Atrial septal defect: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
'''Editors-In-Chief:''' Claudia Hochberg, M.D.; [[User:C Michael Gibson |C. Michael Gibson, M.S., M.D.]] [mailto:mgibson@perfuse.org] Phone:617-525-6884 | '''Editors-In-Chief:''' Claudia Hochberg, M.D.; [[User:C Michael Gibson |C. Michael Gibson, M.S., M.D.]] [mailto:mgibson@perfuse.org] Phone:617-525-6884 | ||
'''Associate Editors-In-Chief:''' {{CZ}}; Keri Shafer, M.D. [mailto:kshafer@bidmc.harvard.edu] | '''Associate Editors-In-Chief:''' {{CZ}}; [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu] | ||
{{Editor Join}} | {{Editor Join}} | ||
Revision as of 21:30, 7 January 2009
| Atrial septal defect | ||
 | ||
|---|---|---|
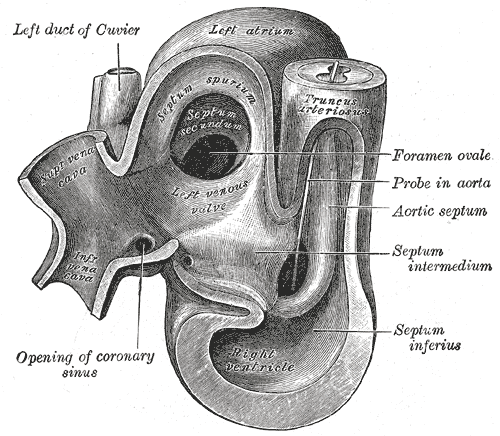
| Heart of human embryo of about thirty-five days | ||
| ICD-10 | Q21.1 | |
| ICD-9 | 745.5-745.6 | |
| OMIM | 108800 | |
| DiseasesDB | 1089 | |
| eMedicine | med/3519 | |
| MeSH | C14.240.400.560.375 | |
| Cardiology Network |
 Discuss Atrial septal defect further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editors-In-Chief: Claudia Hochberg, M.D.; C. Michael Gibson, M.S., M.D. [1] Phone:617-525-6884
Associate Editors-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Keri Shafer, M.D. [3]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Atrial septal defects (ASD) are a group of congenital heart diseases that involve the inter-atrial septum. The inter-atrial septum is the tissue that separates the right and left atria from each other. This tissue prevents arterial and venous blood from mixing with each other. If there is a defect in this septum, a direct communication between the atria can occur, which allows shunting, resulting in mixing of arterial and venous blood. It is possible for blood to travel from the left side of the heart to the right side of the heart, or vice versa. The direction of shunting will depend on a variety of factors, notable the patient's hemodynamics.
It should be noted however, that a "Right-to-left-shunt" typically poses the more dangerous scenario (See Pathophysiology below). Since the right side of the heart contains venous blood with a low oxygen content, and the left side of the heart contains arterial blood with a high oxygen content, right to left shunts can cause hypoxia and result in cyanosis. Additionally, a communication between the two atria may allow blood clots to pass from the venous system to the arterial system and result in a stroke or peripheral embolism.
Embryology
During development of the fetus, the interatrial septum develops to eventually separate the left and right atria. The foramen ovale (pronounced Template:IPA) remains open during fetal development to allow blood from the venous system to bypass the lungs directly and enter the circulatory system. This is so, as prior to birth, the oxygenation of the blood is provided via the mother's placenta as the lungs of the fetus are not breathing air. A layer of tissue begins to cover the foramen ovale during fetal development, in which typically, after birth, the pressure in the pulmonary circulatory system drops, thus causing the foramen ovale to close entirely. In approximately 25% of adults, the foramen ovale does not entirely seal. In this case, elevation of pressure in the pulmonary circulatory system (ie: pulmonary hypertension due to various causes, or transiently during a cough) can cause the foramen ovale to remain open. This is known as a patent foramen ovale (PFO).
Pathophysiology
In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the left ventricle has to produce enough pressure to pump blood throughout the entire body, while the right ventricle only has to produce enough pressure to pump blood to the lungs.
In the case of a large ASD (>9mm), which may result in a clinically remarkable left-to-right shunt, blood will shunt from the left atrium to the right atrium causing excessive interatrial communication (In the case of hemodynamically significant ASD (Qp:Qs > 1.5:1), the patient is often found to be notably symptomatic and ASD repair may be indicated). This extra blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle, which if left untreated, can result in enlargement of the right side of the heart and ultimately heart failure.
Any process that increases the pressure in the left ventricle can cause worsening of the left-to-right shunt. This includes hypertension, which increases the pressure that the left ventricle has to generate in order to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole.
The right ventricle will have to push out more blood than the left ventricle due to the left-to-right shunt. This constant overload of the right side of the heart will cause an overload of the entire pulmonary vasculature. Eventually the pulmonary vasculature will develop pulmonary hypertension to try to divert the extra blood volume away from the lungs.
The pulmonary hypertension will cause the right ventricle to face increased afterload in addition to the increased preload that the shunted blood from the left atrium to the right atrium caused. The right ventricle will be forced to generate higher pressures to try to overcome the pulmonary hypertension. This may lead to right ventricular failure (dilatation and decreased systolic function of the right ventricle) or elevations of the right sided pressures to levels greater than the left sided pressures.
When the pressure in the right atrium rises to the level in the left atrium, there will no longer be a pressure gradient between these heart chambers, and the left-to-right shunt will diminish or cease.
If left uncorrected, the pressure in the right side of the heart will be greater than the left side of the heart. This will cause the pressure in the right atrium to be higher than the pressure in the left atrium. This will reverse the pressure gradient across the ASD, and the shunt will reverse; a right-to-left shunt will exist. This phenomenon is known as Eisenmenger's syndrome.
Once right-to-left shunting occurs, a portion of the oxygen-poor blood will get shunted to the left side of the heart and ejected to the peripheral vascular system. This will cause signs of cyanosis.
Epidemiology
As a group, atrial septal defects are detected in 1 child per 1500 live births. PFO are quite common (appearing in 10 - 20% of adults) but asymptomatic and therefore undiagnosed. ASDs make up 30 to 40% of all congenital heart disease that is seen in adults.[1]
The ostium secundum atrial septal defect accounts for 7% of all congenital heart lesions. This lesion shows a female preponderance, with a male : female ratio of 1:2.[2]
Patent Foramen Ovales (PFOs) are quite common (appearing in 10 - 20% of adults) but are asymptomatic and therefore undiagnosed. ASDs make up 30 to 40% of all congenital heart disease that is seen in adults and are the most common form of congenital heart defect in adults besides bicuspid aortic valve and mitral valve prolapse.
Genetics
ASDs are often associated with other malformations. There is a well described asssociation with primum or secundum ASDs with Down syndrome. Other associated lesions include partially anomalous pulmonary venous return, pulmonary valve stenosis, mitral stenosis or mitral valve prolapse, ventricular septal defects and coarctation of the aorta. There are a small group of ASDs that may have a familial occurence.
Types of atrial septal defects
The different types of atrial septal defects originate in different parts of the septum and are named according to their origins.
There are many types of atrial septal defects. They are differentiated from each other by whether they involve other structures of the heart and how they are formed during the developmental process during early fetal development.
Ostium secundum atrial septal defect
The ostium secundum atrial septal defect is the most common type of atrial septal defect (it accounts for 60%-70% of ASDs), and comprises 6-10% of all congenital heart diseases.
The secundum atrial septal defect usually arises from an enlarged foramen ovale, inadequate growth of the septum secundum, or excessive absorption of the septum primum. 10 to 20 percent of individuals with ostium secundum ASDs also have mitral valve prolapse .[3]
Most individuals with an uncorrected secundum ASD don't have significant symptoms through early adulthood. About 70% develop symptoms by the time they are in their 40s. Symptoms are typically decreased exercise tolerance, easy fatigueability, palpitations, and syncope.
Complications of an uncorrected secundum ASD include pulmonary hypertension, right-sided heart failure, atrial fibrillation or flutter, stroke, and Eisenmenger's syndrome.
While pulmonary hypertension is unusual before 20 years of age, it is seen in 50% of individuals above the age of 40. Progression to Eisenmenger's syndrome occurs in 5 to 10% of individuals late in the disease process.
Patent foramen ovale
A patent foramen ovale (PAY-tent for-amen oh-VALL-ee) (PFO) is a small channel that has little hemodynamic consequence. Clinically it is linked to decompression sickness, paradoxical embolism and migraine. On echocardiography, there may not be any shunting of blood noted except when the patient coughs.
There is a debate within the neurology and cardiology communities about the role of a PFO in cryptogenic (ie of unknown cause) neurologic events, e.g. strokes and transient ischemia attacks (TIAs) without any other potential cause. In addition, there is some data to suggest that PFOs may be involved in the pathogenesis of some migraine headaches. Several clinical trials are currently underway to investigate the role of PFO in these clinical situations..
Ostium primum atrial septal defect
The ostium primum atrial septal defect (also known as an endocardial cushion defect) is a defect in the atrial septum at the level of the tricuspid and mitral valves. This is sometimes known as an endocardial cushion defect because it often involves the endocardial cushion, which is the portion of the heart where the atrial septum meets the ventricular septum and the mitral valve meets the tricuspid valve.
Endocardial cushion defects are associated with abnormalities of the atrioventricular valves (the mitral valve and the tricuspid valve). These include the cleft mitral valve, and the single atrioventricular valve (a single large, deformed valve that flows into both the right ventricle and the left ventricle).
Endocardial cushion defects are the most common congenital heart defect that is associated with Down's syndrome.
Sinus venosus atrial septal defect
A sinus venosus ASD is a type of atrial septum defect in which the defect in the septum involves the venous inflow of either the superior vena cava or the inferior vena cava.
A sinus venosus ASD that involves the superior vena cava makes up 2 to 3% of all interatrial communication. It is located at the junction of the superior vena cava and the right atrium. It is frequently associated with anomalous drainage of the right-sided pulmonary veins into the right atrium (instead of the normal drainage of the pulmonary veins into the left atrium).[4]
Common or single atrium
Common (or single) atrium is a failure of development of the embryologic components that contribute to the atrial septal complex. It is frequently associated with heterotaxy syndrome [5]
Diagnosis
Diagnosis in children
Most individuals with a significant ASD are diagnosed in utero or in early childhood with the use of ultrasonography or auscultation of the heart sounds during physical examination.
Diagnosis in adults
Some individuals with an ASD will have undergone surgical correction of their ASD during childhood. The development of signs and symptoms due to an ASD are related to the size of the intracardiac shunt. Individuals with a larger shunt tend to present with symptoms at a younger age.
Adults with an uncorrected ASD will present with symptoms of dyspnea on exertion (shortness of breath with minimal exercise), congestive heart failure, or cerebrovascular accident (stroke). They may be noted on routine testing to have an abnormal chest x-ray or an abnormal EKG and may have atrial fibrillation.
Physical examination
Auscultation of the heart
The physical findings in an adult with an ASD include those related directly to the intracardiac shunt, and those that are secondary to the right heart failure that may be present in these individuals.
Upon auscultation of the heart sounds, there may be an ejection systolic murmur that is attributed to the pulmonic valve. This is due to the increased flow of blood through the pulmonic valve rather than any structural abnormality of the valve leaflets.
In unaffected individuals, there are respiratory variations in the splitting of the second heart sound (S2). During respiratory inspiration, the negative intrathoracic pressure causes increased blood return into the right side of the heart. The increased blood volume in the right ventricle causes the pulmonic valve to stay open longer during ventricular systole. This causes a normal delay in the P2 component of S2. During expiration, the positive intrathoracic pressure causes decreased blood return to the right side of the heart. The reduced volume in the right ventricle allows the pulmonic valve to close earlier at the end of ventricular systole, causing P2 to occur earlier.
In individuals with an ASD, there is a fixed splitting of S2. The reason why there is a fixed splitting of the second heart sound is that the extra blood return during inspiration gets equalized between the left and right atrium due to the communication that exists between the atria in individuals with ASD.
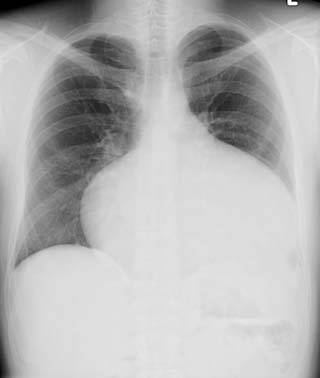
Chest X-ray
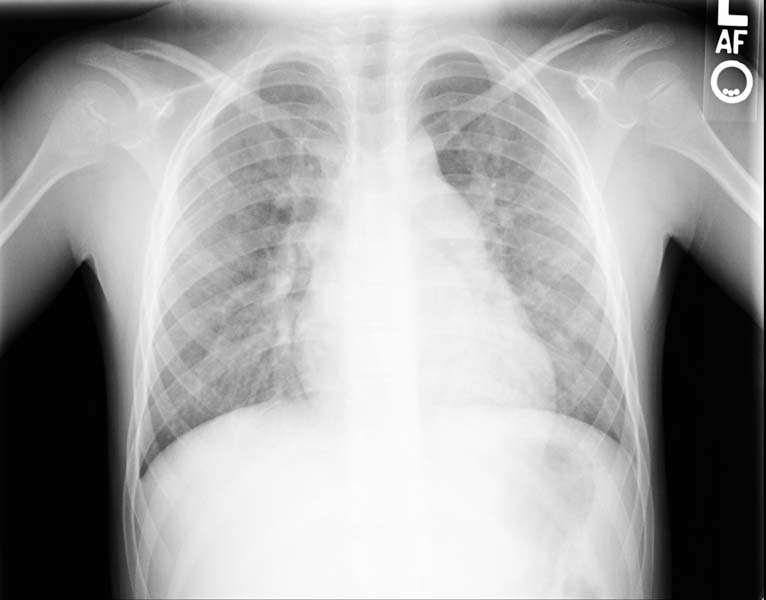
Chest X ray may show an enlarged right atrial border or cardiomegaly if significant pulmonary hypertension is present.
-
Post repair. Enlarged right atrial border and mild cardiomegaly.
-
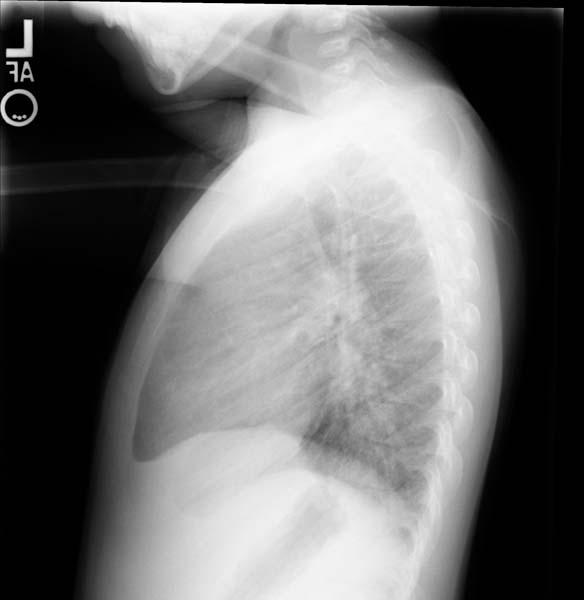
Post repair. Lateral view
-
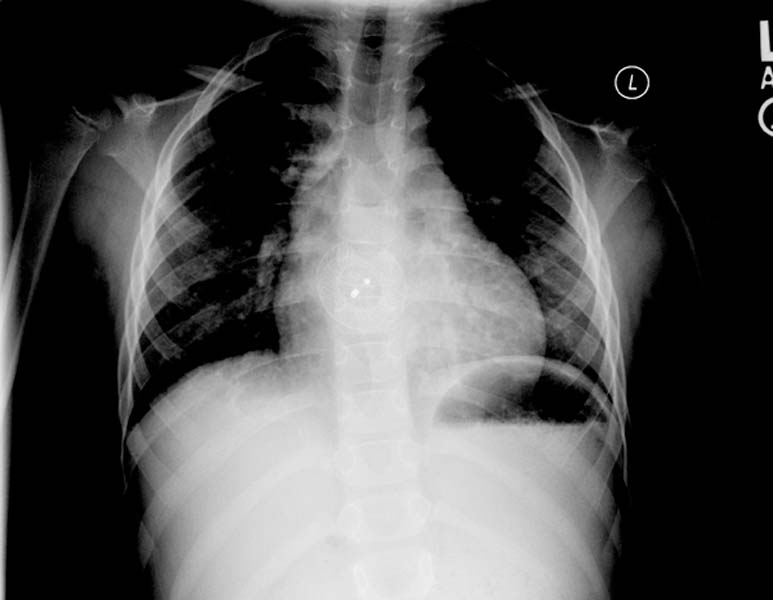
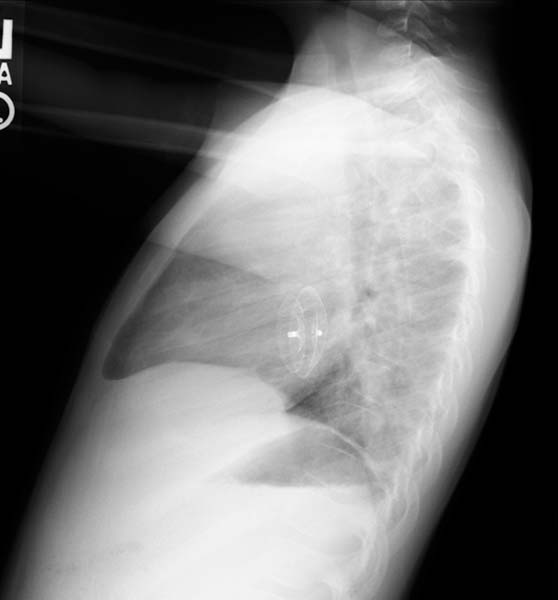
ASD. Another patient. Enlarged right atrial border and advanced cardiomegaly.
Electrocardiography
The ECG findings in atrial septal defect vary with the type of defect the individual has. Individuals with atrial septal defects may have a prolonged PR interval (a first degree heart block). The prolongation of the PR interval is probably due to the enlargement of the atria that is common in ASDs and the increased distance due to the defect itself. Both of these can cause an increased distance of internodal conduction from the SA node to the AV node.[6]
Other EKG findings include the following:
- Incomplete and less frequently complete Right Bundle Branch Block (RBBB) is often present.
- Right Ventricular Hypertrophy (RVH) with strain suggests onset of pulmonary hypertension or associated pulmonic stenosis.
- 2 out of 3 patients with an ostium secundum ASD have right axis deviation.
- Patients with ostium secundum ASDs often develop atrial fibrillation or atrial flutter, and this occurs with a higher incidence with increasing age and with pulmonary hypertension.
- Sinus venosus ASDs are often associated with low atrial and junctional rhythms.
- Ostium primum ASDs are associated with a marked left axis deviation.
- Individuals with a sinus venosus ASD exhibit a left axis deviation of the P wave (not the QRS complex).

Echocardiography
In transthoracic echocardiography, an atrial septal defect may be seen on color flow imaging as a jet of blood from the left atrium to the right atrium.
If agitated saline is injected into a peripheral vein during echocardiography, small air bubbles can be seen on echocardiographic imaging. It may be possible to see bubbles travel across an ASD either at rest or during a cough. (Bubbles will only flow from right atrium to left atrium if the RA pressure is greater than LA).

See Echo in Atrial Septal Defect for more info/images
Because better visualization of the atria is achieved with transesophageal echocardiography, this test may be performed in individuals with a suspected ASD which is not visualized on transthoracic imaging.
Newer techniques to visualize these defects involve intracardiac imaging with special catheters that are typically placed in the venous system and advanced to the level of the heart. This type of imaging is becoming more common and involves only mild sedation for the patient typically.
If the individual has adequate echocardiographic windows, it is possible to use the echocardiogram to measure the cardiac output of the left ventricle and the right ventricle independently. In this way, it is possible to estimate the shunt fraction using echocardiography.
- Atrioventricular septal defects (AVSDs) Rastelli Type A
<googlevideo>8637172269944067306&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A2
<googlevideo>-2527268983131571055&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A3
<googlevideo>1536009221252381368&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A4
<googlevideo>4718874950603401633&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A5
<googlevideo>-2711509694247706297&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A6
<googlevideo>2754627930396522386&hl=en</googlevideo>
- Atrioventricular septal defects (AVSDs) Rastelli Type A7
<googlevideo>-8214352524179603182&hl=en</googlevideo>
Trans-Cranial Doppler (TCD) Ultrasound
This is a less invasive protocol for finding PFO or other ASDs, involves highly-sensitive versions of Trans-Cranial Doppler, or "through the head" doppler ultrasound devices. There is debate as to whether this protocol is the Gold Standard for Finding PFO and also testing for successful closure of PFO after closure procedures. The protocol has been adopted as the testing standard in clinical trials of PFO closure devices and other investigations of the relationship between PFO-stroke-migraine. This protocol requires no sedation and besides a simple intravenous injection of saline. It is otherwise entirely non-invasive and can be administered by clinicians trained in the protocol and using the appropriately sensitive TCD technology, in well under one hour. At least one clinical study has shown this protocol with the appropriate TCD technology, to be highly accurate in comparison to other current standards of care for finding PFO. It is also dramatically less expensive than other tests for PFO.
MRI
Cardiac MRI can be helpful in diagnosing ASDs and can be used to determine defect size, quantify the shunt fraction and detect associated anomalous pulmonary venous connections.
Treatment
Once an individual is found to have an atrial septal defect, a determination of whether it should be corrected has to be made.
Criteria for closure include right ventricular dilatation, pulmonary artery pressures of 50% or less than systemic pressures, history of a cryptogenic stroke.
Surgical mortality due to closure of an ASD is lowest when the procedure is performed prior to the development of significant pulmonary hypertension. The lowest mortality rates are achieved in individuals with a pulmonary artery systolic pressure of less than 40 mm Hg.
If Eisenmenger's syndrome has occurred, there is significant risk of mortality regardless of the method of closure of the ASD. In individuals who have developed Eisenmenger's syndrome, the pressure in the right ventricle has raised high enough to reverse the shunt in the atria. If the ASD is then closed, the afterload that the right ventricle has to act against has suddenly increased. This may cause immediate right ventricular failure, since it may not be able to pump the blood against the pulmonary hypertension.
Closure of an ASD in individuals under age 25 has been shown to have a low risk of complications, and individuals have a normal lifespan (comparable to a healthy age-matched population). Closure of an ASD in individuals between the ages of 25 and 40 who are asymptomatic but have a clinically significant shunt is controversial. Those that perform the procedure believe that they are preventing long-term deterioration in cardiac function and preventing progression of pulmonary hypertension.
Methods of closure of an ASD include surgical closure and percutaneous closure.
Evaluation prior to correction
Prior to correction of an ASD, an evaluation to determine if pulmonary hypertension is present and whether it is reversible. Closure of an ASD may be recommended for prevention purposes, to avoid such a complication in the first place. Pulmonary Hypertension is not always present in adults that are diagnosed with an ASD in adulthood.
If there is a suspicion that pulmonary hypertension is present, the evaluation may include a right heart catheterization. This involves placing a catheter in the venous system of the heart and measuring pressures and oxygen saturations in the SVC, IVC, right atrium, right ventricle, pulmonary artery, and in the wedge position. Individuals with a pulmonary vascular resistance (PVR) of less than 7 wood units show regression of symptoms (including NYHA functional class). On the other hand, individuals with a PVR of greater than 15 wood units have increased mortality associated with closure of the ASD.
If the pulmonary arterial pressure is more than 2/3 the systemic systolic pressure, there should be a net left-to-right shunt of at least 1.5:1 or evidence of reversibility of the shunt when given pulmonary artery vasodilators prior to surgery. If Eisenmenger's physiology has developed, it must be demonstrated that the right-to-left shunt is reversible with pulmonary artery vasodilators prior to surgery.
Surgical ASD closure
Surgical closure of an ASD involves opening up at least one atrium and closing the defect with a patch under direct visualization.
Percutaneous ASD closure
Percutaneous closure of an ASD is currently only indicated for the closure of ostium secundum ASDs with a sufficient rim of tissue around the septal defect so that the closure device does not impinge upon the SVC, IVC, or the tricuspid or mitral valves. The Amplatzer Septal Occluder is commonly used to close ASD's. The ASO consists of two self-expandable round discs connected to each other with a 4-mm waist, made up of 0.004–0.005´´ nitinol wire mesh filled with Dacron fabric. Implantation of the device is relatively easy. The prevalence of residual defect is low. The disadvantages are a thick profile of the device and concern related to a large amount of nitinol (a nickel-titanium compound) in the device and consequent potential for nickel toxicity.
Percutaneous closure is the method of choice in most centers.[7]
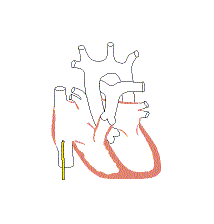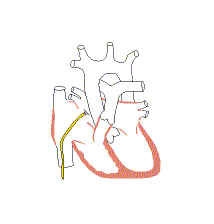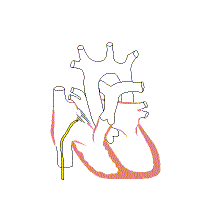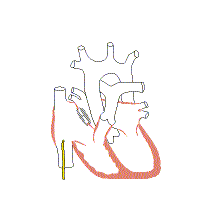
Associated conditions
Due to the communication between the atria that occurs in ASD's, disease entities or complications from the condition, are possible.
Decompression sickness
ASDs, and particularly PFOs, are a predisposing risk factor for decompression sickness in divers because a proportion of venous blood carrying inert gases, such as helium or nitrogen does not pass through the lungs.[8][9] The only way to release the excess inert gases from the body is to pass the blood carrying the inert gases through the lungs to be exhaled. If some of the inert gas-laden blood passes through the PFO, it avoids the lungs and the inert gas is more likely to form large bubbles in the arterial blood stream causing decompression sickness.
Paradoxical emboli
Venous thrombi (clots in the veins) are quite common. Embolization (dislodgement of thrombi) normally go to the lung and cause pulmonary emboli. In an individual with ASD, these emboli can potentially enter the arterial system. This can cause any phenomenon that is attributed to acute loss of blood to a portion of the body, including cerebrovascular accident (stroke), infarction of the spleen or intestines, or even a distal extremity (i.e.: finger or toe).
This is known as a paradoxical embolus because the clot material paradoxically enters the arterial system instead of going to the lungs.
Migraine
Some recent research has suggested that a proportion of cases of migraine may be caused by patent foramen ovale. While the exact mechanism remains unclear, closure of a PFO can reduce symptoms in certain cases.[10][11] This remains controversial. 20% of the general population have a PFO, which for the most part, is asymptomatic. 20% of the female population have migraines. And, the placebo effect in migraine typically averages around 40%. The high frequency of these facts makes statistically significant relationships between PFO and migraine difficult (i.e., the relationship may just be chance or coincidence).
See also
- Atrioventricular septal defect
- Cardiac output
- Congenital heart disease
- Heart sounds
- Pulmonary hypertension
- Vascular resistance
- Ventricular septal defect
References
- ↑ Kaplan S (1993). "Congenital heart disease in adolescents and adults. Natural and postoperative history across age groups". Cardiol Clin. 11 (4): 543–56. PMID 8252558.
- ↑ Feldt R, Avasthey P, Yoshimasu F, Kurland L, Titus J (1971). "Incidence of congenital heart disease in children born to residents of Olmsted County, Minnesota, 1950-1969". Mayo Clin Proc. 46 (12): 794–9. PMID 5128021.
- ↑ Leachman R, Cokkinos D, Cooley D (1976). "Association of ostium secundum atrial septal defects with mitral valve prolapse". Am J Cardiol. 38 (2): 167–9. PMID 952260.
- ↑ Davia J, Cheitlin M, Bedynek J (1973). "Sinus venosus atrial septal defect: analysis of fifty cases". Am Heart J. 85 (2): 177–85. PMID 4569755.
- ↑ Valdes-Cruz LM, Cayre RO (1998). Echocardiographic diagnosis of congenital heart disease. Philadelphia.
- ↑ Clark E, Kugler J (1982). "Preoperative secundum atrial septal defect with coexisting sinus node and atrioventricular node dysfunction". Circulation. 65 (5): 976–80. PMID 7074763.
- ↑ Bjørnstad P (2006). "Is interventional closure the current treatment of choice for selected patients with deficient atrial septation?". Cardiol Young. 16 (1): 3–10. PMID 16454871.
- ↑ Lier H, Schroeder S, Hering R (2004). "[Patent foramen ovale: an underrated risk for divers?]". Dtsch Med Wochenschr. 129 (1–2): 27–30. PMID 14703578.
- ↑ Saary M, Gray G (2001). "A review of the relationship between patent foramen ovale and type II decompression sickness". Aviat Space Environ Med. 72 (12): 1113–20. PMID 11763113.
- ↑ Adams H (2004). "Patent foramen ovale: paradoxical embolism and paradoxical data". Mayo Clin Proc. 79 (1): 15–20. PMID 14708944.
- ↑ Azarbal B, Tobis J, Suh W, Chan V, Dao C, Gaster R (2005). "Association of interatrial shunts and migraine headaches: impact of transcatheter closure". J Am Coll Cardiol. 45 (4): 489–92. PMID 15708691.
External links
- Atrial Septal Defect information from Seattle Children's Hospital Heart Center