Brugada syndrome overview
|
Brugada syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Brugada syndrome overview On the Web |
|
American Roentgen Ray Society Images of Brugada syndrome overview |
|
Risk calculators and risk factors for Brugada syndrome overview |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The Brugada syndrome is a genetic disease that is characterized by abnormal electrocardiogram (EKG) findings and an increased risk of sudden cardiac death in young adults, and occasionally in children and infants.
Historical Perspective
The Brugada brothers were the first to describe the syndrome's characteristic EKG recordings and associate them with sudden death.
Before this association, the syndrome's characteristic EKG findings were often mistaken for a right ventricular myocardial infarction. In 1953 a publication by Oscher and Wolf mentioned that despite being mistaken for right ventricular myocardial infarction, the syndrome's characteristic EKG recordings were not associated with myocardial ischemia.[1]
Although the EKC recordings of Brugada syndrome were first reported[2] among survivors of cardiac arrest in 1989, it was not until 1992 that the Brugada brothers[3] recognized it as a distinct clinical entity that causes sudden death by triggering ventricular fibrillation.
Pathophysiology
Approximately 20% of the cases of Brugada syndrome have been shown to be associated with mutation(s) in the gene that encodes for the sodium ion channel in the cell membranes of the muscle cells of the heart (the myocytes). The gene, named SCN5A, is located on the short arm of the third chromosome (3p21). Loss-of-function mutations in this gene lead to a loss of the action potential dome of some epicardial areas of the right ventricle. This results in transmural and epicardial dispersion of repolarization. Over 160 mutations in the SCN5A gene have been discovered to date, each having varying mechanisms and effects on function, thereby explaining the varying degrees of penetration and expression of this disorder. [4]
Differentiating Brugada Syndrome from other Diseases
Abnormalities that can lead to ST-segment elevation in the right precordial leads include the following:[5]
- Acute myocardial ischemia or infarction
- Acute myocarditis
- Acute pericarditis
- Acute pulmonary thromboemboli
- Arrhythmogenic right ventricular dysplasia / cardiomyopathy (ARVD/C)[6][7]
- Cardioversion. Brugada-like ECG changes can be observed briefly after direct-current cardioversion. It is currently unclear if this is a sign that the patient is a gene carrier for Brugada syndrome.[8][9][10]
- Cocaine intoxication
- Coronary spasm
- Dissecting aortic aneurysm[11]
- Duchenne muscular dystrophy[12]
- Early repolarization
- Friedreich ataxia
- Heterocyclic antidepressant overdose
- Hypercalcemia[13][14]
- Hyperkalemia[15][16][17]
- Hypothermia, can cause an Osborn wave on the ECG which can sometimes resemble Brugada syndrome[18][19]
- Left ventricular hypertrophy
- Pectus excavatum[20]
- Prinzmetal's angina[21]
- Mediastinal tumor compressing the right ventricular outflow tract (RVOT)
- Right or left bundle-branch block (atypical)
- Right ventricular infarction
- Right ventricular ischemia
- Right ventricular outflow tract compression due to a mediastinal tumor[22]or hemopericardium[23]
- Thiamine deficiency[24]
- Various central and autonomic nervous system abnormalities
- Other conditions that can lead to ST-segment elevation in the right precordial leads
- Early repolarization syndrome
- Other normal variants (particularly in males)
Differentiating Brugada Syndrome from Arrhythmogenic Right Ventricular Dysplasia
Although both Brugada syndrome and Arrhythmogenic Right Ventricular Dysplasia are associated with sudden cardiac death in young patients, the two syndromes are fairly easy to distinguish electrocardiographically and clinically.
Genetics
There is only one gene associated with Brugada syndrome, namely the SCN5A gene, and there is no overlap of the genetic abnormalities associated with Arrhythmogenic Right Ventricular Dysplasia.
Structural Abnormalities of the right Ventricle
While Brugada syndrome is not associated with structural abnormalities in the right ventricle, arrhythmogenic right ventricular dysplasia is associated with fibrofatty infiltration.
Precipitant of Ventricular Arrhythmias
Arrhythmogenic right ventricular dysplasia is associated with monomorphic ventricular tachycardia with a left bundle branch morphology and is precipitated by catecholamines or exercise. In contrast, Brugada syndrome is associated with polymorphic ventricular tachycardia and occurs predominantly during sleep or rest.
Response to Pharmacologic Agents
The EKG abnormalities of Brugada syndrome are enhanced by vagotonic agents, beta-adrenergic blockers, and sodium channel blockers whereas the EKG changes of arrhythmogenic right ventricular dysplasia are constant and do not very with vagotonic agents, beta-adrenergic blockers, or sodium channel blockers.
Epidemiology and Demographics
Insofar as Brugada syndrome is a relatively newly recognized syndrome, its incidence and prevalence continues to increase. Brugada syndrome is quite common in Southeast Asia where it is endemic, and affects 50 out of every 10,000 individuals. It is the second leading cause of death after car accidents among young people in these countries. It has been estimated that Brugada syndrome accounts for 4% of all sudden cardiac deaths and 20% of sudden cardiac deaths among patients with structurally normal hearts. It is 8-10 times more common in men.
Prevalence
The prevalence of the Brugada syndrome is estimated at 5-50:10,000, largely depending on geographic location.
Age
The average age at the time of initial diagnosis or sudden death is 40 ± 22 years, with the youngest patient diagnosed at 2 days of age and the oldest at 84 years. Brugada syndrome usually becomes apparent in adulthood, although signs and symptoms, including sudden death, can occur any time from early infancy to old age. The mean age of sudden death is approximately 40 years. This condition may explain some cases of sudden infant death syndrome (SIDS), which is a major cause of death in babies younger than one year. It is characterized by sudden and unexplained death, usually during sleep. Sudden unexplained nocturnal death syndrome (SUNDS) is a condition characterized by unexpected cardiac arrest in young adults, usually at night during sleep. This condition was originally described in Southeast Asian populations, where it is a major cause of death. Researchers have determined that SUNDS and Brugada syndrome are the same disorder.
Race
This condition occurs much more frequently in people of Asian ancestry, particularly in Japanese and Southeast Asian populations. It is the most common cause of sudden death in young men without known underlying cardiac disease in Thailand and Laos[25]. In some southeast Asian countries the disease is considered endemic and believed to be the second cause of death among young men (after car accidents). In these countries Brugada syndrome is believed to underly (in part) the 'Sudden Unexpected Death Syndrome' (SUDS). This relation has, however, not been thoroughly investigated and there are almost no epidemiological studies into Brugada syndrome ECGs (apart from Japan). In different Asian countries, different names have been given to SUDS: in the Phillipines it is called bangungut (to rise and moan in sleep) and in Thailand lai tai (death during sleep).
Gender
Although Brugada syndrome affects both men and women, the condition appears to be 8 to 10 times more common in men. Researchers suspect that testosterone, a sex hormone present at much higher levels in men, may be responsible for this difference.
Risk Factors: Agents and Scenarios that Provoke the Brugada Syndrome Pattern
The EKG changes of Brugada syndrome can vary over time, depending on the autonomic balance and the administration of antiarrhythmic drugs. Adrenergic stimulation decreases the ST segment elevation, while vagal stimulation worsens it. During sleep, there is heightened vagal tone, and the pattern may be exacerbated at that time (as is the risk of sudden cardiac death at that time). The administration of class Ia, Ic and III drugs increases the ST segment elevation, as does fever. The impact of exercise depends upon when the EKG is obtained: during exercise the ST segment elevation may decrease but may increase later after exercise when the body temperature has risen. Similar to early repolarization variant, when the heart rate decreases, the ST segment elevation increases and when the heart rate increases the ST segment elevation decreases. While Brugada syndrome is often associated with polymorphic VT which may be self terminating, in the presence of autonomic imbalance, hypokalemia, fever or exacerbating drugs sustained ventricular fibrillation and sudden cardiac death may result.[26]
The electrocardiographic findings of Brugada syndrome are often concealed, but can be unmasked or modulated by a number of drugs and pathophysiological states including (in alphabetical order)[26]:
- A combination of glucose and insulin[27]. In Thailand large meals of glutinous sticky carbohydrate rich rice have been associated with sudden cardiac death.[28]
- Ajmaline[29] (a diagnostic test agent)
- α-adrenergic agonists[30]
- β-adrenergic blockers[31][30] such as propranolol.
- Calcium channel blockers
- Carotid sinus massage
- Cocaine[32][33][34]
- Dimenhydrinate
- Family History: In large studies, a family history of sudden cardiac death among patients with Brugada syndrome does not appear to be a risk factor for sudden cardiac death in siblings.
- Fever[35].[36][37][38][39] Hot baths and warm climates (such as that in Northeastern Thailand) may be precipitating factors for sudden cardiac death. It is for this reason that antipyretic agents are recommended to aggressively treat a fever in the patient with Brugada syndrome.
- Flecainide[31][40][41][42] (a diagnostic test agent)
- Hypercalcemia[13][14]
- Hyperkalemia[43][44][17]
- Hypokalemia.[45] Hypokalemia in a patient with Brugada syndrome may trigger sustained ventricular fibrillation and sudden cardiac death. In northeastern Thailand where potassium deficiency is widespread, there is a higher incidence of sudden cardiac death than is observed in Bangkok where potassium levels in food are much higher.[28]
- Lithium. Administration of Lithium can result in EKG manifestations of the Brugada syndrome. [46][47]. Syncope and sudden cardiac death have been observed in these patients.[48] The putative role of lithium has been suggested in so far as withdrawal of lithium results in either 1) normalization of the ECG or 2) conversion of the Brugada pattern to type 2 or 3. The appearance of Brugada type EKG patterns does not require toxic lithium levels.
- Phenothiazines
- Potassium channel openers such as nicorandil.
- Procainamide[31] [30](a diagnostic test agent)
- Propranolol intoxication[49]
- Selective serotonin reuptake inhibitors
- Shaving due to vagal stimulation[50][51][52]
- Sleep may exacerbate the electrocardiographic and clinical findings of brugada syndrome due to variations in the balance of sympathetic versus vagal tone, hormonal changes and other metabolic factors.[26][53][54][51]
- Sodium channel blockers[55][31][56][41] (a diagnostic test agent)
- Tetracyclic antidepressants[57]
Natural History
Brugada syndrome usually becomes apparent in adulthood, although signs and symptoms, including sudden death, can occur any time from early infancy to old age. The mean age of sudden death is approximately 40 years. This condition may explain some cases of sudden infant death syndrome (SIDS), which is a major cause of death in babies younger than one year. It is characterized by sudden and unexplained death, usually during sleep. Sudden unexplained nocturnal death syndrome (SUNDS) is a condition characterized by unexpected cardiac arrest in young adults, usually at night during sleep. This condition was originally described in Southeast Asian populations, where it is a major cause of death. Researchers have determined that SUNDS and Brugada syndrome are the same disorder.
Patients with Brugada syndrome frequently develop or are born with supraventricular tachycardias:[61]
- Supraventricular tachycardia: 20% of Brugada patients
- Atrial fibrillation: 10% - 20% of Brugada patients
- Atrioventricular (AV) nodal reentrant tachycardia
- Wolff-Parkinson-White syndrome[62]
Disturbances of atrial conduction and sinus node function have also been reported:
- Prolonged sinus node recovery time and sinoatrial conduction time [63]
- Slowed atrial conduction[5]
- Atrial standstill[5]
The appearance of atrial arrhythmias and impaired atrial conduction are remarkable in so far as these findings are associated with inducibility of ventricular fibrillation.[64] Indeed those patients who undergo implantation of a defibrillator (AICD) have twice the incidence of atrial arrhythmias (27% versus 13%)(p<0.05).
Complications
The following arrhythmias may occur in the patient with Brugada syndrome:
- Polymorphic VT resembling a rapid Torsade de Pointes (TdP) as shown below:

- Monomorphic VT is observed infrequently
- VT/VF often terminates spontaneously in patients with the Brugada syndrome which may explain why patients wake up at night after episodes of agonal respiration caused by the arrhythmia.
Prognosis
Patients who are symptomatic with unexplained syncope, ventricular tachycardia or aborted sudden cardiac death may have a symptom recurrence risk of 2% to 10% per year. In these patients an AICD implant is advisable.
Risk Stratification
In a study of 547 individuals who had confirmed Brugada syndrome who had no prior history of cardiac arrest, Brugada and associates identified the following correlates of future events:[65]
Inducibility on Electrophysiologic Testing
Patients who are inducible at the time electrophysiologic study have an eightfold increased risk of aborted sudden cardiac death compared with those patients who are not inducible.[66] Some groups have advocated that programmed electrical stimulation (PES) be performed to induce ventricular fibrillation for risk assessment in Brugada patients [67][68] Other groups have not reproduced the predictive value of these tests,[69][70] so the value of programmed electrical stimulation (PES) and inducibility remains controversial.
Spontaneous Type I Brugada Pattern
The presence of a spontaneous abnormal Type I pattern of ST segment elevation is associated with a 7.7 fold increased risk of in arrhythmic event during a patient's lifetime compared with those patients who only develop a Type I pattern following sodium blocker infusion.[71]
Male Gender
Male gender is associate with the 5.5 fold increased risk of sudden cardiac death.[72]
Family History
A family history of the disease is not associated with a higher risk of sudden death compared with sporadic occurrence of the disease.[73]
Symptoms
In another study, Brugada has reported that the symptoms of the patient may aid in risk stratification:[74]
- Brugada syndrome patients who present with aborted sudden cardiac death are at particularly high risk of recurrence with an incidence of 69% at 54 months of follow-up in the Brugada series.
- Brugada syndrome patients with syncope and Type 1 ST elevation pattern have a 19% risk of recurrence at 26 months.
- Brugada syndrome patients who are asymptomatic have an 8% risk of cardiac events over the same time period.
Genetic Testing
Genetic testing does not identify patients at high risk of sudden cardiac death and does not aid in risk stratification.[26]
Symptoms
The arrhythmias typically occur when an affected person is resting or asleep:
EKG Characteristics
There are three electrocardiographic patterns associated with Brugada syndrome: Type I, Type II and Type III. The diagnosis of Brugada syndrome is based upon the presence of Type I EKG changes. Patients with Type II or Type III Brugada patterns can convert to a Type I Brugada pattern following the administration of sodium channel blockers such as ajmaline and flecainide. Type 1 Brugada syndrome may always be present on the EKG, or it may be elicited by the administration of particular drugs (e.g., Class IC antiarrythmic drugs that blocks sodium channels such as ajmaline, flecainide) or it may be unmasked by various triggers or risk factors.
Type 1 Brugada pattern is characterized by ST elevations in leadsV1-V3 with a right bundle branch block (RBBB). A prolongation of the PR interval is also frequently seen. The EKG changes of Brugada syndrome can vary over time, depending on the autonomic balance and the administration of antiarrhythmic drugs. Adrenergic stimulation decreases the ST segment elevation, while vagal stimulation worsens it. The administration of class Ia, Ic and III drugs increases the ST segment elevation, as does fever. Exercise decreases ST segment elevation in some patients but increases it in others (after exercise when the body temperature has risen). The changes in heart rate induced by atrial pacing are accompanied by changes in the degree of ST segment elevation. When the heart rate decreases, the ST segment elevation increases and when the heart rate increases the ST segment elevation decreases.
The three patterns of Brugada syndrome (Type I,II,III) are shown below:
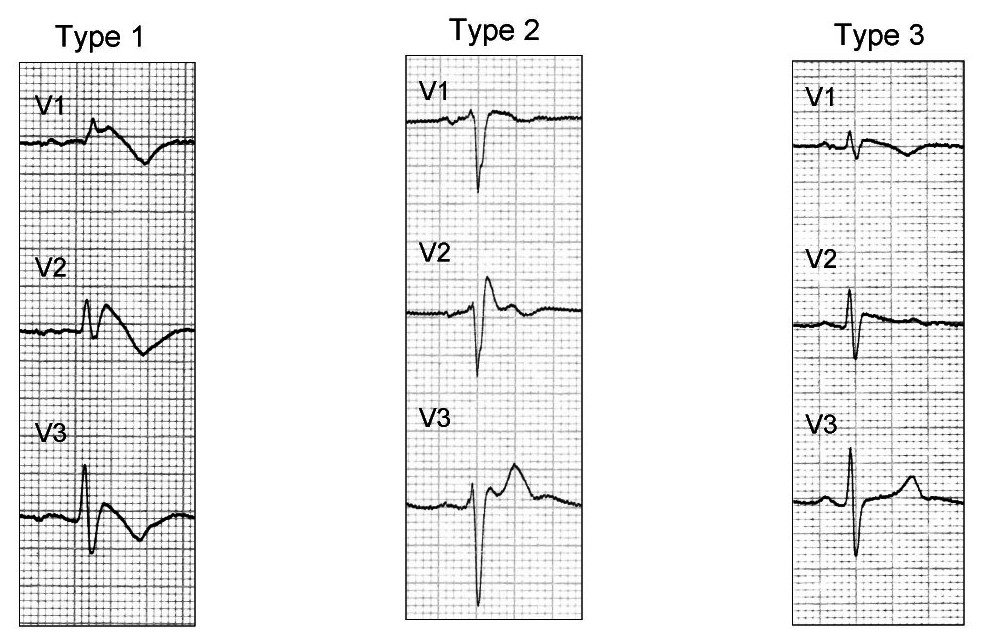
The table below is from ECGpedia and is adapted from Wilde et al.[75]
| Type I | Type II | Type III | |
|---|---|---|---|
| J wave amplitude | >= 2mm | >= 2mm | >= 2mm |
| T wave | Negative | Positive or biphasis | Positive |
| ST-T configuration | Coved type | Saddleback | Saddleback |
| ST segment (terminal portion) | Gradually descending | Elevated >= 1mm | Elevated < 1mm |
Diagnostic Criteria for Brugada Syndrome[26]
Only a Type I Brugada pattern qualifies as one of the required diagnostic criteria of Brugada syndrome. Type II and Type III EKG patterns do not qualify. Furthermore, the presence of the Type I Brugada pattern is necessary, but is not sufficient to make the diagnosis of Brugada syndrome. Other clinical criteria must be met as well. The diagnosis of Brugada syndrome requires that the criteria below be met:
1. The presence of Type 1 ST-segment elevation in more than one right precordial lead (V1-V3). Type I Brugada pattern ST elevation must be observed either spontaneously or following the administration of a sodium channel blocking agent.
2. One or more of the following criteria must also be met:
- Family history of sudden cardiac death (SCD) (<45 years old)
- Documented ventricular fibrillation (VF)
- Polymorphic ventricular tachycardia
- Coved-type ECG changes in family members
- Inducibility of ventricular tachycardia (VT) with programmed electrical stimulation (PES)
3. The patient is also diagnosed as having Brugada syndrome when a Type 2 (saddleback pattern) or Type 3 ST-segment elevation is observed in more than one right precordial lead under baseline conditions that can be converted to the diagnostic Type 1 Brugada pattern following administration of a sodium channel blocker.
Type 1 Brugada Pattern
As shown by the tracing below, the EKG characteristics of Type 1 Brugada syndrome include the following EKG findings in the right precordial leads (V1-V3):
- a) A broad P-wave with some PR prolongation
- b) J point elevation in the right precordial leads (V1-V3)
- c) Coved ST segment elevation
- d) An inverted T wave
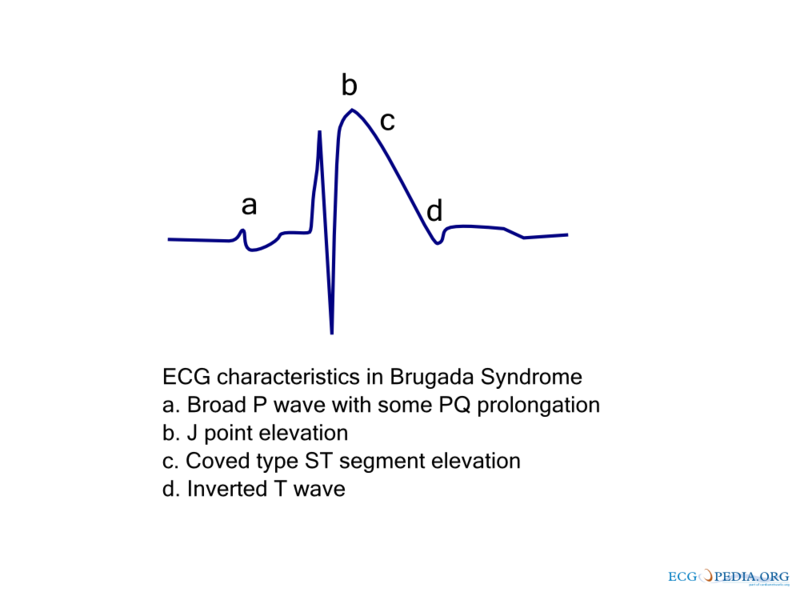
Slight QT prolongation may also be observed, particularly in the right precordial leads.[76][77][78] Typically these changes are in the right precordial leads but these EKG changes can occur in the inferior [79] or left precordial leads[80]. The fact that these cases may represent atypical variants of Brugada syndrome is supported by the observation that these cases were associated with SCN5A genetic abnormalities.
Shown below is an example of the EKG characteristics in Type I Brugada syndrome - a right bundle branch block morphology in leads V1-3 and ST segment elevation in leads V1-3:
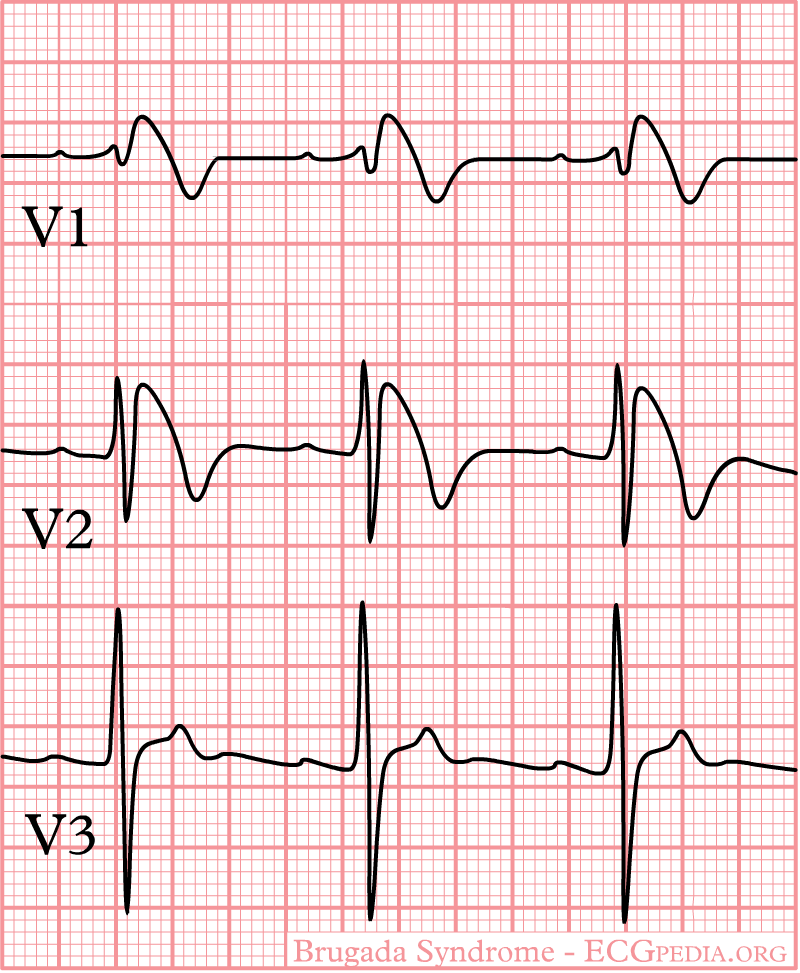
Unmaksing Type 1 Brugada Pattern
The electrocardiographic findings of Type 1 Brugada syndrome are often concealed, but may be unmasked by placing the leads higher on the chest (i.e. using the "Brugada Leads") or by infusion of a sodium channel blockers. Infusion of a sodium channel blocker may also convert a Type II or Type III Brugada pattern to a Type I Brugada pattern to establish a definitive diagnosis of the syndrome.
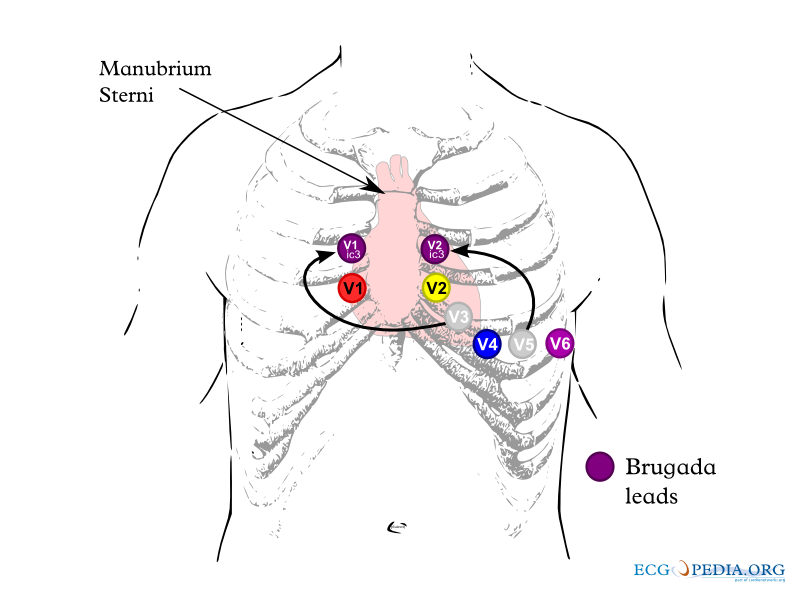
Unmasking Brugada Syndrome by Positioning the EKG Leads Higher on the Chest Wall: The Brugada Leads
The electrocardiographic findings of Brugada syndrome can be unmasked by placing the electrocardiographic leads higher on the chest. The EKG leads should be placed on the second and third intercostal space rather than the fourth intercostal space as shown below. When the electrodes are placed in this higher position they are called Brugada leads.[81][82]
Sodium Channel Blocker Challenge
Agents
Several sodium channel blockers are effective in unmasking Type 1 Brugada syndrome and in converting Type II and III Brugada syndrome to Type I to establish the diagnosis of Brugada syndrome. These agents include:
- Ajmaline 1 mg/kg/5 min IV
- Flecainide 2 mg/kg/10 min IV or 400 mg PO
- Procainamide 10 mg/kg/10 min IV
- Pilsicainide 1 mg/kg/10 min IV
When to Terminate the Sodium Channel Blocker Infusion
The sodium challenge should be terminated when:
- A diagnostic Type 1 Brugada pattern ST-segment elevation develops
- The ST segment elevation in Type 2 increases by ≥ 2 mm
- Premature ventricular beats or other arrhythmias develop
- the QRS widens to ≥ 130% of baseline
Precautions
The infusion should be carried out in a highly monitored area equipped to perform resuscitation. In an elderly patient with prolongation of the electrocardiographic intervals, the test is best performed in the electrophysiology laboratory. Exercise caution in the presence of pre-existing conduction abnormalities, or in the presence of QRS prolongation as the infusion may cause complete AV block. Isoproteronol can be used as an antidote should this complication should occur.
Type II Brugada Pattern
The Type II Brugada pattern is not diagnostic of Brugada syndrome. The Type II Brugada pattern is characterized by a "saddleback appearance" to the ST segment. The ST segment must be elevated greater than 2 mm, and the trough of the ST segment elevation at the bottom of the saddle must be elevated > 1 mm. The Type II Brugada pattern may alternate with the Type I Brugada pattern at different times in the same patient.
In order for a patient with type II Brugada pattern to be diagnosed as having Brugada syndrome, there must be a conversion of the Type II pattern to a Type I pattern with greater than 2 mm of ST segment elevation in the right precordial leads (either spontaneously or following infusion of a sodium channel blocking agent). In addition to these electrocardiographic changes, the required clinical criteria to establish the diagnosis of Brugada syndrome described above for the Type I Brugada pattern must also be present.
Shown below are examples of the Type II Brugada pattern demonstrating J point elevation, and a "saddle shaped" ST segment:
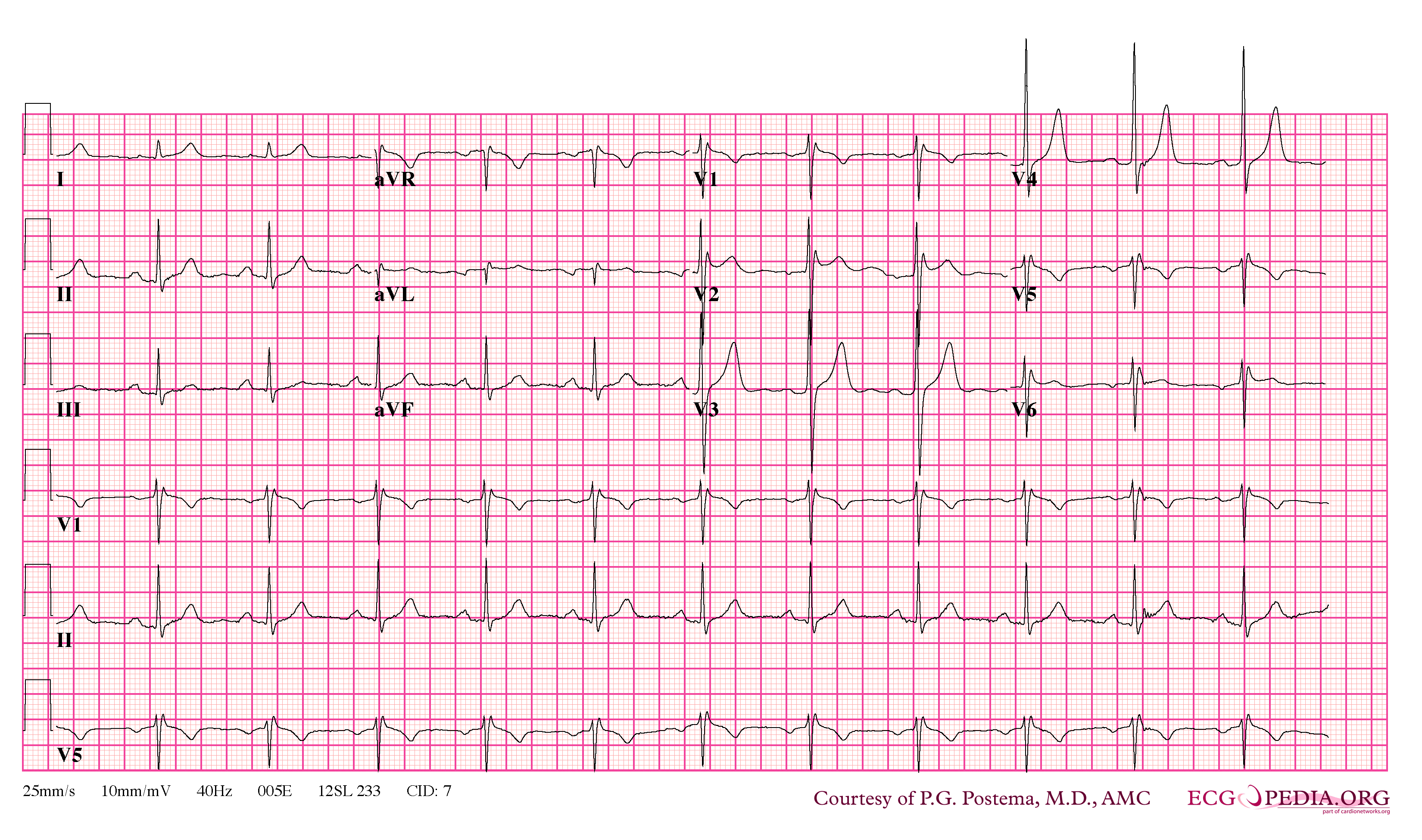
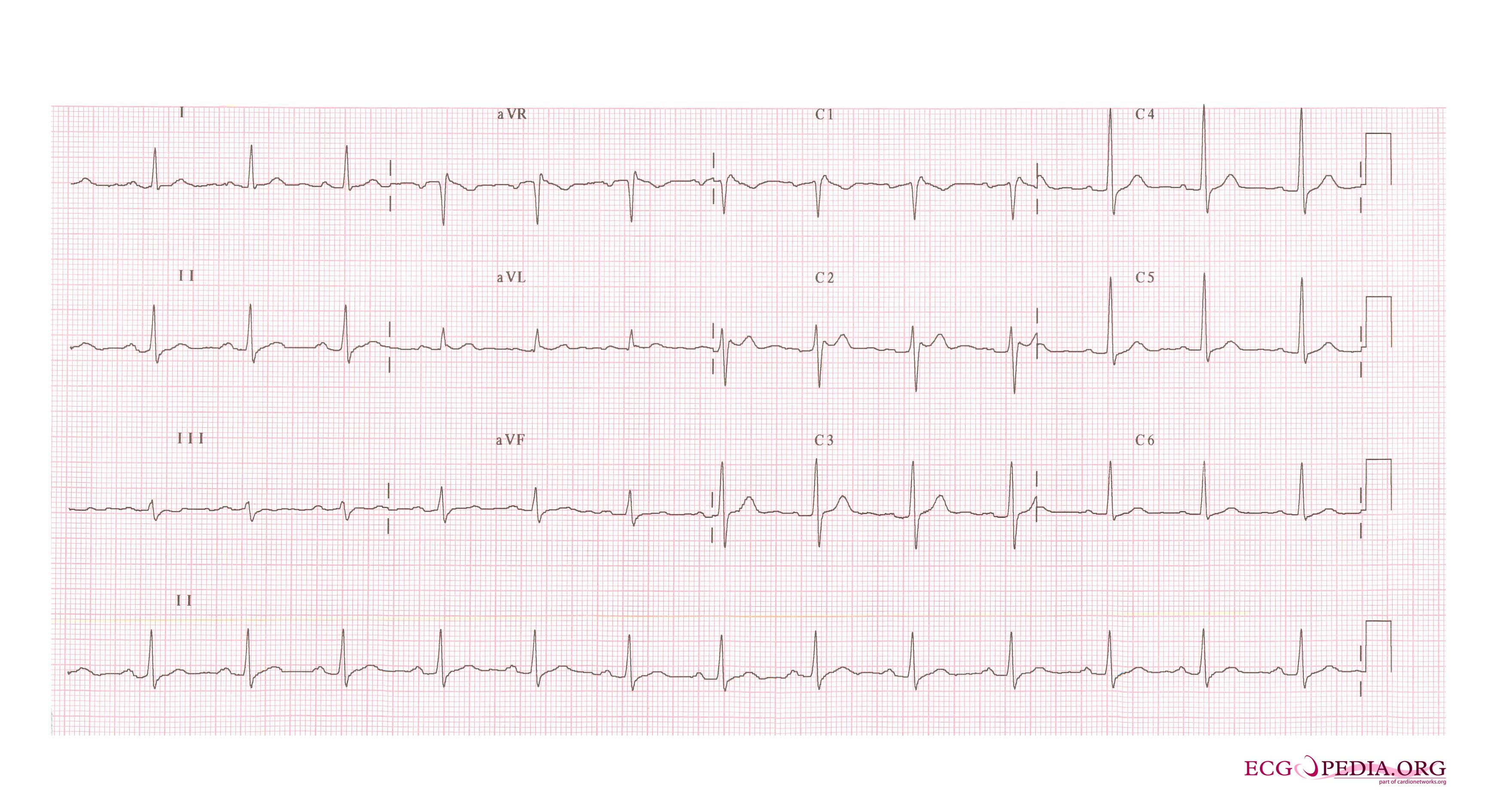
Type III Brugada Pattern
The Type 3 Brugada pattern is associated with either a "saddleback" or a "coved" appearance but the magnitude of ST-segment elevation is <1 mm.
In order for a patient with type II Brugada pattern to be diagnosed as having Brugada syndrome, there must be a conversion of the Type II pattern to a Type I pattern with greater than 2 mm of ST segment elevation in the right precordial leads (either spontaneously or following infusion of a sodium channel blocking agent). In addition to these electrocardiographic changes, the required clinical criteria to establish the diagnosis of Brugada syndrome described above for the Type I Brugada pattern must also be present.
A sodium channel blocker infusion can be administered to convert a type III Brugada pattern to a type I Brugada pattern to facilitate the definitive diagnosis of Brugada syndrome, however the conversion of a type III Brugada pattern to a type II Brugada pattern is not considered diagnostic of the Brugada syndrome.[26]
Treatment
Implantation of a cardiac defibrillator is the only proven method of treatment in Brugada syndrome. Patients with aborted sudden cardiac death are at high risk for recurrence and should undergo AICD implantation, and do not require an electrophysiologic study to assess inducibility. Patients with symptoms (either syncope, seizures or nocturnal agonal respirations) should undergo implantation of a defibrillator if no other cause of their symptoms can be identified. Asymptomatic patients should undergo electrophysiologic testing, and if VT / VF can be induced, they should undergo implantation of an ICD. Asymptomatic patients who cannot be induced should followed-up closely. Patients who are asymptomatic with no family history of Brugada syndrome can be followed-up closely.
The 2005 consensus statement divides patients into two groups:
- Higher risk patients with spontaneous Type I Brugada pattern
- A less high risk cohort of patients who require infusion of a sodium channel blocker to induce a Type I Brugada pattern.
The management of these two groups of patients will be discussed separately.
Management of Patients with a Spontaneous Type I Brugada Pattern
Implantation of a cardiac defibrillator should be considered in the following patients:
Symptomatic Patients
- Patients with aborted sudden cardiac death
- Patients with syncope, seizures or nocturnal agonal respirations who have no other identifiable cause for their symptoms
Asymptomatic Patients
- Patients with a family history of sudden cardiac death that is suspected to be due to Brugada syndrome in whom VT VF can be induced on electrophysiologic testing.
- Patients with no family history of sudden cardiac death in whom VT VF can be induced on electrophysiologic testing.
In essence, if VT VF can be induced on electrophysiologic testing in these patients, a cardiac defibrillator should be implanted. It is unclear if the same recommendations apply to those patients who require that the electrodes be placed one to two intercostal spaces higher to demonstrate a Brugada type I electrocardiographic pattern.
The flowchart below summarizes the recommendations of the 2005 consensus panel.
Management of Patients with a Sodium Channel Induced Type I Brugada Pattern
Implantation of a cardiac defibrillator should be considered in the following patients:
Symptomatic Patients
- Patients with aborted sudden cardiac death
- Patients with syncope, seizures or nocturnal agonal respirations who have no other identifiable cause for their symptoms
Asymptomatic Patients
- Patients with a family history of sudden cardiac death that is suspected to be due to Brugada syndrome in whom VT VF can be induced on electrophysiologic testing.
The flowchart below summarizes the recommendations of the 2005 consensus panel.
Pharmacotherapy
Pharmacotherapy alone may not be sufficient to treat Brugada syndrome, but it may be required in regions of the world where ICD implantation is cost prohibitive or in infants. Quinidine reduces the number of VF episodes and corrects spontaneous ECG changes, possibly via inhibiting Ito channels.[83] No drug has demonstrated long term efficacy in the prevention of sudden cardiac death.
Drugs with Potential Antiarrhythmic Effect
(Alphabetical order generic name)
| Generic name | Brand name® | Class / Clinical use | References | Recommendation |
| Cilostazol | e.g. Pletal® |
Phosphodiesterase inhibitor | Tsuchiya 2002 Abud 2006 Matsui 1999 |
Class IIb |
| Isoproterenol Isoprenaline |
e.g. Isuprel® |
Beta-adrenergic receptor stimulation | Miyazaki 1996 Suzuki 2000 Watanabe 2006 Ohgo 2007 Ganesan 2006 |
Class I |
| Orciprenaline | e.g. Alotec® Metaprel® Novasmasol® |
Beta-adrenergic receptor stimulation | Kyriazis 2009 | Class IIa |
| Quinidine | e.g. Quinalan® Chinidin® |
Antiarrhythmic Agent | Suzuki 2000 Alings 2001 Belhassen 2004 Mizusawa 2006 Probst 2007 Ohgo 2007 Yan 1999 |
Class I |
Recommendation: Class I: convincing evidence/opinion; Class IIa: evidence/opinion less clear; Class IIb: conflicting evidence/opinion; Class III: very little evidence.
Treatment of VT Storm
VT storm has been successfully treated with Isoproterenol. The mechanism is thought to be augmenting the cardiac L type channel.
Treatment of Coronary Ischemia
Patients with risk factors for coronary artery disease may require an angiogram before ICD implantation.
Treatment of Factors that may Precipitate Brugada Type EKG Changes and Clinical Symptoms
- Fever in a Brugada syndrome patient should be treated with an antipyretic.
- Brugada syndrome patients should avoid hot tubs, very hot baths or extremely hot climates.
- Hypokalemia, hyperkalemia, and hypercalcemia should be treated aggressively.
- Carbohydrate loading should be avoided.
ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death (DO NOT EDIT) [84]
Recommendations for Brugada Syndrome
| Class I |
| "1. An ICD is indicated for Brugada syndrome patients with previous cardiac arrest receiving chronic optimal medical therapy and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: C)" |
| Class IIa |
| "1. An ICD is reasonable for Brugada syndrome patients with spontaneous ST-segment elevation in V1, V2, or V3 who have had syncope with or without mutations demonstrated in the SCN5A gene and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: C)" |
| "2. Clinical monitoring for the development of a spontaneous ST-segment elevation pattern is reasonable for the management of patients with ST-segment elevation induced only with provocative pharmacological challenge with or without symptoms. (Level of Evidence: C)" |
| "3. An ICD is reasonable for Brugada syndrome patients with documented VT that has not resulted in cardiac arrest and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: C)" |
| "4. Isoproterenol can be useful to treat an electrical storm in the Brugada syndrome. (Level of Evidence: C)" |
| Class IIb |
| "1. EP testing may be considered for risk stratification in asymptomatic Brugada syndrome patients with spontaneous ST elevation with or without a mutation in the SCN5A gene. (Level of Evidence: C)" |
| "2. Quinidine might be reasonable for the treatment of electrical storm in patients with Brugada syndrome.(Level of Evidence: C)" |
References
- ↑ OSHER HL, WOLFF L (1953). "Electrocardiographic pattern simulating acute myocardial injury". The American Journal of the Medical Sciences. 226 (5): 541–5. PMID 13104407. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Martini B, Nava A, Thiene G, Buja GF, Canciani B, Scognamiglio R, Daliento L, Dalla Volta S. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J 1989 Dec;118(6):1203-9 PMID 2589161
- ↑ Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992 Nov 15;20(6):1391-6. PMID 1309182
- ↑ Napolitano C, Priori SG (2006). "Brugada syndrome". Orphanet journal of rare diseases. 1: 35. doi:10.1186/1750-1172-1-35. PMID 16972995.
- ↑ 5.0 5.1 5.2 Takehara N, Makita N, Kawabe J, Sato N, Kawamura Y, Kitabatake A, Kikuchi K (2004). "A cardiac sodium channel mutation identified in Brugada syndrome associated with atrial standstill". Journal of Internal Medicine. 255 (1): 137–42. PMID 14687250. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Corrado D, Nava A, Buja G, Martini B, Fasoli G, Oselladore L, Turrini P, Thiene G. Familial cardiomyopathy underlies syndrome of right bundle branch block, ST segment elevation and sudden death. J Am Coll Cardiol. 1996; 27: 443–448.
- ↑ Corrado D, Basso C, Buja G, Nava A, Rossi L, Thiene G. Right bundle branch block, right precordial ST-segment elevation, and sudden death in young people. Circulation. 2001; 103: 710–717.
- ↑ Kok LC, Mitchell MA, Haines DE, Mounsey JP, DiMarco JP (2000). "Transient ST elevation after transthoracic cardioversion in patients with hemodynamically unstable ventricular tachyarrhythmia". The American Journal of Cardiology. 85 (7): 878–81, A9. PMID 10758932. Retrieved 2012-10-14. Unknown parameter
|month=ignored (help) - ↑ Gurevitz O, Glikson M (2003). "Cardiac resynchronization therapy: a new frontier in the management of heart failure". The Israel Medical Association Journal : IMAJ. 5 (8): 571–5. PMID 12929296. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Gurevitz O, Lipchenca I, Yaacoby E, Segal E, Perel A, Eldar M, Glikson M (2002). "ST-segment deviation following implantable cardioverter defibrillator shocks: incidence, timing, and clinical significance". Pacing and Clinical Electrophysiology : PACE. 25 (10): 1429–32. PMID 12418739. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Myers GB. Other QRS-T patterns that may be mistaken for myocardial infarction; IV. Alterations in blood potassium; myocardial ischemia; subepicardial myocarditis; distortion associated with arrhythmias. Circulation. 1950; 2: 75–93.
- ↑ Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation. 1984; 69: 33–42.
- ↑ 13.0 13.1 Douglas PS, Carmichael KA, Palevsky PM (1984). "Extreme hypercalcemia and electrocardiographic changes". The American Journal of Cardiology. 54 (6): 674–5. PMID 6475795. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 14.0 14.1 Sridharan MR, Horan LG (1984). "Electrocardiographic J wave of hypercalcemia". The American Journal of Cardiology. 54 (6): 672–3. PMID 6475794. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Myers GB. Other QRS-T patterns that may be mistaken for myocardial infarction; IV. Alterations in blood potassium; myocardial ischemia; subepicardial myocarditis; distortion associated with arrhythmias. Circulation. 1950; 2: 75–93.
- ↑ Merrill JP, Levine HD, Somerville W, Smith S. Clinical recognition and treatment of acute potassium intoxication. Ann Intern Med. 1950; 33: 797–830.
- ↑ 17.0 17.1 Ortega-Carnicer J, Benezet J, Ruiz-Lorenzo F, Alcázar R (2002). "Transient Brugada-type electrocardiographic abnormalities in renal failure reversed by dialysis". Resuscitation. 55 (2): 215–9. PMID 12413761. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953; 175: 389–398.
- ↑ Noda T, Shimizu W, Tanaka K, Chayama K (2003). "Prominent J wave and ST segment elevation: serial electrocardiographic changes in accidental hypothermia". Journal of Cardiovascular Electrophysiology. 14 (2): 223. PMID 12693512. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Kataoka H. Electrocardiographic patterns of the Brugada syndrome in right ventricular infarction/ischemia. Am J Cardiol. 2000; 86: 1056.
- ↑ Wang K, Asinger RW, Marriott HJ (2003). "ST-segment elevation in conditions other than acute myocardial infarction". The New England Journal of Medicine. 349 (22): 2128–35. doi:10.1056/NEJMra022580. PMID 14645641. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Tarín N, Farré J, Rubio JM, Tuñón J, Castro-Dorticós J (1999). "Brugada-like electrocardiographic pattern in a patient with a mediastinal tumor". Pacing and Clinical Electrophysiology : PACE. 22 (8): 1264–6. PMID 10461308. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Tomcsanyi J, Simor T, Papp L. Images in cardiology. Haemopericardium and Brugada-like ECG pattern in rheumatoid arthritis. Heart. 2002; 87: 234.
- ↑ Read DH, Harrington DD (1981). "Experimentally induced thiamine deficiency in beagle dogs: clinical observations". American Journal of Veterinary Research. 42 (6): 984–91. PMID 7197132. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Brugada J, Brugada P, Brugada R. The syndrome of right bundle branch block ST segment elevation in V1 to V3 and sudden death--the Brugada syndrome. Europace. 1999 Jul;1(3):156-66. PMID 11225790
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A (2005). "Brugada syndrome: report of the second consensus conference". Heart Rhythm : the Official Journal of the Heart Rhythm Society. 2 (4): 429–40. PMID 15898165. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Nogami A, Nakao M, Kubota S, Sugiyasu A, Doi H, Yokoyama K; et al. (2003). "Enhancement of J-ST-segment elevation by the glucose and insulin test in Brugada syndrome". Pacing Clin Electrophysiol. 26 (1 Pt 2): 332–7. PMID 12687840.
- ↑ 28.0 28.1 Nimmannit S, Malasit P, Chaovakul V, Susaengrat W, Vasuvattakul S, Nilwarangkur S (1991). "Pathogenesis of sudden unexplained nocturnal death (lai tai) and endemic distal renal tubular acidosis". Lancet. 338 (8772): 930–2. PMID 1681278. Retrieved 2012-10-14. Unknown parameter
|month=ignored (help) - ↑ Rolf S, Bruns HJ, Wichter T, Kirchhof P, Ribbing M, Wasmer K; et al. (2003). "The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol". Eur Heart J. 24 (12): 1104–12. PMID 12804924.
- ↑ 30.0 30.1 30.2 Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S (1996). "Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome". J Am Coll Cardiol. 27 (5): 1061–70. doi:10.1016/0735-1097(95)00613-3. PMID 8609322.
- ↑ 31.0 31.1 31.2 31.3 Brugada P, Brugada J, Brugada R (2000). "Arrhythmia induction by antiarrhythmic drugs". Pacing Clin Electrophysiol. 23 (3): 291–2. PMID 10750126.
- ↑ Ortega-Carnicer J, Bertos-Polo J, Gutiérrez-Tirado C (2001). "Aborted sudden death, transient Brugada pattern, and wide QRS dysrrhythmias after massive cocaine ingestion". J Electrocardiol. 34 (4): 345–9. PMID 11590577.
- ↑ 33.0 33.1 Rouleau F, Asfar P, Boulet S, Dube L, Dupuis JM, Alquier P; et al. (2001). "Transient ST segment elevation in right precordial leads induced by psychotropic drugs: relationship to the Brugada syndrome". J Cardiovasc Electrophysiol. 12 (1): 61–5. PMID 11204086.
- ↑ Littmann L, Monroe MH, Svenson RH (2000). "Brugada-type electrocardiographic pattern induced by cocaine". Mayo Clin Proc. 75 (8): 845–9. doi:10.4065/75.8.845. PMID 10943241.
- ↑ Antzelevitch C, Brugada R (2002). "Fever and Brugada syndrome". Pacing Clin Electrophysiol. 25 (11): 1537–9. PMID 12494608.
- ↑ González Rebollo JM, Hernández Madrid A, García A, García de Castro A, Mejías A, Moro C (2000). "[Recurrent ventricular fibrillation during a febrile illness in a patient with the Brugada syndrome]". Rev Esp Cardiol. 53 (5): 755–7. PMID 10816181.
- ↑ Saura D, García-Alberola A, Carrillo P, Pascual D, Martínez-Sánchez J, Valdés M (2002). "Brugada-like electrocardiographic pattern induced by fever". Pacing Clin Electrophysiol. 25 (5): 856–9. PMID 12049381.
- ↑ Porres JM, Brugada J, Urbistondo V, García F, Reviejo K, Marco P (2002). "Fever unmasking the Brugada syndrome". Pacing Clin Electrophysiol. 25 (11): 1646–8. PMID 12494626.
- ↑ Kum LC, Fung JW, Sanderson JE (2002). "Brugada syndrome unmasked by febrile illness". Pacing Clin Electrophysiol. 25 (11): 1660–1. PMID 12494630.
- ↑ Fujiki A, Usui M, Nagasawa H, Mizumaki K, Hayashi H, Inoue H (1999). "ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome". J Cardiovasc Electrophysiol. 10 (2): 214–8. PMID 10090224.
- ↑ 41.0 41.1 Krishnan SC, Josephson ME (1998). "ST segment elevation induced by class IC antiarrhythmic agents: underlying electrophysiologic mechanisms and insights into drug-induced proarrhythmia". J Cardiovasc Electrophysiol. 9 (11): 1167–72. PMID 9835260.
- ↑ Gasparini M, Priori SG, Mantica M, Napolitano C, Galimberti P, Ceriotti C; et al. (2003). "Flecainide test in Brugada syndrome: a reproducible but risky tool". Pacing Clin Electrophysiol. 26 (1 Pt 2): 338–41. PMID 12687841.
- ↑ MYERS GB (1950). "Other QRS-T patterns that may be mistaken for myocardial infarction; IV. alterations in blood potassium; myocardial ischemia; subepicardial myocarditis; distortion associated with arrhythmias". Circulation. 2 (1): 75–93. PMID 15427197.
- ↑ MERRILL JP, LEVINE HD, SOMERVILLE W, SMITH S (1950). "Clinical recognition and treatment of acute potassium intoxication". Ann Intern Med. 33 (4): 797–830. PMID 14771753.
- ↑ Araki T, Konno T, Itoh H, Ino H, Shimizu M (2003). "Brugada syndrome with ventricular tachycardia and fibrillation related to hypokalemia". Circ J. 67 (1): 93–5. PMID 12520160.
- ↑ Pirotte MJ, Mueller JG, Poprawski T. A case report of Brugada-type electrocardiographic changes in a patient taking lithium. Am J Emerg Med. 2008; 26: 113.
- ↑ Wright D, Salehian O. Brugada-Type Electrocardiographic Changes Induced by Long-Term Lithium Use. Circulation, FRCPC2010;122:e418-e419
- ↑ Laske C, Soekadar SR, Laszlo R, Plewnia C. Brugada syndrome in a patient treated with lithium. Am J Psychiatry. 2007; 164: 1440–1441.
- ↑ Aouate P, Clerc J, Viard P, Seoud J (2005). "Propranolol intoxication revealing a Brugada syndrome". J Cardiovasc Electrophysiol. 16 (3): 348–51. doi:10.1046/j.1540-8167.2005.40564.x. PMID 15817098.
- ↑ 50.0 50.1 Kasanuki H, Ohnishi S, Ohtuka M, Matsuda N, Nirei T, Isogai R; et al. (1997). "Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease". Circulation. 95 (9): 2277–85. PMID 9142005.
- ↑ 51.0 51.1 51.2 Mizumaki K, Fujiki A, Tsuneda T, Sakabe M, Nishida K, Sugao M; et al. (2004). "Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome". J Cardiovasc Electrophysiol. 15 (6): 667–73. doi:10.1046/j.1540-8167.2004.03601.x. PMID 15175062.
- ↑ 52.0 52.1 Litovsky SH, Antzelevitch C (1990). "Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium". Circ Res. 67 (3): 615–27. PMID 2397572.
- ↑ Kasanuki H, Ohnishi S, Ohtuka M, Matsuda N, Nirei T, Isogai R, Shoda M, Toyoshima Y, Hosoda S. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997; 95: 2277–2285.
- ↑ Proclemer A, Facchin D, Feruglio GA, Nucifora R (1993). "[Recurrent ventricular fibrillation, right bundle-branch block and persistent ST segment elevation in V1-V3: a new arrhythmia syndrome? A clinical case report]". Giornale Italiano Di Cardiologia (in Italian). 23 (12): 1211–8. PMID 8174872. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA; et al. (2000). "Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts". Circulation. 101 (5): 510–5. PMID 10662748.
- ↑ Shimizu W, Antzelevitch C, Suyama K, Kurita T, Taguchi A, Aihara N; et al. (2000). "Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome". J Cardiovasc Electrophysiol. 11 (12): 1320–9. PMID 11196553.
- ↑ 57.0 57.1 Bolognesi R, Tsialtas D, Vasini P, Conti M, Manca C (1997). "Abnormal ventricular repolarization mimicking myocardial infarction after heterocyclic antidepressant overdose". Am J Cardiol. 79 (2): 242–5. PMID 9193039.
- ↑ Goldgran-Toledano D, Sideris G, Kevorkian JP (2002). "Overdose of cyclic antidepressants and the Brugada syndrome". N Engl J Med. 346 (20): 1591–2. doi:10.1056/NEJM200205163462020. PMID 12015405.
- ↑ Tada H, Sticherling C, Oral H, Morady F (2001). "Brugada syndrome mimicked by tricyclic antidepressant overdose". J Cardiovasc Electrophysiol. 12 (2): 275. PMID 11232630.
- ↑ Babaliaros VC, Hurst JW (2002). "Tricyclic antidepressants and the Brugada syndrome: an example of Brugada waves appearing after the administration of desipramine". Clin Cardiol. 25 (8): 395–8. PMID 12173907.
- ↑ Morita H, Kusano-Fukushima K, Nagase S, Fujimoto Y, Hisamatsu K, Fujio H, Haraoka K, Kobayashi M, Morita ST, Nakamura K, Emori T, Matsubara H, Hina K, Kita T, Fukatani M, Ohe T. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J Am Coll Cardiol. 2002; 40: 1437–1444.
- ↑ Eckardt L, Kirchhof P, Johna R, Haverkamp W, Breithardt G, Borggrefe M (2001). "Wolff-Parkinson-White syndrome associated with Brugada syndrome". Pacing and Clinical Electrophysiology : PACE. 24 (9 Pt 1): 1423–4. PMID 11584469. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Morita H, Fukushima-Kusano K, Nagase S, Miyaji K, Hiramatsu S, Banba K, Nishii N, Watanabe A, Kakishita M, Takenaka-Morita S, Nakamura K, Saito H, Emori T, Ohe T (2004). "Sinus node function in patients with Brugada-type ECG". Circulation Journal : Official Journal of the Japanese Circulation Society. 68 (5): 473–6. PMID 15118291. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Eur Heart J (2004) 25;(10): 879-884. doi: 10.1016/j.ehj.2004.01.004
- ↑ Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003; 108: 3092–3096.
- ↑ Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003; 108: 3092–3096.
- ↑ Brugada J, Brugada R, Antzelevitch C, Towbin J, Nademanee K, Brugada P (2002). "Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3". Circulation. 105 (1): 73–8. PMID 11772879. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Brugada P, Brugada R, Mont L, Rivero M, Geelen P, Brugada J (2003). "Natural history of Brugada syndrome: the prognostic value of programmed electrical stimulation of the heart". Journal of Cardiovascular Electrophysiology. 14 (5): 455–7. PMID 12776858. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Priori SG, Napolitano C, Gasparini M, Pappone C, Della Bella P, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, Ronchetti E, Faggiano G, Nastoli J (2002). "Natural history of Brugada syndrome: insights for risk stratification and management". Circulation. 105 (11): 1342–7. PMID 11901046. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Eckardt L, Probst V, Smits JP, Bahr ES, Wolpert C, Schimpf R, Wichter T, Boisseau P, Heinecke A, Breithardt G, Borggrefe M, LeMarec H, Böcker D, Wilde AA (2005). "Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome". Circulation. 111 (3): 257–63. doi:10.1161/01.CIR.0000153267.21278.8D. PMID 15642768. Retrieved 2012-10-13. Unknown parameter
|month=ignored (help) - ↑ Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003; 108: 3092–3096.
- ↑ Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003; 108: 3092–3096.
- ↑ Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003; 108: 3092–3096.
- ↑ Brugada J, Brugada R, Antzelevitch C, Towbin J, Nademanee K, Brugada P. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002; 105: 73–78.
- ↑ Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA (2002). "Proposed diagnostic criteria for the Brugada syndrome: consensus report". Circulation. 106 (19): 2514–9. PMID 12417552. Retrieved 2012-10-14. Unknown parameter
|month=ignored (help) - ↑ Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999; 99: 666–673.
- ↑ Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, Van Langen IM, Tan-Sindhunata G, Bink-Boelkens MT, van Der Hout AH, Mannens MM, Wilde AA. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999; 85: 1206–1213.
- ↑ Priori SG, Napolitano C, Gasparini M, Pappone C, Della Bella P, Brignole M, Giordano U, Giovannini T, Menozzi C, Bloise R, Crotti L, Terreni L, Schwartz PJ. Clinical and genetic heterogeneity of right bundle branch block and ST-segment elevation syndrome: a prospective evaluation of 52 families. Circulation. 2000; 102: 2509–2515.
- ↑ Kalla H, Yan GX, Marinchak R (2000). "Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant?". Journal of Cardiovascular Electrophysiology. 11 (1): 95–8. PMID 10695469. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Horigome H, Shigeta O, Kuga K, Isobe T, Sakakibara Y, Yamaguchi I, Matsui A (2003). "Ventricular fibrillation during anesthesia in association with J waves in the left precordial leads in a child with coarctation of the aorta". Journal of Electrocardiology. 36 (4): 339–43. PMID 14661171. Retrieved 2012-10-14. Unknown parameter
|month=ignored (help) - ↑ Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S (2000). "Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms". Journal of Cardiovascular Electrophysiology. 11 (4): 396–404. PMID 10809492. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ sukhowong P, Tungsanga K. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001; 22: 2290–2296.
- ↑ Belhassen B, Glick A, Viskin S (2004). "Efficacy of quinidine in high-risk patients with Brugada syndrome". Circulation. 110 (13): 1731–7. doi:10.1161/01.CIR.0000143159.30585.90. PMID 15381640.
- ↑ Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M; et al. (2006). "ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (10): e385–484. doi:10.1161/CIRCULATIONAHA.106.178233. PMID 16935995.