Islet cell transplantation
Islet transplantation is the transplantation of isolated islets from a donor pancreas and into another person. It is an experimental treatment for type 1 diabetes mellitus. Once transplanted, the islets begin to produce insulin, actively regulating the level of glucose in the blood.
Islets are usually infused into the patient's liver,[1]. The patient's body, however, will treat the infused islets just as it would any other introduction of foreign tissue: the immune system will attack the islets as it would a viral infection, leading to the risk of transplant rejection. Thus, the patient needs to undergo treatment involving immunosuppressants, which reduce immune system activity. Recent studies have shown that islet transplantation has progressed to the point that 58% of the patients in one study were insulin independent one year after the operation.[2]
In the period from 1999 to 2004, 471 patients with type 1 diabetes have received islet transplants at 43 institutions worldwide.[3]
History
The concept of islet transplantation is not new. Investigators as early as the English surgeon Charles Pybus (1882–1975) attempted to graft pancreatic tissue to cure diabetes. Most, however, credit the recent era of islet transplantation research to Paul Lacy's studies dating back more than three decades. In 1967, Lacy's group described a novel collagenase-based method (later modified by Dr. Camillo Ricordi, then working with Dr. Lacy) to isolate islets, paving the way for future in vitro and in vivo islet experiments.[4] Subsequent studies showed that transplanted islets could reverse diabetes in both rodents and non-human primates.[5][6] In a summary of the 1977 Workshop on Pancreatic Islet Cell Transplantation in Diabetes, Lacy commented on the feasibility of “islet cell transplantation as a therapeutic approach [for] the possible prevention of the complications of diabetes in man”.[7] Improvements in isolation techniques and immunosuppressive regimens ushered in the first human islet transplantation clinical trials in the mid-1980s. The first successful trial of human islet allotransplantation resulting in long-term reversal of diabetes was performed at the University of Pittsburgh in 1990.[8] Yet despite continued procedural improvements, only about 10% of islet recipients in the late 1990s achieved euglycemia (normal blood glucose). In 2000, Dr. James Shapiro and colleagues published a report describing seven consecutive patients who achieved euglycemia following islet transplantation using a steroid-free protocol and large numbers of donor islets, since referred to as the Edmonton protocol.[9] This protocol has been adapted by islet transplant centers around the world and has greatly increased islet transplant success.
Goals
The goal of islet transplantation is to infuse enough islets to control the blood glucose level removing the need for insulin injections. For an average-size person (70 kg), a typical transplant requires about one million islets, isolated from two donor pancreases. Because good control of blood glucose can slow or prevent the progression of complications associated with diabetes, such as nerve or eye damage, a successful transplant may reduce the risk of these complications. But a transplant recipient will need to take immunosuppressive drugs that stop the immune system from rejecting the transplanted islets.
Procedure
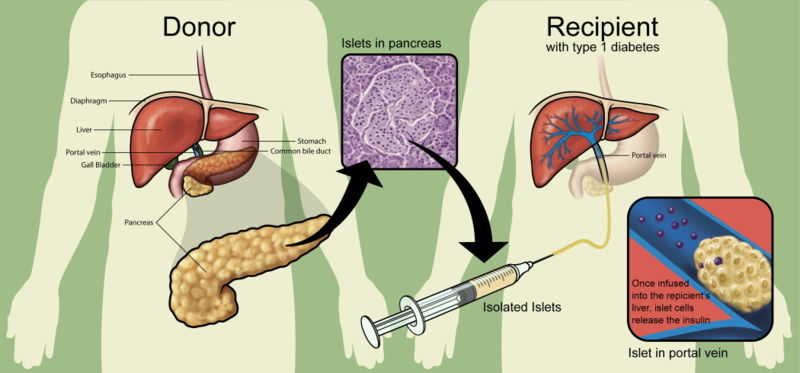
Researchers use a mixture of highly purified enzymes (Collagenase) to isolate islets from the pancreas of a deceased donor. Collagenase solution is injected into the pancreatic duct which runs through the head, body and tail of the pancreas. Delivered this way, the enzyme solution causes distension of the pancreas, which is subsequently cut into small chunks and transferred into so-called Ricordi's chamber, where digestion takes place until the islets are liberated and removed from the solution. Isolated islets are then separated from the exocrine tissue and debris in a process called purification.
During the transplant, a radiologist uses ultrasound and radiography to guide placement of a catheter through the upper abdomen and into the portal vein of the liver. The islets are then infused through the catheter into the liver. The patient will receive a local anesthetic. If a patient cannot tolerate local anesthesia, the surgeon may use general anesthesia and do the transplant through a small incision. Possible risks of the procedure include bleeding or blood clots.
It takes time for the islets to attach to new blood vessels and begin releasing insulin. The doctor will order many tests to check blood glucose levels after the transplant, and insulin may be needed until control is achieved.
-
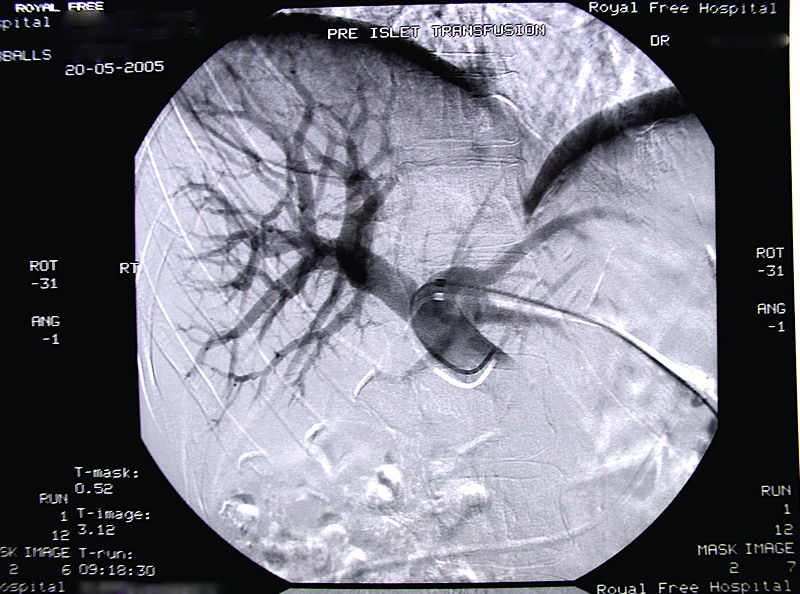
Radiographic image of the portal vein and its branches in the transplant recipient before infusion of isolated islets.
-
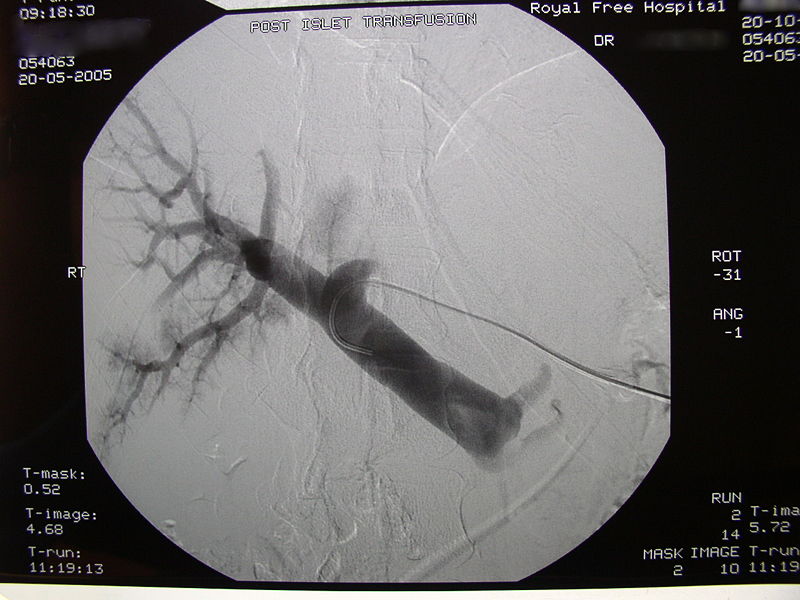
Post-transplant radiographic image of the recipient's portal tree.
Immunosuppression
The Edmonton protocol uses a combination of immunosuppressive drugs, also called antirejection drugs, including dacliximab (Zenapax), sirolimus (Rapamune) and tacrolimus (Prograf). Dacliximab is given intravenously right after the transplant and then discontinued. Sirolimus and tacrolimus, the two main drugs that keep the immune system from destroying the transplanted islets, must be taken for life.
Limitations
While significant progress has been made in the islet transplantation field [10], many obstacles remain that currently preclude its widespread application. Two of the most important limitations are the currently inadequate means for preventing islet rejection, and the limited supply of islets for transplantation. Current immunosuppressive regimens are capable of preventing islet failure for months to years, but the agents used in these treatments are expensive and may increase the risk for specific malignancies and opportunistic infections. In addition, and somewhat ironically, the most commonly used agents (like calcineurin inhibitors and rapamycin) are also known to impair normal islet function and/or insulin action. Further, like all medications, the agents have other associated toxicities, with side effects such as oral ulcers, peripheral edema, anemia, weight loss, hypertension, hyperlipidemia, diarrhea and fatigue.[11] Perhaps of greatest concern to the patient and physician is the harmful effect of certain widely employed immunosuppressive agents on renal function. For the patient with diabetes, renal function is a crucial factor in determining long-term outcome, and calcineurin inhibitors (tacrolimus and cyclosporin) are significantly nephrotoxic. Thus, while some patients with a pancreas transplant tolerate the immunosuppressive agents well, and for such patients diabetic nephropathy can gradually improve, in other patients the net effect (decreased risk due to the improved blood glucose control, increased risk from the immunosuppressive agents) may worsen kidney function. Indeed, Ojo et al. have published an analysis indicating that among patients receiving other-than-kidney allografts, 7%–21% end up with renal failure as a result of the transplant and/or subsequent immunosuppression.[12]
Seen another way, patients with heart, liver, lung, or kidney failure have a dismal prognosis for survival, so the toxicity associated with immunosuppression is warranted (the benefits of graft survival outweigh the risks associated with the medications). But for the subset of patients with diabetes and preserved kidney function, even those with long-standing and difficult-to-control disease, the prognosis for survival is comparatively much better. In addition to the immunosuppressive toxicities, other risks are associated with the islet transplant procedure itself, including intra-abdominal hemorrhage following the transplant, and portal vein thrombosis. The fact that there is already a good alternative to islet transplantation (i.e. the modern intensive insulin regimen) forces us to regard any newer, riskier interventions with a critical eye.
Like all transplantation therapies, islet transplantation is also handicapped by the limited donor pool. The numbers are striking; at least 1 million Americans have type 1 diabetes mellitus, and only a few thousand donor pancreata are available each year. To circumvent this organ shortage problem, researchers continue to look for ways to "grow" islets—or at least cells capable of physiologically regulated insulin secretion—in vitro, but currently only islets from cadaveric donors can be used to restore euglycemia. Further exacerbating the problem (and unlike kidney, liver, and heart transplants, where only one donor is needed for each recipient) most islet transplant patients require islets from two or more donors to achieve euglycemia. Lastly, the current methods for islet isolation need improvement, since only about half of attempted isolations produce transplant-ready islets.
While islet transplantation research has made important progress and the success stories are encouraging, the long-term safety and efficacy of the procedure remain unclear. Other concerns relating to the field include questions about the impact of having insulin-producing foreign cells within the hepatic parenchyma, the long-term consequences of elevated portal pressures resulting from the islet infusion, and the fact that islet recipients can be sensitized against donor tissue types, making it more difficult to find a suitable donor should another life-saving transplant be required in the future. Also, very few islet transplant recipients have remained euglycemic without the use of any exogenous insulin beyond four years post-transplant. Thus, while most islet recipients achieve better glycemia control and suffer less serious hypoglycemia, islet transplantation continues to fall short of the definitive diabetes cure.
Future
Just as early studies showed islet transplantation's promise, research must now overcome the hurdles revealed by the recent islet transplant experience. New immunomodulatory agents offer the greatest hope of revolutionizing the field. New drug regimens capable of inducing tolerance to the transplanted islets would allow recipients to maintain their grafts without general immunosuppression and its associated toxicities. While many targets are currently under investigation, none are ready for clinical use.
References
- ↑ Lakey J, Burridge P, Shapiro A (2003). "Technical aspects of islet preparation and transplantation". Transpl Int. 16 (9): 613–632. PMID 12928769.
- ↑ Close N, Hering B, Eggerman T (2005). "Results from the inaugural year of the Collaborative Islet Transplant Registry". Transplant Proc. 37 (2): 1305–1308. PMID 15848704.
- ↑ Shapiro A, Lakey J, Paty B, Senior P, Bigam D, Ryan E (2005). "Strategic opportunities in clinical islet transplantation". Transplantation. 79 (10): 1304–1307. PMID 15912095.
- ↑ Lacy P, Kostianovsky M (1967). "Method for the isolation of intact islets of Langerhans from the rat pancreas". Diabetes. 16 (1): 35–39. PMID 5333500.
- ↑ Kemp C, Knight M, Scharp D, Lacy P, Ballinger W (1973). "Transplantation of isolated pancreatic islets into the portal vein of diabetic rats". Nature. 244 (5416): 447. PMID 4200461.
- ↑ Scharp D, Murphy J, Newton W, Ballinger W, Lacy P (1975). "Transplantation of islets of Langerhans in diabetic rhesus monkeys". Surgery. 77 (1): 100–105. PMID 122797.
- ↑ Lacy P (1978). "Workshop on Pancreatic Islet Cell Transplantation in Diabetes sponsored by the National Institute of Arthritis, Metabolism, and Digestive Diseases and held at the National Institutes of Health in Bethesda, Maryland, on November 29 and 30, 1977". Diabetes. 27 (4): 427–429. PMID 416985.
- ↑ Tzakis A, Ricordi C, Alejandro R, Zeng Y, Fung J, Todo S, Demetris A, Mintz D, Starzl T (1990). "Pancreatic islet transplantation after upper abdominal exenteration and liver replacement". Lancet. 336 (8712): 402–405. PMID 1974944.
- ↑ Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, Kneteman N, Rajotte R (2000). "Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen". N Engl J Med. 343 (4): 230–238. PMID 10911004.
- ↑ Robertson R (2004). "Islet transplantation as a treatment for diabetes - a work in progress". N Engl J Med. 350 (7): 694–705. PMID 14960745.
- ↑ Hirshberg B, Rother K, Digon B, Lee J, Gaglia J, Hines K, Read E, Chang R, Wood B, Harlan D (2003). "Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience". Diabetes Care. 26 (12): 3288–3295. PMID 14633816. Full text
- ↑ Ojo A, Held P, Port F, Wolfe R, Leichtman A, Young E, Arndorfer J, Christensen L, Merion R (2003). "Chronic renal failure after transplantation of a nonrenal organ". N Engl J Med. 349 (10): 931–940. PMID 12954741.
- Lakey J, Mirbolooki M, Shapiro A. "Current status of clinical islet cell transplantation". Methods Mol Biol. 333: 47–104. PMID 16790847.
- Merani S, Shapiro A (2006). "Current status of pancreatic islet transplantation". Clin Sci (Lond). 110 (6): 611–625. PMID 16689680.
- Naftanel M, Harlan D (2004). "Pancreatic islet transplantation". PLoS Med. 1 (3): e58, quiz e75. PMID 15630467. Full text
External links
- VIDEO: Update on Islet Transplantation at the University of Wisconsin Dr. Luis Fernandez, November 2007.
- Diabetes Research Institute [1]
- Miami Islet Recipients [2]
- Mayo Clinic: Islet cell transplant: Emerging treatment for type 1 diabetes
- Clinical Islet Transplant Program - University of Alberta
- Immune Tolerance Network
Template:Organ transplantation