Diabetes mellitus type 1 medical therapy
|
Diabetes mellitus type 1 Microchapters |
|
Differentiating Diabetes mellitus type 1 from other Diseases |
|
Diagnosis |
|
Treatment |
|
Cardiovascular Disease and Risk Management |
|
Case Studies |
|
Diabetes mellitus Main page |
|
Patient Information |
|---|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.B.B.S. [2]; Cafer Zorkun, M.D., Ph.D. [3]
Overview
Treatment
Type 1 is treated with insulin replacement therapy — usually by injection or insulin pump, dietary control, typically including carbohydrate tracking, and careful monitoring of blood glucose levels using Glucose meters.
Untreated Type 1 diabetes can lead to one form of diabetic coma, diabetic ketoacidosis, which can be fatal. At present, insulin treatment must be continued for a lifetime; this will change if better treatment, or a cure, is discovered. Continuous glucose monitors have been developed which alert to the presence of dangerously high or low blood sugar levels.
In some extreme cases, a pancreas transplant can help restore proper glucose regulation. However, the surgery and accompanying immunosuppression required is considered by many physicians to be more dangerous than continued insulin replacement therapy and is therefore often used only as a last resort (such as when a kidney must also be transplanted or in cases where the patient's blood glucose levels are extremely control resistant). Experimental replacement of beta cells (by transplant or from stem cells) is being investigated in several research programs and may become clinically available in the future. Thus far, beta cell replacement has only been performed on patients over age 18, and with tantalizing successes amidst nearly universal failure.
Research foundations
The major charitable organization in the USA devoted to type 1 diabetes research is the Juvenile Diabetes Research Foundation (JDRF), whose mission is to cure type 1 diabetes and its complications through the support of research. Since its founding in 1970, JDRF has contributed more than $1 billion to diabetes research, including more than $137 million in FY 2007. In FY2007, the Foundation funded 700 centers, grants and fellowships in 20 countries.
The International Diabetes Federation is a worldwide alliance of over 160 countries to address diabetes research and treatment.
The American Diabetes Association funds some work on type 1 but devotes much of its resources to type 2 diabetes due to the increasing prevalence of the latter type.
Diabetes Australia is involved in promoting research and education in Australia on both type 1 and type 2 diabetes.
The Canadian Diabetes Association is also involved in educating, researching, and sustaining sufferers of Type 1 Diabetics in Canada.
Cure
As of 2008, there is no known cure for diabetes mellitus type 1 used by modern medical insitutes or hospitals.[1] There is ongoing research on various approaches to curing diabetes type 1.
Diabetes type 1 is caused by the non-existence of a sufficient number of beta cells in the body; these cells, which are found in the Langerhans islets in the pancreas, produce and secrete insulin, the single hormone responsible for allowing glucose to enter from the blood into cells. Hence, the phrase "curing diabetes type 1" means "causing a maintenance or restoration of the endogenous ability of the body to produce insulin in response to the level of blood glucose". This section does not deal with approaches other than that (for instance, closed-loop integrated glucometer/insulin pump products), which may also greatly increase quality of life for those who have diabetes type 1, and may by some be termed "artificial pancreas". Instead, it only deals with such approaches for thoroughly curing the underlying condition of diabetes type 1, by enabling the body to endogenously, in vivo, produce insulin in response to the level of blood glucose.
Reversion
Encapsulation approach
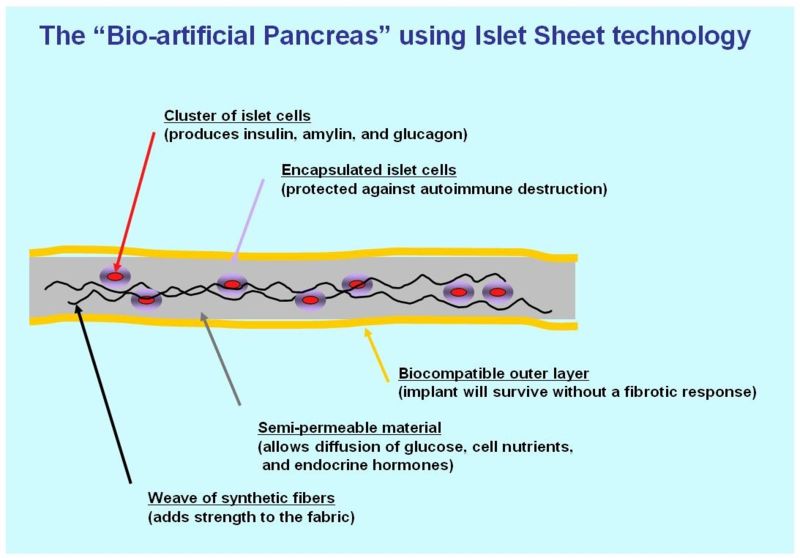
A biological approach to the artificial pancreas is to implant bioengineered tissue containing islet cells, which would secrete the amounts of insulin, amylin and glucagon needed in response to sensed glucose.
When islet cells have been transplanted via the Edmonton protocol, insulin production (and glycemic control) was restored, but at the expense of immunosuppression. Encapsulation of the islet cells in a protective coating has been developed to block the immune response to transplanted cells, which relieves the burden of immunosuppression and benefits the longevity of the transplant.[2]
One concept of the bio-artificial pancreas uses encapsulated islet cells to build an islet sheet which can be surgically implanted to function as an artificial pancreas.[3]
This islet sheet design consists of:
- an inner mesh of fibers to provide strength for the islet sheet;
- islet cells, encapsulated to avoid triggering a proliferating immune response, adhered to the mesh fibers;
- a semi-permeable protective layer around the sheet, to allow the diffusion of nutrients and secreted hormones;
- a protective coating, to prevent a foreign body response resulting in a fibrotic reaction which walls off the sheet and causes failure of the islet cells.
Islet sheet with encapsulation research is pressing forward with large animal studies at the present, with plans for human clinical trials within a few years.
Islet cell transplantation approach
Less invasive than a pancreas transplant, islet cell transplantation is considered a very promising approach to curing type 1 diabetes.
In one variant of this procedure, islet cells are injected into the patient's liver, where they take up residence and begin to produce insulin. The liver is expected to be the most reasonable choice because it is more accessible than the pancreas, and the islet cells seem to produce insulin well in that environment. The patient's body, however, will treat the new cells just as it would any other introduction of foreign tissue. The immune system will attack the cells as it would a bacterial infection or a skin graft. Thus, the patient also needs to undergo treatment involving immunosuppressants, which reduce immune system activity.
Recent studies have shown that islet cell transplants have progressed to the point that 58% of the patients in one study were insulin independent one year after the operation.[4] It would be best to use islet cells which will not provoke this immune reaction.
Islet cell regeneration approach
Research undertaken at the Massachusetts General Hospital in Boston Masschusetts from 2001 and 2003 demonstrated a protocol to reverse type 1 diabetes in mice.[5] Three other institutions have had similar results, published in the March 24, 2006 issue of Science. A fourth study by the National Institutes of Health further confirmed the approach, and also sheds light on the biological mechanisms involved.[6]
Stem cells approach
Research is being done at several locations in which islet cells are developed from stem cells.
In January 2006, a team of South Korean scientists has grown pancreatic beta cells, which can help treat diabetes, from stem cells taken from the umbilical cord blood of newborn babies.
In April 2007, it was reported by the Times Online that 15 young Brazilian patients diagnosed with Type 1 diabetes were able to naturally produce insulin once again after undergoing mild chemotherapy to temporarily weaken their immune systems and then injection of their own stem cells. This allowed the pancreatic beta cells to produce insulin. Since white blood cells were blocking the pancreas from producing insulin, Dr. Voltarelli and colleagues killed the immune cells, allowing the pancreas to secrete insulin once more.
However, there were no control subjects, which means that all of the processes could have been completely or partially natural. Secondly, no theory for the mechanism of cure has been promoted. It is too early to say whether the results will be positive or negative in the long run.[7]
Gene therapy approach
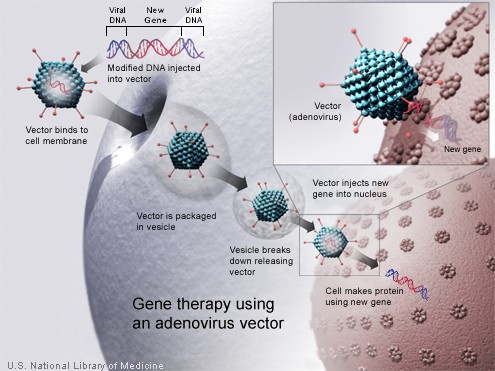
Technology for gene therapy is advancing rapidly such that there are multiple pathways possible to support endocrine function, with potential to practically cure diabetes.[8]
- Gene therapy can be used to manufacture insulin directly: an oral medication, consisting of viral vectors containing the insulin sequence, is digested and delivers its genes to the upper intestines. Those intestinal cells will then behave like any viral infected cell, and will reproduce the insulin protein. The virus can be controlled to infect only the cells which respond to the presence of glucose, such that insulin is produced only in the presence of high glucose levels. Due to the limited numbers of vectors delivered, very few intestinal cells would actually be impacted and would die off naturally in a few days. Therefore by varying the amount of oral medication used, the amount of insulin created by gene therapy can be increased or decreased as needed. As the insulin producing intestinal cells die off, they are boosted by additional oral medications.[9]
- Gene therapy might eventually be used to cure the cause of beta cell destruction, thereby curing the new diabetes patient before the beta cell destruction is complete and irreversible.[10]
- Gene therapy can be used to turn duodenum cells and duodenum adult stem cells into beta cells which produce insulin and amylin naturally. By delivering beta cell DNA to the intestine cells in the duodenum, a few intestine cells will turn into beta cells, and subsequently adult stem cells will develop into beta cells. This makes the supply of beta cells in the duodenum self replenishing, and the beta cells will produce insulin in proportional response to carbohydrates consumed.[11]
Yonsei University study
Scientists in the South Korean university of Yonsei have, in 2000, succeeded in reversing diabetes in mice and rats. Using a viral vector, a DNA encoding the production of an insulin analog was injected to the animals, which remained non-diabetic for at least the eight months duration of the study.[12]
Nanotechnology approach
Under the nanotechnological approach to curing diabetes type 1, many "nanobots" would be injected into the patient's bloodstream. These nanobots would be able to synthesize insulin, and to secrete it according to the level of glucose they would sense.[13]
Nano Mist
An American body called "Nano Mist" claims to be involved in a diabetes cure-related nanotechnology project. Their product is at least 10 years behind FDA approval.[14]
References
- ↑ Without the use of large doses of immunosuppressants, that causes a multitude of other medical issues.
- ↑ Cerco Medical: Science: Methods
- ↑ Cerco Medical: Company: Islet Sheet Research
- ↑ "Islet cell transplant: Experimental treatment for type 1 diabetes - MayoClinic.com". Retrieved 2007-06-04.
- ↑ "November 13, 2003 Regeneration of insulin-producing islets may lead to diabetes cure". Retrieved 2007-06-04.
- ↑ Faustman DL, Tran SD, Kodama S; et al. (2006). "Comment on papers by Chong et al., Nishio et al., and Suri et al. on diabetes reversal in NOD mice". Science. 314 (5803): 1243, author reply 1243. doi:10.1126/science.1129811. PMID 17124308.
- ↑ Voltarelli JC, Couri CE, Stracieri AB; et al. (2007). "Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA. 297 (14): 1568–76. doi:10.1001/jama.297.14.1568. PMID 17426276.
- ↑ Gene Therapy Approaches to Diabetes
- ↑ Mary Ann Liebert, Inc. - Cookie absent
- ↑ http://www.hopkinsbayview.org/healthcarenews06/060605diabetes.html
- ↑ Engene Inc
- ↑ Gene Therapy for Diabetes: Scientific American
- ↑ http://ieeexplore.ieee.org/iel5/6/29742/1353792/13537927.html
- ↑ The Nano Mist - Curing Diabetes
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Disease state
- Medicine
- Endocrinology
- Autoimmune diseases
- Mature chapter
- Diabetes
- Aging-associated diseases
- Medical conditions related to obesity
- Emergency medicine
- Primary care
- Emergency medicine patient information
- Overview complete
- For review