Epidermal growth factor
| Epidermal growth factor (beta-urogastrone) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
File:PBB Protein EGF image.jpg PDB rendering based on 1ivo. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | EGF ; URG | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 1483 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Epidermal growth factor or EGF is a growth factor that plays an important role in the regulation of cell growth, proliferation, and differentiation. Human EGF is a 6045-Da protein with 53 amino acid residues and three intramolecular disulfide bonds.[1]
Function
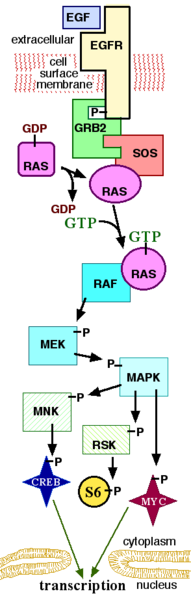
EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface and stimulating the intrinsic protein-tyrosine kinase activity of the receptor (see the second diagram). The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell - a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes including the gene for EGFR - that ultimately lead to DNA synthesis and cell proliferation.[2]
EGF-family
EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. Besides EGF itself other family members include:[3]
- Heparin-binding EGF-like growth factor (HB-EGF)
- transforming growth factor-α (TGF-α)
- Amphiregulin (AR)
- Epiregulin (EPR)
- Epigen
- Betacellulin (BTC)
- neuregulin-1 (NRG1)
- neuregulin-2 (NRG2)
- neuregulin-3 (NRG3)
- neureguline-4 (NRG4).
All family members contain one or more repeats of the conserved amino acid sequence:
Where X represents any amino acid.[3]
This sequence contains 6 cysteine residues that form three intramolecular disulphide bonds. Disulphide bond formation generates three structural loops that are essential for high-affinity binding between members of the EGF-family and their cell-surface receptors.[4]
EGF as Therapeutic Protein
EGF is currently being marketed as a therapeutic protein for the treatment of diabetic foot ulcers by at least three companies. Bharat Biotech International, a company based in India, is marketing EGF as REGEN-D, Daewoong Pharmaceutical, based in South Korea, is marketing EGF as Easyef, and the Center for Genetic Engineering and Biotechnology, in Cuba, is marketing EGF as CITOPROT-P.[5] [6] EGF is also used in a burn treatment cream product, Hebermin, manufactured by Heber Biotec S. A. in Cuba.[6]
References
- ↑ Carpenter G, and Cohen S. (1990). "Epidermal growth factor". J. Biol. Chem. 265 (14): 7709–7712. PMID 2186024.
- ↑ Fallon JH, Seroogy KB.; et al. (1984). "Epidermal growth factor immunoreactive material in the central nervous system: location and development". Science. 224 (4653): 1107–1109. PMID 6144184.
- ↑ 3.0 3.1 Dreux AC, Lamb DJ.; et al. (2006). "The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis". Atherosclerosis. 186 (1): 38–53. PMID 16076471.
- ↑ Harris RC, Chung E, and Coffey RJ. (2003). "EGF receptor ligands". Exp. Cell. Res. 284 (1): 2–13. PMID 12648462.
- ↑ Frew S, Rezaie R.; et al. (2007). "India's health biotech sector at a crossroads". Nature Biotechnology. 25 (4).
- ↑ 6.0 6.1 Lopez E, Acevedo B.; et al. (2002). "Development of Cuban Biotechnology". Journal of Commercial Biotechnology. 9 (2).
External links
- EGF at the Human Protein Reference Database.
- Epidermal+growth+factor at the US National Library of Medicine Medical Subject Headings (MeSH)
Further reading
- Boonstra J, Rijken P, Humbel B; et al. (1995). "The epidermal growth factor". Cell Biol. Int. 19 (5): 413–30. PMID 7640657.
- Dvorak B (2004). "Epidermal growth factor and necrotizing enterocolitis". Clinics in perinatology. 31 (1): 183–92. doi:10.1016/j.clp.2004.03.015. PMID 15183666.
- Howell WM (2004). "Epidermal growth factor gene polymorphism and development of cutaneous melanoma". J. Invest. Dermatol. 123 (4): xx–xxi. doi:10.1111/j.0022-202X.2004.23308.x. PMID 15373802.