Aprepitant (patient information)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aprepitant (patient information) is an antiemetic, neurokinin-1 receptor antagonist that is FDA approved for the prophylaxis of in combination with other antiemetic agents for the:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin
- of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC)
Also, indicated for the prevention of postoperative nausea and vomiting (PONV). Common adverse reactions include alopecia, anorexia, asthenia/fatigue, constipation, diarrhea, headache, hiccups, nausea, hypotension, pruritus, pyrexia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
Capsules of Aprepitant (aprepitant) are given for 3 days as part of a regimen that includes a corticosteroid and a 5-HT3 antagonist. The recommended dose of Aprepitant is 125 mg orally 1 hour prior to chemotherapy treatment (Day 1) and 80 mg orally once daily in the morning on Days 2 and 3.
- Aprepitant may be taken with or without food.
- Aprepitant (fosaprepitant dimeglumine) for Injection (115 mg) is a prodrug of aprepitant and may be substituted for oral Aprepitant (125 mg), 30 minutes prior to chemotherapy, on Day 1 only of the CINV regimen as an intravenous infusion administered over 15 minutes.
In clinical studies with Aprepitant, the following regimen was used for the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy:

In a clinical study with Aprepitant, the following regimen was used for the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy:
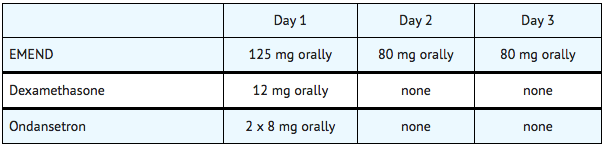
Prevention of Postoperative Nausea and Vomiting (PONV)
The recommended oral dosage of Aprepitant is 40 mg within 3 hours prior to induction of anesthesia. Aprepitant may be taken with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aprepitant (patient information) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aprepitant (patient information) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Aprepitant (patient information) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aprepitant (patient information) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aprepitant (patient information) in pediatric patients.
Contraindications
Aprepitant is contraindicated in patients who are hypersensitive to any component of the product. Aprepitant is a dose-dependent inhibitor of cytochrome P450 isoenzyme 3A4 (CYP3A4). Aprepitant should not be used concurrently with pimozide, terfenadine, astemizole, or cisapride. Inhibition of CYP3A4 by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions.
Warnings
CYP3A4 Interactions
- Aprepitant (aprepitant), a dose-dependent inhibitor of CYP3A4, should be used with caution in patients receiving concomitant medications that are primarily metabolized through CYP3A4. Moderate inhibition of CYP3A4 by aprepitant, 125 mg/80 mg regimen, could result in elevated plasma concentrations of these concomitant medications.
- Weak inhibition of CYP3A4 by a single 40 mg dose of aprepitant is not expected to alter the plasma concentrations of concomitant medications that are primarily metabolized through CYP3A4 to a clinically significant degree.
- When aprepitant is used concomitantly with another CYP3A4 inhibitor, aprepitant plasma concentrations could be elevated. When Aprepitant is used concomitantly with medications that induce CYP3A4 activity aprepitant plasma concentrations could be reduced and this may result in decreased efficacy of Aprepitant.
- Chemotherapy agents that are known to be metabolized by CYP3A4 include docetaxel, paclitaxel, etoposide, irinotecan, ifosfamide, imatinib, vinorelbine, vinblastine and vincristine. In clinical studies, Aprepitant (125 mg/80 mg regimen) was administered commonly with etoposide, vinorelbine, or paclitaxel. The doses of these agents were not adjusted to account for potential drug interactions.
- In separate pharmacokinetic studies no clinically significant change in docetaxel or vinorelbine pharmacokinetics was observed when Aprepitant (125 mg/80 mg regimen) was co-administered.
- Due to the small number of patients in clinical studies who received the CYP3A4 substrates vinblastine, vincristine, or ifosfamide, particular caution and careful monitoring are advised in patients receiving these agents or other chemotherapy agents metabolized primarily by CYP3A4 that were not studied.
Coadministration with Warfarin (a CYP2C9 substrate)
Coadministration of Aprepitant with warfarin may result in a clinically significant decrease in International Normalized Ratio (INR) of prothrombin time. In patients on chronic warfarin therapy, the INR should be closely monitored in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of Aprepitant with each chemotherapy cycle, or following administration of a single 40 mg dose of Aprepitant for the prevention of postoperative nausea and vomiting.
Coadministration with Hormonal Contraceptives
Upon coadministration with Aprepitant, the efficacy of hormonal contraceptives during and for 28 days following the last dose of Aprepitant may be reduced. Alternative or back-up methods of contraception should be used during treatment with Aprepitant and for 1 month following the last dose of Aprepitant.
Patients with Severe Hepatic Impairment
There are no clinical or pharmacokinetic data in patients with severe hepatic impairment ([Child-Pugh score]] >9). Therefore, caution should be exercised when Aprepitant is administered in these patients.
Chronic Continuous Use
Chronic continuous use of Aprepitant for prevention of nausea and vomiting is not recommended because it has not been studied; and because the drug interaction profile may change during chronic continuous use.
Adverse Reactions
Clinical Trials Experience
In 2 well-controlled clinical trials in patients receiving highly emetogenic cancer chemotherapy, 544 patients were treated with aprepitant during Cycle 1 of chemotherapy and 413 of these patients continued into the Multiple-Cycle extension for up to 6 cycles of chemotherapy. Aprepitant was given in combination with ondansetron and dexamethasone. In Cycle 1, clinical adverse experiences were reported in approximately 69% of patients treated with the aprepitant regimen compared with approximately 68% of patients treated with standard therapy. Table 1 shows the percent of patients with clinical adverse experiences reported at an incidence ≥3%.

In a combined analysis of these two studies, isolated cases of serious adverse experiences were similar in the two treatment groups.
Highly and Moderately Emetogenic Chemotherapy
The following additional clinical adverse experiences (incidence >0.5% and greater than standard therapy), regardless of causality, were reported in patients treated with aprepitant regimen in either HEC or MEC studies:
- Infections and infestations: candidiasis, herpes simplex, lower respiratory infection, oral candidiasis, pharyngitis, septic shock, upper respiratory infection, urinary tract infection.
- Neoplasms benign, malignant and unspecified (including cysts and polyps): malignant neoplasm, non-small cell lung carcinoma.
- Blood and lymphatic system disorders: anemia, febrile neutropenia, thrombocytopenia.
- Metabolism and nutrition disorders: appetite decreased, diabetes mellitus, hypokalemia.
- Psychiatric disorders: anxiety disorder, confusion, depression.
- Nervous system: peripheral neuropathy, sensory neuropathy, taste disturbance, tremor.
- Eye disorders: conjunctivitis.
- Cardiac disorders: myocardial infarction, palpitations, tachycardia.
- Vascular disorders: deep venous thrombosis, flushing, hot flush, hypertension, hypotension.
- Respiratory, thoracic and mediastinal disorders: cough, dyspnea, nasal secretion, pharyngolaryngeal pain, pneumonitis, pulmonary embolism, respiratory insufficiency, vocal disturbance.
- Gastrointestinal disorders: abdominal pain upper, acid reflux, deglutition disorder, dry mouth, dysgeusia, dysphagia, eructation, flatulence, obstipation, salivation increased.
- Skin and subcutaneous tissue disorders: acne, diaphoresis, pruritus, rash.
- Musculoskeletal and connective tissue disorders: arthralgia, back pain, muscular weakness, musculoskeletal pain, myalgia.
- Renal and urinary disorders: dysuria, renal insufficiency.
- Reproductive system and breast disorders: pelvic pain.
- General disorders and administrative site conditions: edema, malaise, pain, rigors.
- Investigations: weight loss.
- Stevens-Johnson syndrome was reported as a serious adverse experience in a patient receiving aprepitant with cancer chemotherapy in another CINV study.
Laboratory Adverse Experiences Table 3 shows the percent of patients with laboratory adverse experiences reported at an incidence ≥3% in patients receiving highly emetogenic chemotherapy.

The following additional laboratory adverse experiences (incidence >0.5% and greater than standard therapy), regardless of causality, were reported in patients treated with aprepitant regimen: alkaline phosphatase increased, hyperglycemia, hyponatremia, leukocytes increased, erythrocyturia, leukocyturia.
The adverse experience profiles in the Multiple-Cycle extensions of HEC and MEC studies for up to 6 cycles of chemotherapy were generally similar to that observed in Cycle 1.
Postoperative Nausea and Vomiting
In well-controlled clinical studies in patients receiving general anesthesia, 564 patients were administered 40 mg aprepitant orally and 538 patients were administered 4 mg ondansetron IV.
Clinical adverse experiences were reported in approximately 60% of patients treated with 40 mg aprepitant compared with approximately 64% of patients treated with 4 mg ondansetron IV. Table 4 shows the percent of patients with clinical adverse experiences reported at an incidence ≥3% of the combined studies.

The following additional clinical adverse experiences (incidence >0.5% and greater than ondansetron), regardless of causality, were reported in patients treated with aprepitant:
- Infections and infestations: postoperative infection
- Metabolism and nutrition disorders: hypokalemia, hypovolemia.
- Nervous system disorders: dizziness, hypoesthesia, syncope.
- Vascular disorders: hematoma
- Respiratory, thoracic and mediastinal disorders: dyspnea, hypoxia, respiratory depression.
- Gastrointestinal disorders: abdominal pain, abdominal pain upper, dry mouth, dyspepsia.
- Skin and subcutaneous tissue disorders: urticaria
- General disorders and administrative site conditions: hypothermia, pain.
- Investigations: hypotension
Injury, poisoning and procedural complications: operative hemorrhage, wound dehiscence.
Other adverse experiences (incidence ≤0.5%) reported in patients treated with aprepitant 40 mg for postoperative nausea and vomiting included:
- Nervous system disorders: dysarthria, sensory disturbance.
- Eye disorders: miosis, visual acuity reduced.
- Respiratory, thoracic and mediastinal disorders: wheezing
- Gastrointestinal disorders: bowel sounds abnormal, stomach discomfort.
There were no serious adverse drug-related experiences reported in the postoperative nausea and vomiting clinical studies in patients taking 40 mg aprepitant.
=Laboratory Adverse Experiences
One laboratory adverse experience, hemoglobin decreased (40 mg aprepitant 3.8%, ondansetron 4.2%), was reported at an incidence ≥3% in a patient receiving general anesthesia.
The following additional laboratory adverse experiences (incidence >0.5% and greater than ondansetron), regardless of causality, were reported in patients treated with aprepitant 40 mg: blood albumin decreased, blood bilirubin increased, blood glucose increased, blood potassium decreased, glucose urine present. The adverse experience of ALT increased occurred with similar incidence in patients treated with aprepitant 40 mg (1.1%) as in patients treated with ondansetron 4 mg (1.0%).
Other Studies
- In addition, two serious adverse experiences were reported in postoperative nausea and vomiting (PONV) clinical studies in patients taking a higher dose of aprepitant: one case of constipation, and one case of sub-ileus.
- Angioedema and urticaria were reported as serious adverse experiences in a patient receiving aprepitant in a non-CINV/non-PONV study.
Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of aprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to the drug.
Immune system disorders: hypersensitivity reactions including anaphylactic reactions.
Drug Interactions
Effect of Aprepitant on the Pharmacokinetics of Other Agents
CYP3A4 Substrates
- Weak inhibition of CYP3A4 by a single 40 mg dose of aprepitant is not expected to alter the plasma concentrations of concomitant medications that are primarily metabolized through CYP3A4 to a clinically significant degree. However, higher aprepitant doses or repeated dosing at any aprepitant dose may have a clinically significant effect.
- As a moderate inhibitor of CYP3A4 at a dose of 125 mg/80 mg, aprepitant can increase plasma concentrations of concomitantly administered oral medications that are metabolized through CYP3A4. The use of fosaprepitant may increase CYP3A4 substrate plasma concentrations to a lesser degree than the use of oral aprepitant (125 mg).
5-HT3 antagonists
In clinical drug interaction studies, aprepitant did not have clinically important effects on the pharmacokinetics of ondansetron, granisetron, or hydrodolasetron (the active metabolite of dolasetron).
Corticosteroids
- Dexamethasone: Aprepitant, when given as a regimen of 125 mg with dexamethasone coadministered orally as 20 mg on Day 1, and Aprepitant when given as 80 mg/day with dexamethasone coadministered orally as 8 mg on Days 2 through 5, increased the AUC of dexamethasone, a CYP3A4 substrate, by 2.2-fold on Days 1 and 5. The oral dexamethasone doses should be reduced by approximately 50% when coadministered with Aprepitant (125 mg/80 mg regimen), to achieve exposures of dexamethasone similar to those obtained when it is given without Aprepitant. The daily dose of dexamethasone administered in clinical chemotherapy induced nausea and vomiting studies with Aprepitant reflects an approximate 50% reduction of the dose of dexamethasone. A single dose of Aprepitant (40 mg) when coadministered with a single oral dose of dexamethasone 20 mg, increased the AUC of dexamethasone by 1.45-fold. Therefore, no dose adjustment is recommended.
- Methylprednisolone: Aprepitant, when given as a regimen of 125 mg on Day 1 and 80 mg/day on Days 2 and 3, increased the AUC of methylprednisolone, a CYP3A4 substrate, by 1.34-fold on Day 1 and by 2.5-fold on Day 3, when methylprednisolone was coadministered intravenously as 125 mg on Day 1 and orally as 40 mg on Days 2 and 3. The IV methylprednisolone dose should be reduced by approximately 25%, and the oral methylprednisolone dose should be reduced by approximately 50% when coadministered with Aprepitant (125 mg/80 mg regimen) to achieve exposures of methylprednisolone similar to those obtained when it is given without Aprepitant Although the concomitant administration of methylprednisolone with the single 40 mg dose of aprepitant has not been studied, a single 40 mg dose of Aprepitant produces a weak inhibition of CYP3A4 (based on midazolam interaction study) and it is not expected to alter the plasma concentrations of methylprednisolone to a clinically significant degree. Therefore, no dose adjustment is recommended.
Chemotherapeutic agents
- Docetaxel: In a pharmacokinetic study, Aprepitant (125 mg/80 mg regimen) did not influence the pharmacokinetics of docetaxel.
- Vinorelbine: In a pharmacokinetic study, Aprepitant (125 mg/80 mg regimen) did not influence the pharmacokinetics of vinorelbine to a clinically significant degree.
CYP2C9 Substrates (Warfarin, Tolbutamide)
Aprepitant has been shown to induce the metabolism of S(-) warfarin and tolbutamide, which are metabolized through CYP2C9. Coadministration of Aprepitant with these drugs or other drugs that are known to be metabolized by CYP2C9, such as phenytoin, may result in lower plasma concentrations of these drugs.
Warfarin
A single 125-mg dose of Aprepitant was administered on Day 1 and 80 mg/day on Days 2 and 3 to healthy subjects who were stabilized on chronic warfarin therapy. Although there was no effect of Aprepitant on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin (a CYP2C9 substrate) trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with Aprepitant In patients on chronic warfarin therapy, the prothrombin time (INR) should be closely monitored in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of Aprepitant with each chemotherapy cycle, or following administration of a single 40 mg dose of Aprepitant for the prevention of postoperative nausea and vomiting.
Tolbutamide
- Aprepitant, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide (a CYP2C9 substrate) by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered orally prior to the administration of the 3-day regimen of Aprepitant and on Days 4, 8, and 15.
- Aprepitant, when given as a 40-mg single oral dose on Day 1, decreased the AUC of tolbutamide (a CYP2C9 substrate) by 8% on Day 2, 16% on Day 4, 15% on Day 8, and 10% on Day 15, when a single dose of tolbutamide 500 mg was administered orally prior to the administration of Aprepitant 40 mg and on Days 2, 4, 8, and 15. This effect was not considered clinically important.
Oral contraceptives
Aprepitant, when given once daily for 14 days as a 100-mg capsule with an oral contraceptive containing 35 mcg of ethinyl estradiol and 1 mg of norethindrone, decreased the AUC of ethinyl estradiol by 43%, and decreased the AUC of norethindrone by 8%.
In another study, a daily dose of an oral contraceptive containing ethinyl estradiol and norethindrone was administered on Days 1 through 21, and Aprepitant was given as a 3-day regimen of 125 mg on Day 8 and 80 mg/day on Days 9 and 10 with ondansetron 32 mg IV on Day 8 and oral dexamethasone given as 12 mg on Day 8 and 8 mg/day on Days 9, 10, and 11. In the study, the AUC of ethinyl estradiol decreased by 19% on Day 10 and there was as much as a 64% decrease in ethinyl estradiol trough concentrations during Days 9 through 21. While there was no effect of Aprepitant on the AUC of norethindrone on Day 10, there was as much as a 60% decrease in norethindrone trough concentrations during Days 9 through 21.
In another study, a daily dose of an oral contraceptive containing ethinyl estradiol and norgestimate (which is converted to norelgestromin) was administered on Days 1 through 21, and Aprepitant 40 mg was given on Day 8. In the study, the AUC of ethinyl estradiol decreased by 4% and 29% on Day 8 and Day 12, respectively, while the AUC of norelgestromin increased by 18% on Day 8 and decreased by 10% on Day 12. In addition, the trough concentrations of ethinyl estradiol and norelgestromin on Days 8 through 21 were generally lower following coadministration of the oral contraceptive with Aprepitant 40 mg on Day 8 compared to the trough levels following administration of the oral contraceptive alone.
The coadministration of Aprepitant may reduce the efficacy of hormonal contraceptives (these can include birth control pills, skin patches, implants, and certain IUDs) during and for 28 days after administration of the last dose of Aprepitant. Alternative or back-up methods of contraception should be used during treatment with Aprepitant and for 1 month following the last dose of Aprepitant.
Midazolam
Aprepitant increased the AUC of midazolam, a sensitive CYP3A4 substrate, by 2.3-fold on Day 1 and 3.3-fold on Day 5, when a single oral dose of midazolam 2 mg was coadministered on Day 1 and Day 5 of a regimen of Aprepitant 125 mg on Day 1 and 80 mg/day on Days 2 through 5. The potential effects of increased plasma concentrations of midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) should be considered when coadministering these agents with Aprepitant (125 mg/80 mg). A single dose of Aprepitant (40 mg) increased the AUC of midazolam by 1.2-fold on Day 1, when a single oral dose of midazolam 2 mg was coadministered on Day 1 with Aprepitant 40 mg; this effect was not considered clinically important.
In another study with intravenous administration of midazolam, Aprepitant was given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, and midazolam 2 mg IV was given prior to the administration of the 3-day regimen of Aprepitant and on Days 4, 8, and 15. Aprepitant increased the AUC of midazolam by 25% on Day 4 and decreased the AUC of midazolam by 19% on Day 8 relative to the dosing of Aprepitant on Days 1 through 3. These effects were not considered clinically important. The AUC of midazolam on Day 15 was similar to that observed at baseline.
An additional study was completed with intravenous administration of midazolam and Aprepitant. Intravenous midazolam 2 mg was given 1 hour after oral administration of a single dose of Aprepitant 125 mg. The plasma AUC of midazolam was increased by 1.5-fold. Depending on clinical situations (e.g., elderly patients) and degree of monitoring available, dosage adjustment for intravenous midazolam may be necessary when it is coadministered with Aprepitant for the chemotherapy induced nausea and vomiting indication (125 mg on Day 1 followed by 80 mg on Days 2 and 3).
Effect of Other Agents on the Pharmacokinetics of Aprepitant
Aprepitant is a substrate for CYP3A4; therefore, coadministration of Aprepitant with drugs that inhibit CYP3A4 activity may result in increased plasma concentrations of aprepitant. Consequently, concomitant administration of Aprepitant with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavir) should be approached with caution. Because moderate CYP3A4 inhibitors (e.g., diltiazem) result in a 2-fold increase in plasma concentrations of aprepitant, concomitant administration should also be approached with caution.
Aprepitant is a substrate for CYP3A4; therefore, coadministration of Aprepitant with drugs that strongly induce CYP3A4 activity (e.g., rifampin, carbamazepine, phenytoin) may result in reduced plasma concentrations of aprepitant that may result in decreased efficacy of Aprepitant.
Ketoconazole
When a single 125-mg dose of Aprepitant was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold. Concomitant administration of Aprepitant with strong CYP3A4 inhibitors should be approached cautiously.
Rifampin
When a single 375-mg dose of Aprepitant was administered on Day 9 of a 14-day regimen of 600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold. Coadministration of Aprepitant with drugs that induce CYP3A4 activity may result in reduced plasma concentrations and decreased efficacy of Aprepitant.
Additional Interactions
Aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter, as demonstrated by the lack of interaction of Aprepitant with digoxin in a clinical drug interaction study.
- Diltiazem: In patients with mild to moderate hypertension, administration of aprepitant once daily, as a tablet formulation comparable to 230 mg of the capsule formulation, with diltiazem 120 mg 3 times daily for 5 days, resulted in a 2-fold increase of aprepitant AUC and a simultaneous 1.7-fold increase of diltiazem AUC. These pharmacokinetic effects did not result in clinically meaningful changes in ECG, heart rate or blood pressure beyond those changes induced by diltiazem alone.
- Paroxetine: Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in rats at oral doses up to 1000 mg/kg twice daily (plasma AUC0‑24hr of 31.3 mcg•hr/mL, about 1.6 times the human exposure at the recommended dose) and in rabbits at oral doses up to 25 mg/kg/day (plasma AUC0‑24hr of 26.9 mcg•hr/mL, about 1.4 times the human exposure at the recommended dose) and have revealed no evidence of impaired fertility or harm to the fetus due to aprepitant. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aprepitant (patient information) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aprepitant (patient information) during labor and delivery.
Nursing Mothers
Aprepitant is excreted in the milk of rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for possible serious adverse reactions in nursing infants from aprepitant and because of the potential for tumorigenicity shown for aprepitant in rodent carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of Aprepitant in pediatric patients have not been established.
Geriatic Use
In 2 well-controlled chemotherapy-induced nausea and vomiting clinical studies, of the total number of patients (N=544) treated with Aprepitant 31% were 65 and over, while 5% were 75 and over. In well-controlled postoperative nausea and vomiting clinical studies, of the total number of patients (N=1120) treated with Aprepitant 7% were 65 and over, while 2% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment in the elderly is not necessary.
Gender
There is no FDA guidance on the use of Aprepitant (patient information) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aprepitant (patient information) with respect to specific racial populations.
Renal Impairment
No dosage adjustment is necessary for patients with renal impairment or for patients with end stage renal disease (ESRD) undergoing hemodialysis.
Hepatic Impairment
No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There are no clinical data in patients with severe hepatic impairment (Child-Pugh score >9).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aprepitant (patient information) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aprepitant (patient information) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aprepitant (patient information) Administration in the drug label.
Monitoring
There is limited information regarding Aprepitant (patient information) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Aprepitant (patient information) and IV administrations.
Overdosage
No specific information is available on the treatment of overdosage. Drowsiness and headache were reported in one patient who ingested 1440 mg of aprepitant. In the event of overdose, Aprepitant should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of aprepitant, drug-induced emesis may not be effective. Aprepitant cannot be removed by hemodialysis.
Pharmacology
Mechanism of Action
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting (PONV).
Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies show that aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
Structure
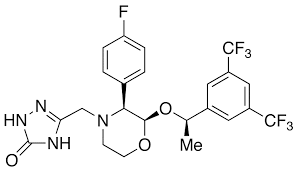
Chemically described as 5-(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one.
Its empirical formula is C23H21F7N4O3, and its structural formula is:
Pharmacodynamics
NK1 Receptor Occupancy
In two single-blind, multiple-dose, randomized, and placebo control studies, healthy young men received oral aprepitant doses of 10 mg (N=2), 30 mg (N=3), 100 mg (N=3) or 300 mg (N=5) once daily for 14 days with 2 or 3 subjects on placebo. Both plasma aprepitant concentration and NK1 receptor occupancy in the corpus striatum by positron emission tomography were evaluated, at predose and 24 hours after the last dose. At aprepitant plasma concentrations of ~10 ng/mL and ~100 ng/mL, the NK1 receptor occupancies were ~50% and ~90%, respectively. The oral aprepitant regimen for CINV produces mean trough plasma aprepitant concentrations >500 ng/mL, which would be expected to, based on the fitted curve with the Hill equation, result in >95% brain NK1 receptor occupancy. However, the receptor occupancy for either CINV or PONV dosing regimen has not been determined. In addition, the relationship between NK1 receptor occupancy and the clinical efficacy of aprepitant has not been established.
Cardiac Electrophysiology
In a randomized, double-blind, positive-controlled, thorough QTc study, a single 200-mg dose of fosaprepitant had no effect on the QTc interval. QT prolongation with the oral dosing regimens for CINV and PONV are not expected.
Pharmacokinetics
Absorption
Following oral administration of a single 40 mg dose of Aprepitant in the fasted state, mean area under the plasma concentration-time curve (AUC0‑∞) was 7.8 mcg•hr/mL and mean peak plasma concentration (Cmax) was 0.7 mcg/mL, occurring at approximately 3 hours postdose (Tmax). The absolute bioavailability at the 40‑mg dose has not been determined.
Following oral administration of a single 125-mg dose of Aprepitant on Day 1 and 80 mg once daily on Days 2 and 3, the AUC0-24hr was approximately 19.6 mcg•hr/mL and 21.2 mcg•hr/mL on Day 1 and Day 3, respectively. The Cmax of 1.6 mcg/mL and 1.4 mcg/mL were reached in approximately 4 hours (Tmax) on Day 1 and Day 3, respectively. At the dose range of 80-125 mg, the mean absolute oral bioavailability of aprepitant is approximately 60 to 65%. Oral administration of the capsule with a standard high-fat breakfast had no clinically meaningful effect on the bioavailability of aprepitant.
The pharmacokinetics of aprepitant are non-linear across the clinical dose range. In healthy young adults, the increase in AUC0‑∞ was 26% greater than dose proportional between 80‑mg and 125‑mg single doses administered in the fed state.
Distribution
Aprepitant is greater than 95% bound to plasma proteins. The mean apparent volume of distribution at steady state (Vdss) is approximately 70 L in humans. Aprepitant crosses the placenta in rats and rabbits and crosses the blood brain barrier in humans.
Metabolism
Aprepitant undergoes extensive metabolism. In vitro studies using human liver microsomes indicate that aprepitant is metabolized primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19. Metabolism is largely via oxidation at the morpholine ring and its side chains. No metabolism by CYP2D6, CYP2C9, or CYP2E1 was detected. In healthy young adults, aprepitant accounts for approximately 24% of the radioactivity in plasma over 72 hours following a single oral 300-mg dose of [14C]-aprepitant, indicating a substantial presence of metabolites in the plasma. Seven metabolites of aprepitant, which are only weakly active, have been identified in human plasma.
Excretion
Following administration of a single IV 100-mg dose of [14C]-aprepitant prodrug to healthy subjects, 57% of the radioactivity was recovered in urine and 45% in feces. A study was not conducted with radiolabeled capsule formulation. The results after oral administration may differ.
Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. The apparent plasma clearance of aprepitant ranged from approximately 62 to 90 mL/min. The apparent terminal half-life ranged from approximately 9 to 13 hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat carcinogenicity studies, animals were treated with oral doses ranging from 0.05 to 1000 mg/kg twice daily. The highest dose produced a systemic exposure to aprepitant (plasma AUC0‑24hr) of 0.7 to 1.6 times the human exposure (AUC0‑24hr = 19.6 mcg•hr/mL) at the recommended dose of 125 mg/day. Treatment with aprepitant at doses of 5 to 1000 mg/kg twice daily caused an increase in the incidences of thyroid follicular cell adenomas and thyroid carcinomas in male rats. In female rats, it produced hepatocellular adenomas at 5 to 1000 mg/kg twice daily and hepatocellular carcinomas and thyroid follicular cell adenomas at 125 to 1000 mg/kg twice daily. In the mouse carcinogenicity studies, the animals were treated with oral doses ranging from 2.5 to 2000 mg/kg/day. The highest dose produced a systemic exposure of about 2.8 to 3.6 times the human exposure at the recommended dose. Treatment with aprepitant produced skin fibrosarcomas at 125 and 500 mg/kg/day doses in male mice.
Aprepitant was not genotoxic in the Ames test, the human lymphoblastoid cell (TK6) mutagenesis test, the rat hepatocyte DNA strand break test, the Chinese hamster ovary (CHO) cell chromosome aberration test and the mouse micronucleus test.
Aprepitant did not affect the fertility or general reproductive performance of male or female rats at doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended human dose and exposure in female rats at about 1.6 times the human exposure).
Clinical Studies
Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
Oral administration of Aprepitant in combination with ondansetron and dexamethasone (aprepitant regimen) has been shown to prevent acute and delayed nausea and vomiting associated with highly emetogenic chemotherapy including high-dose cisplatin, and nausea and vomiting associated with moderately emetogenic chemotherapy.
Highly Emetogenic Chemotherapy (HEC)
In 2 multicenter, randomized, parallel, double-blind, controlled clinical studies, the aprepitant regimen (see Table 6) was compared with standard therapy in patients receiving a chemotherapy regimen that included cisplatin >50 mg/m2 (mean cisplatin dose = 80.2 mg/m2). Of the 550 patients who were randomized to receive the aprepitant regimen, 42% were women, 58% men, 59% White, 3% Asian, 5% Black, 12% Hispanic American, and 21% Multi-Racial. The aprepitant-treated patients in these clinical studies ranged from 14 to 84 years of age, with a mean age of 56 years. 170 patients were 65 years or older, with 29 patients being 75 years or older. Patients (N = 1105) were randomized to either the aprepitant regimen (N = 550) or standard therapy (N = 555). The treatment regimens are defined in Table 5.

During these studies 95% of the patients in the aprepitant group received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common chemotherapeutic agents and the number of aprepitant patients exposed follows: etoposide (106), fluorouracil (100), gemcitabine (89), vinorelbine (82), paclitaxel (52), cyclophosphamide (50), doxorubicin (38), docetaxel (11).
The antiemetic activity of Aprepitant was evaluated during the acute phase (0 to 24 hours post-cisplatin treatment), the delayed phase (25 to 120 hours post-cisplatin treatment) and overall (0 to 120 hours post-cisplatin treatment) in Cycle 1. Efficacy was based on evaluation of the following endpoints:
Primary endpoint:
- complete response (defined as no emetic episodes and no use of rescue therapy)
Other prespecified endpoints:
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [[[VAS]]] score <25 mm on a 0 to 100 mm scale)
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- no nausea (maximum VAS <5 mm on a 0 to 100 mm scale)
- no significant nausea (maximum VAS <25 mm on a 0 to 100 mm scale)


In both studies, a statistically significantly higher proportion of patients receiving the aprepitant regimen in Cycle 1 had a complete response in the overall phase (primary endpoint), compared with patients receiving standard therapy. A statistically significant difference in complete response in favor of the aprepitant regimen was also observed when the acute phase and the delayed phase were analyzed separately.
In both studies, the estimated time to first emesis after initiation of cisplatin treatment was longer with the aprepitant regimen, and the incidence of first emesis was reduced in the aprepitant regimen group compared with standard therapy group as depicted in the Kaplan-Meier curves in Figure 1.

- Patient-Reported Outcomes: The impact of nausea and vomiting on patients’ daily lives was assessed in Cycle 1 of both Phase III studies using the Functional Living Index–Emesis (FLIE), a validated nausea- and vomiting-specific patient-reported outcome measure. Minimal or no impact of nausea and vomiting on patients’ daily lives is defined as a FLIE total score >108. In each of the 2 studies, a higher proportion of patients receiving the aprepitant regimen reported minimal or no impact of nausea and vomiting on daily life (Study 1: 74% versus 64%; Study 2: 75% versus 64%).
- Multiple-Cycle Extension: In the same 2 clinical studies, patients continued into the Multiple-Cycle extension for up to 5 additional cycles of chemotherapy. The proportion of patients with no emesis and no significant nausea by treatment group at each cycle is depicted in Figure 2. Antiemetic effectiveness for the patients receiving the aprepitant regimen is maintained throughout repeat
Moderately Emetogenic Chemotherapy (MEC)
In a multicenter, randomized, double-blind, parallel-group, clinical study in breast cancer patients, the aprepitant regimen (see Table 9) was compared with a standard of care therapy in patients receiving a moderately emetogenic chemotherapy regimen that included cyclophosphamide 750-1500 mg/m2; or cyclophosphamide 500-1500 mg/m2 and doxorubicin (≤60 mg/m2) or epirubicin (≤100 mg/m2). In this study, the most common combinations were cyclophosphamide + doxorubicin (60.6%); and cyclophosphamide + epirubicin + fluorouracil (21.6%). Of the 438 patients who were randomized to receive the aprepitant regimen, 99.5% were women. Of these, approximately 80% were White, 8% Black, 8% Asian, 4% Hispanic, and <1% Other. The aprepitant-treated patients in this clinical study ranged from 25 to 78 years of age, with a mean age of 53 years; 70 patients were 65 years or older, with 12 patients being over 74 years. Patients (N = 866) were randomized to either the aprepitant regimen (N = 438) or standard therapy (N = 428). The treatment regimens are defined in Table 8.

The antiemetic activity of Aprepitant was evaluated based on the following endpoints:
- Primary endpoint:
- Complete response (defined as no emetic episodes and no use of rescue therapy) in the overall phase (0 to 120 hours post-chemotherapy)
- Other prespecified endpoints:
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- no nausea (maximum VAS <5 mm on a 0 to 100 mm scale)
- no significant nausea (maximum VAS <25 mm on a 0 to 100 mm scale)
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [VAS] score <25 mm on a 0 to 100 mm scale)
complete response during the acute and delayed phases.
A summary of the key results from this study is shown in Table 9.

In this study, a statistically significantly (p=0.015) higher proportion of patients receiving the aprepitant regimen (51%) in Cycle 1 had a complete response (primary endpoint) during the overall phase compared with patients receiving standard therapy (42%). The difference between treatment groups was primarily driven by the “No Emesis Endpoint”, a principal component of this composite primary endpoint. In addition, a higher proportion of patients receiving the aprepitant regimen in Cycle 1 had a complete response during the acute (0-24 hours) and delayed (25-120 hours) phases compared with patients receiving standard therapy; however, the treatment group differences failed to reach statistical significance, after multiplicity adjustments.
- Patient-Reported Outcomes: In a phase III study in patients receiving moderately emetogenic chemotherapy, the impact of nausea and vomiting on patients’ daily lives was assessed in Cycle 1 using the FLIE. A higher proportion of patients receiving the aprepitant regimen reported minimal or no impact on daily life (64% versus 56%). This difference between treatment groups was primarily driven by the “No Vomiting Domain” of this composite endpoint.
- Multiple-Cycle Extension: Patients receiving moderately emetogenic chemotherapy were permitted to continue into the Multiple-Cycle extension of the study for up to 3 additional cycles of chemotherapy. Antiemetic effect for patients receiving the aprepitant regimen is maintained during all cycles.
- Postmarketing Trial: In a postmarketing, multicenter, randomized, double-blind, parallel-group, clinical study in 848 cancer patients, the aprepitant regimen (N = 430) was compared with a standard of care therapy (N = 418) in patients receiving a moderately emetogenic chemotherapy regimen that included any IV dose of oxaliplatin, carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, daunorubicin, doxorubicin; cyclophosphamide IV (<1500 mg/m2); or cytarabine IV (>1 g/m2).
Of the 430 patients who were randomized to receive the aprepitant regimen, approximately 76% were women and 24% were men. The distribution by race was 67% White, 6% Black or African American, 11% Asian, and 12% multiracial. Classified by ethnicity, 36% were Hispanic and 64% were non-Hispanic. The aprepitant-treated patients in this clinical study ranged from 22 to 85 years of age, with a mean age of 57 years; approximately 59% of the patients were 55 years or older with 32 patients being over 74 years. Patients receiving the aprepitant regimen were receiving chemotherapy for a variety of tumor types including 50% with breast cancer, 21% with gastrointestinal cancers including colorectal cancer, 13% with lung cancer and 6% with gynecological cancers.
The antiemetic activity of Aprepitant was evaluated based on no vomiting (with or without rescue therapy) in the overall period (0 to 120 hours post-chemotherapy) and complete response (defined as no vomiting and no use of rescue therapy) in the overall period.
A summary of the key results from this study is shown in Table 10.

In this study, a statistically significantly higher proportion of patients receiving the aprepitant regimen (76%) in Cycle 1 had no vomiting during the overall phase compared with patients receiving standard therapy (62%). In addition, a higher proportion of patients receiving the aprepitant regimen (69%) in Cycle 1 had a complete response in the overall phase (0-120 hours) compared with patients receiving standard therapy (56%). In the acute phase (0 to 24 hours following initiation of chemotherapy), a higher proportion of patients receiving aprepitant compared to patients receiving standard therapy were observed to have no vomiting (92% and 84%, respectively) and complete response (89% and 80%, respectively). In the delayed phase (25 to 120 hours following initiation of chemotherapy), a higher proportion of patients receiving aprepitant compared to patients receiving standard therapy were observed to have no vomiting (78% and 67%, respectively) and complete response (71% and 61%, respectively).
In a subgroup analysis by tumor type, a numerically higher proportion of patients receiving aprepitant were observed to have no vomiting and complete response compared to patients receiving standard therapy. For gender, the difference in complete response rates between the aprepitant and standard regimen groups was 14% in females (64.5% and 50.3%, respectively) and 4% in males (82.2% and 78.2%, respectively) during the overall phase. A similar difference for gender was observed for the no vomiting endpoint.
Prevention of Postoperative Nausea and Vomiting (PONV)
In two multicenter, randomized, double-blind, active comparator-controlled, parallel-group clinical studies (PONV Studies 1 and 2), aprepitant was compared with ondansetron for the prevention of postoperative nausea and vomiting in 1658 patients undergoing open abdominal surgery. Patients were randomized to receive 40 mg aprepitant, 125 mg aprepitant, or 4 mg ondansetron. Aprepitant was given orally with 50 mL of water 1 to 3 hours before anesthesia. Ondansetron was given intravenously immediately before induction of anesthesia. A comparison between the 125 mg dose and the 40 mg dose did not demonstrate any additional clinical benefit. The remainder of this section will focus on the results in the 40 mg aprepitant dose recommended for PONV.
Of the 564 patients who received 40 mg aprepitant, 92% were women and 8% were men; of these, 58% were White, 13% Hispanic American, 7% Multi-Racial, 14% Black, 6% Asian, and 2% Other. The age of patients treated with 40 mg aprepitant ranged from 19 to 84 years, with a mean age of 46.1 years. 46 patients were 65 years or older, with 13 patients being 75 years or older. The antiemetic activity of Aprepitant was evaluated during the 0 to 48 hour period following the end of surgery. The two pivotal studies were of similar design; however, they differed in terms of study hypothesis, efficacy analyses and geographic location. PONV Study 1 was a multinational study including the U.S., whereas, PONV Study 2 was conducted entirely in the U.S.
Efficacy measures in PONV Study 1 included:
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- complete response (defined as no emetic episodes and no use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 48 hours following the end of surgery (secondary)
- time to first use of rescue medication in the 0 to 24 hours following the end of surgery (exploratory)
- time to first emesis in the 0 to 48 hours following the end of surgery (exploratory).
A closed testing procedure was applied to control the type I error for the primary endpoints.
The results of the primary and secondary endpoints for 40 mg aprepitant and 4 mg ondansetron are described in Table 11:
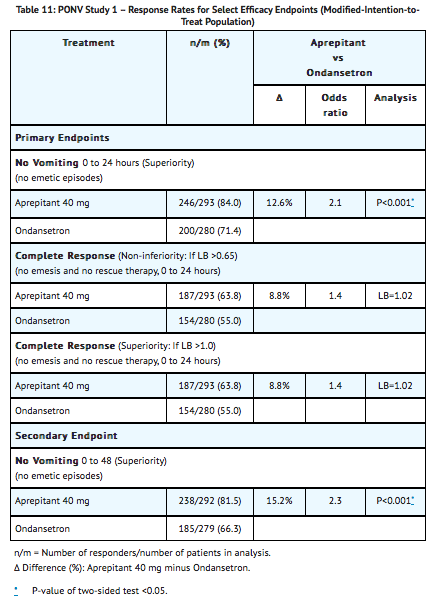
The use of aprepitant did not affect the time to first use of rescue medication when compared to ondansetron. However, compared to the ondansetron group, use of aprepitant delayed the time to first vomiting, as depicted in Figure 3.

- Efficacy measures in PONV Study 2 included:
- complete response (defined as no emetic episodes and no use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 24 hours following the end of surgery (secondary)
- no use of rescue therapy in the 0 to 24 hours following the end of surgery (secondary)
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 48 hours following the end of surgery (secondary).
- PONV Study 2 failed to satisfy its primary hypothesis that aprepitant is superior to ondansetron in the prevention of PONV as measured by the proportion of patients with complete response in the 24 hours following end of surgery.
The study demonstrated that both dose levels of aprepitant had a clinically meaningful effect with respect to the secondary endpoint “no vomiting” during the first 24 hours after surgery and showed that the use of 40 mg aprepitant was associated with a 16% improvement over ondansetron for the no vomiting endpoint.

How Supplied
No. 3854 — 80 mg capsules: White, opaque, hard gelatin capsule with “461” and “80 mg” printed radially in black ink on the body. They are supplied as follows:
- NDC 54868-5231-2 unit-of-use BiPack of 2
- NDC 54868-5231-3 unit-of-use BiPack of 4
- NDC 54868-5231-1 unit-dose package of 6.
Storage
Store at 20-25°C (68-77°F)
Images
Drug Images
{{#ask: Page Name::Aprepitant (patient information) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Aprepitant (patient information) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Aprepitant (patient information) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Aprepitant (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Aprepitant (patient information) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Aprepitant (patient information) |Label Name=Aprep PAckage.png
}}
{{#subobject:
|Label Page=Aprepitant (patient information) |Label Name=Aprep Package2.png
}}