infection.
History and Symptoms
Symptoms of infectious mononucleosis are fever, sore throat, and swollen lymph glands. Sometimes, a splenomegaly or hepatomegaly may develop. Heart problems or involvement of the central nervous system occurs only rarely, and infectious mononucleosis is almost never fatal. There are no known associations between active EBV infection and problems during pregnancy, such as miscarriages or birth defects. Although the symptoms of infectious mononucleosis usually resolve in 1 or 2 months, EBV remains dormant or latent in a few cells in the throat and blood for the rest of the person's life. Periodically, the virus can reactivate and is commonly found in the saliva of infected persons. This reactivation usually occurs without symptoms of illness.
Laboratory Findings
Laboratory tests are not always foolproof. For various reasons, false-positive and false-negative results can occur for any test. However, the laboratory tests for EBV are for the most part accurate and specific. Because the antibody response in primary EBV infection appears to be quite rapid, in most cases testing paired acute- and convalescent-phase serum samples will not demonstrate a significant change in antibody level. Effective laboratory diagnosis can be made on a single acute-phase serum sample by testing for antibodies to several EBV-associated antigens simultaneously. In most cases, a distinction can be made as to whether a person is susceptible to EBV, has had a recent infection, has had infection in the past, or has a reactivated EBV infection.
Antibodies to several antigen complexes may be measured. These antigens are the viral capsid antigen, the early antigen, and the EBV nuclear antigen (EBNA). In addition, differentiation of immunoglobulin G and M subclasses to the viral capsid antigen can often be helpful for confirmation. When the "mono spot" test is negative, the optimal combination of EBV serologic testing consists of the antibody titration of four markers: IgM and IgG to the viral capsid antigen, IgM to the early antigen, and antibody to EBNA.
IgM to the viral capsid antigen appears early in infection and disappears within 4 to 6 weeks. IgG to the viral capsid antigen appears in the acute phase, peaks at 2 to 4 weeks after onset, declines slightly, and then persists for life. IgG to the early antigen appears in the acute phase and generally falls to undetectable levels after 3 to 6 months. In many people, detection of antibody to the early antigen is a sign of active infection, but 20% of healthy people may have this antibody for years.
Antibody to EBNA determined by the standard immunofluorescent test is not seen in the acute phase, but slowly appears 2 to 4 months after onset, and persists for life. This is not true for some EBNA enzyme immunoassays, which detect antibody within a few weeks of onset.
Finally, even when EBV antibody tests, such as the early antigen test, suggest that reactivated infection is present, this result does not necessarily indicate that a patient's current medical condition is caused by EBV infection. A number of healthy people with no symptoms have antibodies to the EBV early antigen for years after their initial EBV infection.
Therefore, interpretation of laboratory results is somewhat complex and should be left to physicians who are familiar with EBV testing and who have access to the entire clinical picture of a person. To determine if EBV infection is associated with a current illness, consult with an experienced physician.
Pathological Findings
Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology
-
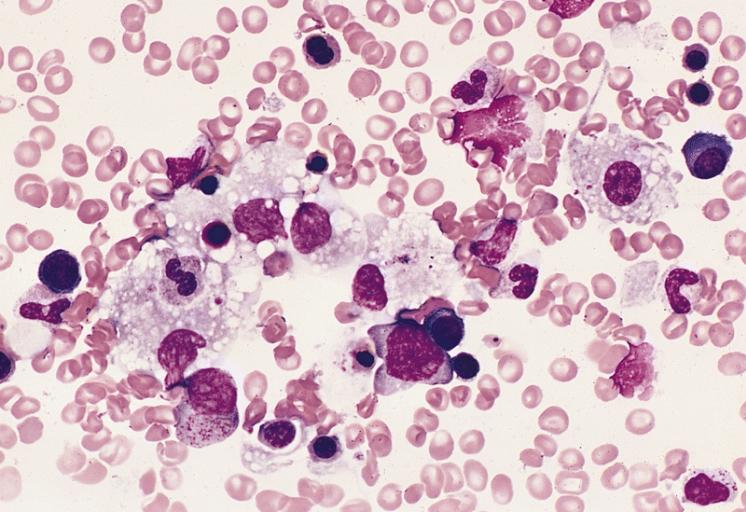
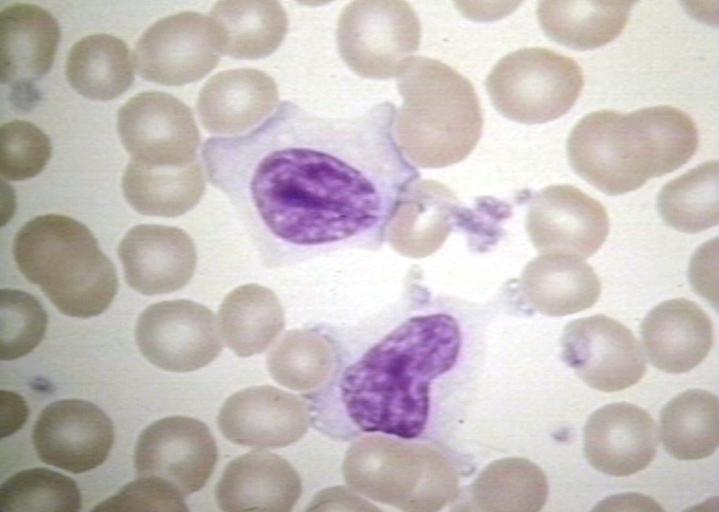
BONE MARROW: INFECTION-ASSOCIATED HEMOPHAGOCYTIC SYNDROME A bone marrow aspirate smear from a child with infection-associated hemophagocytic syndrome secondary to an Epstein-Barr virus infection. On this field there are several large histiocytes. Phagocytosis of nucleated red blood cells, neutrophils, and platelets is evident. The histiocytes have the appearance of reactive cells and should be readily distinguishable from neoplastic histiocytes. (Wright-Giemsa stain)
-
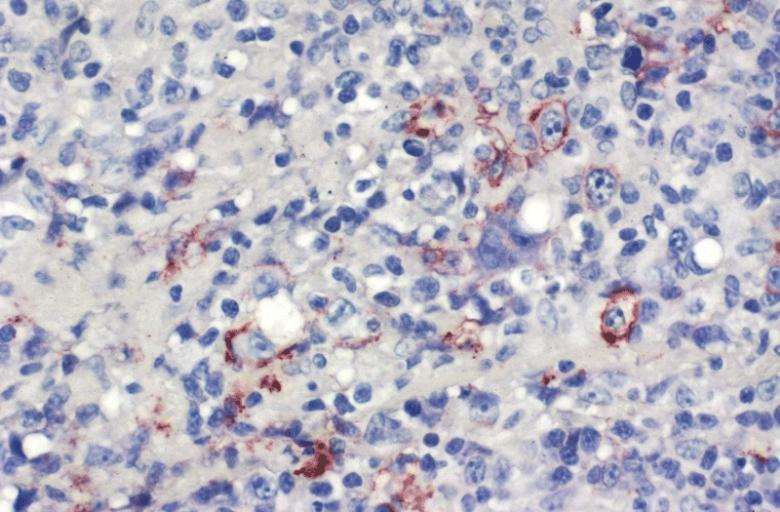
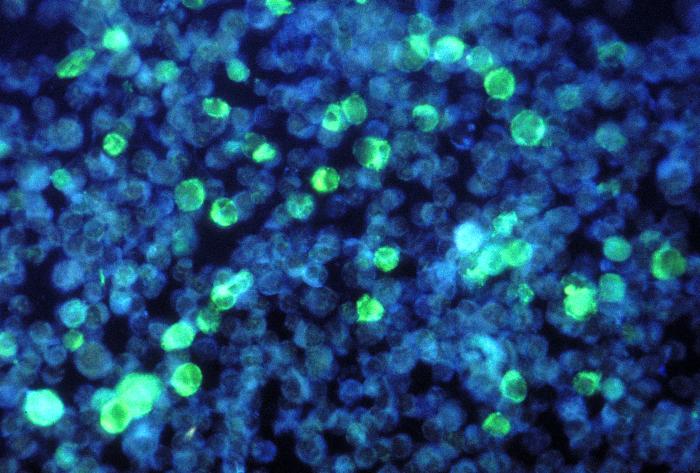
LOWER RESPIRATORY TRACT: (Supplement) AIL/LYG CD20 staining (for B cells) in the viable tissue shows positive staining of scattered large lymphoid cells (C) which were also the cells that were positive for Epstein-Barr virus by in situ hybridization
-
LOWER RESPIRATORY TRACT: (Supplement) AIL/LYG CD20 staining (for B cells) in the viable tissue shows positive staining of scattered large lymphoid cells (C) which were also the cells that were positive for Epstein-Barr virus by in situ hybridization
-
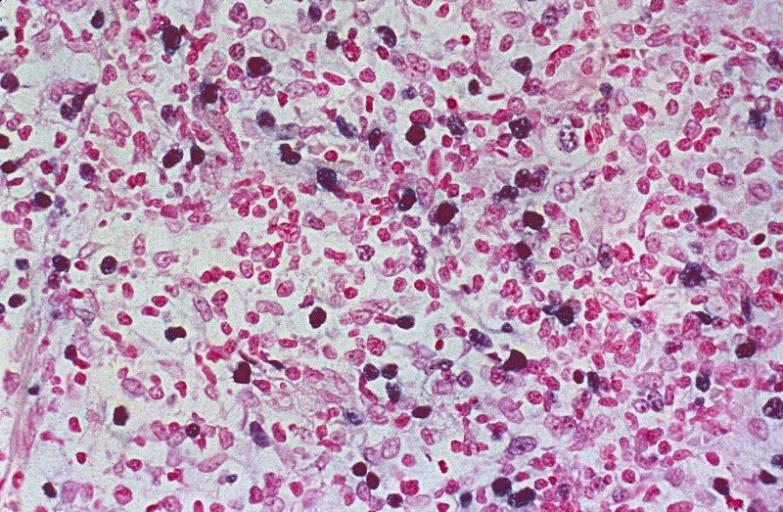
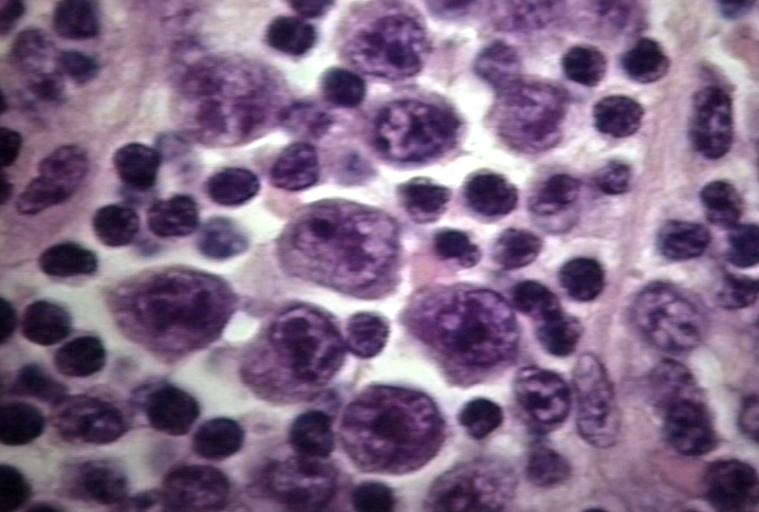
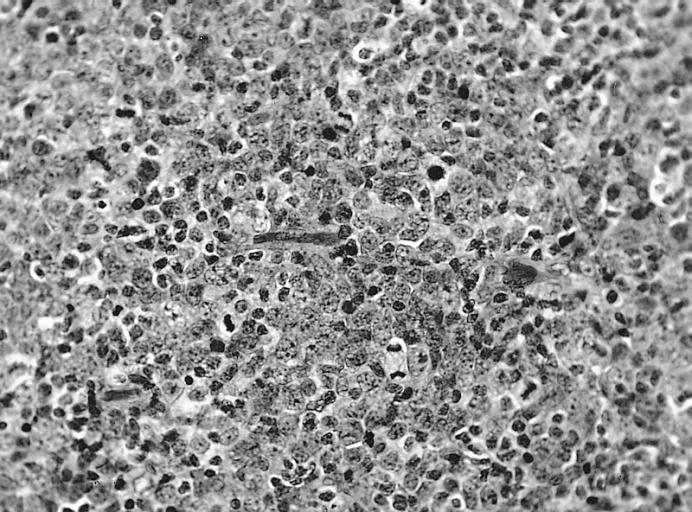
LYMPH NODES-SPLEEN: EPSTEIN-BARR VIRUS-ASSOCIATED INFECTIOUS MONONUCLEOSIS A heterogeneous population is present, including cells mimicking Hodgkin cells. However, the spectrum of cell types and the extensive apoptosis present would not be found in Hodgkin's disease.
-
LYMPH NODES-SPLEEN: METASTATIC NASOPHARYNGEAL CARCINOMA IN LYMPH NODE PRESENTING AS METASTATIC CARCINOMA OF UNKNOWN PRIMARY Nuclear labeling of the carcinoma cells for Epstein-Barr virus-encoded RNA strongly suggests that the primary is nasopharyngeal.
-
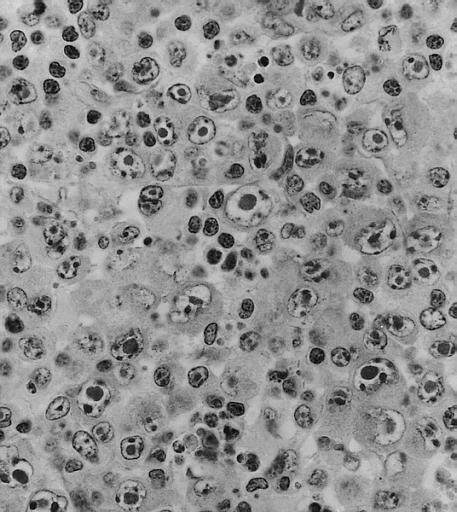
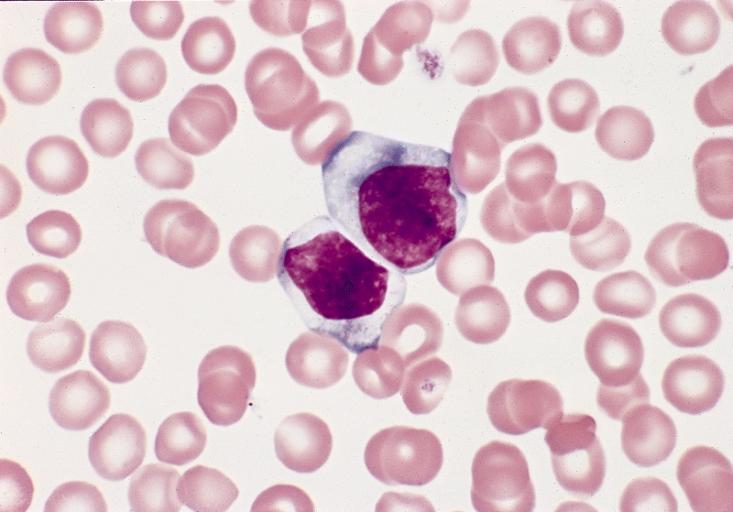
BONE MARROW: REACTIVE (ATYPICAL) LYMPHOCYTES This blood smear is from a 19-year-old male college student with infectious mononucleosis. The two reactive (atypical) lymphocytes are large, with abundant cytoplasm and coarse nuclear chromatin, and lack a nucleolus. Cytoplasmic basophilia is radial in distribution and accentuated at the cell margin. In contrast, lymphoblasts are generally smaller, have less cytoplasm with uniform basophilia and more dispersed nuclear chromatin, and may contain a nucleolus. (Wright-Giemsa stain)
-
LYMPH NODE: INFECTIOUS MONONUCLEOSIS, LYMPH NODE
-
BLOOD: INFECTIOUS MONONUCLEOSIS; PERIPHERAL BLOOD
-
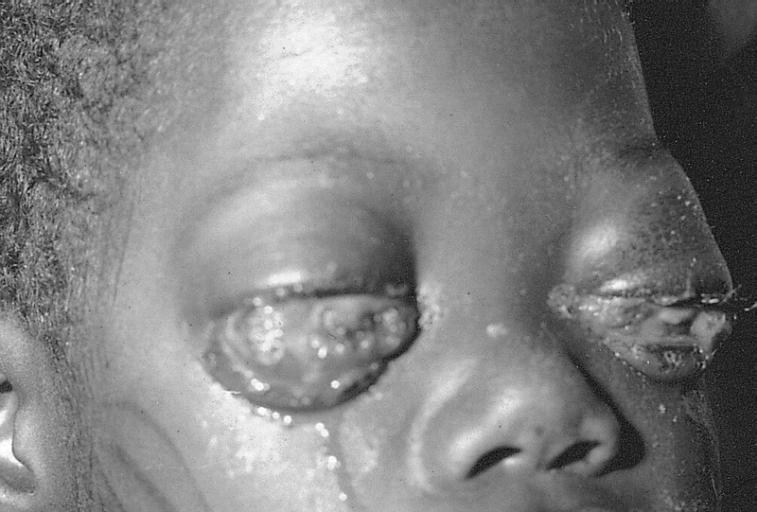
EYE AND OCULAR ADNEXA: BURKITT LYMPHOMA Bilateral involvement.
-
EYE AND OCULAR ADNEXA: BURKITT LYMPHOMA Lymphoblastic tumor with numerous mitotic figures.
Treatment
There is no specific treatment for infectious mononucleosis, other than treating the symptoms. No antiviral drugs or vaccines are available. Some physicians have prescribed a 5-day course of steroids to control the swelling of the throat and tonsils. The use of steroids has also been reported to decrease the overall length and severity of illness, but these reports have not been published.
References
- ↑ Epstein MA, Achong BG, Barr YM (1964). "Virus particles in cultured lymphblasts from Burkitt's Lymphoma". Lancet. 1: 702–3. PMID 14107961.
- ↑ The nomenclature used here is that of the Kieff lab. Other laboratories use different nomenclatures.
- ↑ Yates JL, Warren N, Sugden B (1985). "Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells". Nature. 313 (6005): 812–5. PMID 2983224.
- ↑ Gratama JW, Oosterveer MA, Zwaan FE, Lepoutre J, Klein G, Ernberg I (1988). "Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency". Proc. Natl. Acad. Sci. U.S.A. 85 (22): 8693–6. PMID 2847171.
- ↑ Levitskaya J, Coram M, Levitsky V; et al. (1995). "Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1". Nature. 375 (6533): 685–8. doi:10.1038/375685a0. PMID 7540727.
- ↑ Knight JS, Sharma N, Robertson ES (2005). "SCFSkp2 complex targeted by Epstein-Barr virus essential nuclear antigen". Mol. Cell. Biol. 25 (5): 1749–63. doi:10.1128/MCB.25.5.1749-1763.2005. PMID 15713632.
- ↑ Knight JS, Sharma N, Robertson ES (2005). "Epstein-Barr virus latent antigen 3C can mediate the degradation of the retinoblastoma protein through an SCF cellular ubiquitin ligase". Proc. Natl. Acad. Sci. U.S.A. 102 (51): 18562–6. doi:10.1073/pnas.0503886102. PMID 16352731.
- ↑ Sara J. Molesworth, Cathleen M. Lake, Corina M. Borza, Susan M. Turk, and Lindsey M. Hutt-Fletcher. "Epstein-Barr Virus gH Is Essential for Penetration of B Cells but Also Plays a Role in Attachment of Virus to Epithelial Cells". Journal of Virology. July 2000, p. 6324-6332, Vol. 74, No. 14. External link in
|title=(help) - ↑ [1]
- ↑ Epstein-Barr Virus and Infectious Mononucleosis - National Center for Infectious Diseases
- ↑ Lerner AM, Beqaj SH, Deeter RG, Fitzgerald JT (2004). "IgM serum antibodies to Epstein-Barr virus are uniquely present in a subset of patients with the chronic fatigue syndrome". In Vivo. 18 (2): 101–6. PMID 15113035.
- ↑ Lünemann JD, Münz C (2007). "Epstein-Barr virus and multiple sclerosis". Current neurology and neuroscience reports. 7 (3): 253–8. PMID 17488592.
- ↑ Weiss SW (2002). "Smooth muscle tumors of soft tissue". Adv Anat Pathol. 9 (6): 351–9. PMID 12409644.
- ↑ [2] Nasopharyngeal carcinoma information at OncologyChannel.com
- ↑ Deyrup AT, Lee VK, Hill CE, Cheuk W, Toh HC, Kesavan S, Chan EW, Weiss SW. "Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients". Am J Surg Pathol. 2006 Jan;30(1):75-82. PMID 16330945.
External links
- Article in The Scientist 13(6):1, Mar. 15, 1999 (registration required)
- CDC website on EBV
- Epstein Barr Virus Editor: Erle S. Robertson Department of Microbiology and the Abramson Comprehensive Cancer Center, University of Pennsylvania Medical School, Philadelphia, Pennsylvania 19104, USA
- International Association for Research on EBV Body that organizes the two-yearly EBV research meeting.
- http://www.cdc.gov/ncidod/diseases/ebv.htm
- http://en.wikipedia.org/wiki/Epstein-Barr_virus
Template:Baltimore classification Template:Viral diseases
Template:SIB bg:Вирус на Епщайн-Бар de:Epstein-Barr-Virus it:Virus di Epstein-Barr he:וירוס אפשטיין בר nl:Epstein-Barr virus id:Virus Epstein-Barr fi:Epstein-Barrin virus sv:Epstein-Barr-virus