Diazepam (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diazepam (oral) is a general anesthetic that is FDA approved for the treatment of anxiety disorders or for the short-term relief of the symptoms of anxiety, acute alcohol withdrawal. It is also approved as an adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma), spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia), athetosis, stiff-man syndrome, and convulsive disorders. Common adverse reactions include hypotension, rash, diarrhea, muscle weakness, ataxia, incoordination, somnolence, euphoria, respiratory depression, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Diazepam Tablets USP are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
- In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
- Diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma ; spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia); athetosis; and stiff-man syndrome.
- Oral diazepam may be used adjunctively in convulsive disorders, although it has not proved useful as the sole therapy.
- The effectiveness of diazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Anesthesia; Adjunct.
- Benzodiazepine withdrawal.
- Sedation for a mechanically ventilated patient, Intensive care unit.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diazepam (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Diazepam (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diazepam (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diazepam (oral) in pediatric patients.
Contraindications
- Diazepam Tablets USP are contraindicated in patients with a known hypersensitivity to this drug and, because of lack of sufficient clinical experience, in pediatric patients under 6 months of age. Diazepam is also contraindicated in patients with myasthenia gravis, severe respiratory insufficiency, severe hepatic insufficiency, and sleep apnea syndrome. It may be used in patients with open-angle glaucoma who are receiving appropriate therapy, but is contraindicated in acute narrow-angle glaucoma.
Warnings
- Diazepam is not recommended in the treatment of psychotic patients and should not be employed instead of appropriate treatment.
- Since diazepam has a central nervous system depressant effect, patients should be advised against the simultaneous ingestion of alcohol and other CNS-depressant drugs during diazepam therapy.
- As with other agents which have anticonvulsant activity, when diazepam is used as an adjunct in treating convulsive disorders, the possibility of an increase in the frequency and/or severity of grand mal seizures may require an increase in the dosage of standard anticonvulsant medication. Abrupt withdrawal of diazepam in such cases may also be associated with a temporary increase in the frequency and/or severity of seizures.
Pregnancy
- An increased risk of congenital malformations and other developmental abnormalities associated with the use of benzodiazepine drugs during pregnancy has been suggested. There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy. There have been reports of neonatal flaccidity respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy. In addition, children born to mothers receiving benzodiazepines on a regular basis late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period.
- Diazepam has been shown to be teratogenic in mice and hamsters when given orally at daily doses of 100 mg/kg or greater (approximately eight times the maximum recommended human dose [MRHD=1 mg/kg/day] or greater on a mg/m2 basis). Cleft palate and encephalopathy are the most common and consistently reported malformations produced in these species by administration of high, maternally toxic doses of diazepam during organogenesis. Rodent studies have indicated that prenatal exposure to diazepam doses similar to those used clinically can produce long-term changes in cellular immune responses, brain neurochemistry, and behavior.
- In general, the use of diazepam in women of childbearing potential, and more specifically during known pregnancy, should be considered only when the clinical situation warrants the risk to the fetus. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should also be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
Labor and Delivery
- Special care must be taken when diazepam is used during labor and delivery, as high single doses may produce irregularities in the fetal heart rate and hypotonia, poor sucking, hypothermia, and moderate respiratory depression in the neonates. With newborn infants it must be remembered that the enzyme system involved in the breakdown of the drug is not yet fully developed (especially in premature infants).
Nursing Mothers
- Diazepam passes into breast milk. Breastfeeding is therefore not recommended in patients receiving diazepam.
Adverse Reactions
Clinical Trials Experience
- Side effects most commonly reported were drowsiness, fatigue, muscle weakness, and ataxia. The following have also been reported:
- Central Nervous System: confusion, depression, dysarthria, headache, slurred speech, tremor, vertigo
- Gastrointestinal System: constipation, nausea, gastrointestinal disturbances
Special Senses: blurred vision, diplopia, dizziness
- Cardiovascular System: hypotension
- Psychiatric and Paradoxical Reactions: stimulation, restlessness, acute hyperexcited states, anxiety, agitation, aggressiveness, irritability, rage, hallucinations, psychoses, delusions, increased muscle spasticity, insomnia, sleep disturbances, and nightmares. Inappropriate behavior and other adverse behavioral effects have been reported when using benzodiazepines. Should these occur, use of the drug should be discontinued. They are more likely to occur in children and in the elderly.
- Urogenital System: incontinence, changes in libido, urinary retention
Skin and Appendages: skin reactions
- Laboratories: elevated transaminases and alkaline phosphatase
- Other: changes in salivation, including dry mouth, hypersalivation
- Antegrade amnesia may occur using therapeutic dosages, the risk increasing at higher dosages. Amnestic effects may be associated with inappropriate behavior.
- Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during and after diazepam therapy and are of no known significance.
- Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy.
Postmarketing Experience
- Injury, Poisoning and Procedural Complications: There have been reports of falls and fractures in benzodiazepine users. The risk is increased in those taking concomitant sedatives (including alcohol), and in the elderly.
Drug Interactions
Centrally Acting Agents
- If diazepam is to be combined with other centrally acting agents, careful consideration should be given to the pharmacology of the agents employed particularly with compounds that may potentiate or be potentiated by the action of diazepam, such as phenothiazines, antipsychotics, anxiolytics/sedatives, hypnotics, anticonvulsants, narcotic analgesics, anesthetics, sedative antihistamines, narcotics, barbiturates, MAO inhibitors and other antidepressants.
Alcohol
- Concomitant use with alcohol is not recommended due to enhancement of the sedative effect.
Antacids
- Diazepam peak concentrations are 30% lower when antacids are administered concurrently. However, there is no effect on the extent of absorption. The lower peak concentrations appear due to a slower rate of absorption, with the time required to achieve peak concentrations on average 20 - 25 minutes greater in the presence of antacids. However, this difference was not statistically significant.
Compounds Which Inhibit Certain Hepatic Enzymes
- There is a potentially relevant interaction between diazepam and compounds which inhibit certain hepatic enzymes (particularly cytochrome P450 3A and 2C19). Data indicate that these compounds influence the pharmacokinetics of diazepam and may lead to increased and prolonged sedation. At present, this reaction is known to occur with cimetidine, ketoconazole, fluvoxamine, fluoxetine, and omeprazole.
Phenytoin
- There have also been reports that the metabolic elimination of phenytoin is decreased by diazepam.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diazepam (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diazepam (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Diazepam (oral) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Diazepam (oral) with respect to pediatric patients.
Geriatic Use
- In elderly patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (2 mg to 2.5 mg once or twice daily, initially to be increased gradually as needed and tolerated).
- Extensive accumulation of diazepam and its major metabolite, desmethyldiazepam, has been noted following chronic administration of diazepam in healthy elderly male subjects. Metabolites of this drug are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Diazepam (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diazepam (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diazepam (oral) in patients with renal impairment.
Hepatic Impairment
- Decreases in clearance and protein binding, and increases in volume of distribution and half-life has been reported in patients with cirrhosis. In such patients, a 2- to 5- fold increase in mean half-life has been reported. Delayed elimination has also been reported for the active metabolite desmethyldiazepam. Benzodiazepines are commonly implicated in hepatic encephalopathy. Increases in half-life have also been reported in hepatic fibrosis and in both acute and chronic hepatitis
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diazepam (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diazepam (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Diazepam (oral) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Diazepam (oral) in the drug label.
Overdosage
- Overdose of benzodiazepines is usually manifested by central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, confusion, and lethargy. In more serious cases, symptoms may include ataxia, diminished reflexes, hypotonia, hypotension, respiratory depression, coma (rarely), and death (very rarely). Overdose of benzodiazepines in combination with other CNS depressants (including alcohol) may be fatal and should be closely monitored.
Management of Overdosage
- Following overdose with oral benzodiazepines, general supportive measures should be employed including the monitoring of respiration, pulse, and blood pressure. Vomiting should be induced (within 1 hour) if the patient is conscious. Gastric lavage should be undertaken with the airway protected if the patient is unconscious. Intravenous fluids should be administered. If there is no advantage in emptying the stomach, activated charcoal should be given to reduce absorption. Special attention should be paid to respiratory and cardiac function in intensive care. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Should hypotension develop, treatment may include intravenous fluid therapy, repositioning, judicious use of vasopressors appropriate to the clinical situation, if indicated, and other appropriate countermeasures. Dialysis is of limited value.
- As with the management of intentional overdosage with any drug, it should be considered that multiple agents may have been ingested.
- Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. Caution should be observed in the use of flumazenil in epileptic patients treated with benzodiazepines.
Pharmacology
Mechanism of Action
- Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
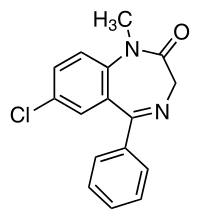
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Diazepam (oral) in the drug label.
Pharmacokinetics
Absorption
- After oral administration >90% of diazepam is absorbed and the average time to achieve peak plasma concentrations is 1 – 1.5 hours with a range of 0.25 to 2.5 hours. Absorption is delayed and decreased when administered with a moderate fat meal. In the presence of food mean lag times are approximately 45 minutes as compared with 15 minutes when fasting. There is also an increase in the average time to achieve peak concentrations to about 2.5 hours in the presence of food as compared with 1.25 hours when fasting. This results in an average decrease in Cmax of 20% in addition to a 27% decrease in AUC (range 15% to 50%) when administered with food.
Distribution
- Diazepam and its metabolites are highly bound to plasma proteins (diazepam 98%). Diazepam and its metabolites cross the blood-brain and placental barriers and are also found in breast milk in concentrations approximately one tenth of those in maternal plasma (days 3 to 9 post-partum). In young healthy males, the volume of distribution at steady-state is 0.8 to 1.0 L/kg. The decline in the plasma concentration-time profile after oral administration is biphasic. The initial distribution phase has a half-life of approximately 1 hour, although it may range up to >3 hours.
Metabolism
- Diazepam is N-demethylated by CYP3A4 and 2C19 to the active metabolite N-desmethyldiazepam, and is hydroxylated by CYP3A4 to the active metabolite temazepam. N-desmethyldiazepam and temazepam are both further metabolized to oxazepam. Temazepam and oxazepam are largely eliminated by glucuronidation.
Elimination
- The initial distribution phase is followed by a prolonged terminal elimination phase (half-life up to 48 hours). The terminal elimination half-life of the active metabolite N-desmethyldiazepam is up to 100 hours. Diazepam and its metabolites are excreted mainly in the urine, predominantly as their glucuronide conjugates. The clearance of diazepam is 20 to 30 mL/min in young adults. Diazepam accumulates upon multiple dosing and there is some evidence that the terminal elimination half-life is slightly prolonged.
Pharmacokinetics in Special Populations
Children
- In children 3 - 8 years old the mean half-life of diazepam has been reported to be 18 hours.
Newborns
- In full term infants, elimination half-lives around 30 hours have been reported, with a longer average half-life of 54 hours reported in premature infants of 28 - 34 weeks gestational age and 8 - 81 days post-partum. In both premature and full term infants the active metabolite desmethyldiazepam shows evidence of continued accumulation compared to children. Longer half-lives in infants may be due to incomplete maturation of metabolic pathways.
Geriatric
- Elimination half-life increases by approximately 1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age. This appears to be due to an increase in volume of distribution with age and a decrease in clearance. Consequently, the elderly may have lower peak concentrations, and on multiple dosing higher trough concentrations. It will also take longer to reach steady-state. Conflicting information has been published on changes of plasma protein binding in the elderly. Reported changes in free drug may be due to significant decreases in plasma proteins due to causes other than simply aging.
Hepatic Insufficiency
- In mild and moderate cirrhosis, average half-life is increased. The average increase has been variously reported from 2-fold to 5-fold, with individual half-lives over 500 hours reported. There is also an increase in volume of distribution, and average clearance decreases by almost half. Mean half-life is also prolonged with hepatic fibrosis to 90 hours (range 66 - 104 hours), with chronic active hepatitis to 60 hours (range 26 - 76 hours), and with acute viral hepatitis to 74 hours (range 49 - 129). In chronic active hepatitis, clearance is decreased by almost half.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Diazepam (oral) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Diazepam (oral) in the drug label.
How Supplied
- Diazepam Tablets USP 2 mg are scored, round, white tablets imprinted DAN 5621 and 2 supplied in bottles of 100, 500 and 1000.
- Diazepam Tablets USP 5 mg are scored, round, yellow tablets imprinted DAN 5619 and 5 supplied in bottles of 100, 500 and 1000.
- Diazepam Tablets USP 10 mg are scored, round, blue tablets imprinted DAN 5620 and 10 supplied in bottles of 100, 500 and 1000.
- Dispense in tight, light-resistant container with child-resistant closure.
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Diazepam (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Diazepam (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Diazepam (oral) in the drug label.
Precautions with Alcohol
- Concomitant use with alcohol is not recommended due to enhancement of the sedative effect.
Brand Names
- DIAZEPAM®[1]
Look-Alike Drug Names
There is limited information regarding Diazepam (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.