Antithrombin III
| Antithrombin III |
|
WikiDoc Resources for Antithrombin III |
|
Articles |
|---|
|
Most recent articles on Antithrombin III Most cited articles on Antithrombin III |
|
Media |
|
Powerpoint slides on Antithrombin III |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Antithrombin III at Clinical Trials.gov Trial results on Antithrombin III Clinical Trials on Antithrombin III at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Antithrombin III NICE Guidance on Antithrombin III
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Antithrombin III Discussion groups on Antithrombin III Patient Handouts on Antithrombin III Directions to Hospitals Treating Antithrombin III Risk calculators and risk factors for Antithrombin III
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Antithrombin III |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
| Cardiology Network |
 Discuss Antithrombin III further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Antithrombin (AT) is a small protein molecule that inactivates several enzymes of the coagulation system. It is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites. α-antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin.[1]
Reference Range
| Level | 0.14-0.39 g/l |
| Activity | 70-120% of Normal Activity |
Nomenclature
Antithrombin is officially termed antithrombin III (AT III) and it is a member of a larger family of antithrombins, numbered antithrombin I (AT I) through to antithrombin VI (AT VI). All antithrombins are serpins, however only AT III and possibly AT I are medically significant. AT III is generally referred to solely as antithrombin and it is antithrombin III that is discussed in this article.
Structure
Antithrombin has a half life in blood plasma of around 3 days.[2]
The normal antithrombin concentration in human blood plasma is high at approximately 0.12 mg/ml, which is equivalent to a molar concentration of 2.3 μM.[3]
Antithrombin has been isolated from the plasma of a large number of species additional to humans.[4]
As deduced from protein and cDNA sequencing, cow, sheep, rabbit and mouse antithrombins are all 433 amino acids in length, which is one amino acid longer than human antithrombin III. The extra amino acid is thought to occur at amino acid position 6. Cow, sheep, rabbit, mouse and human antithrombins share between 84 and 89% amino acid sequence identity.[5]
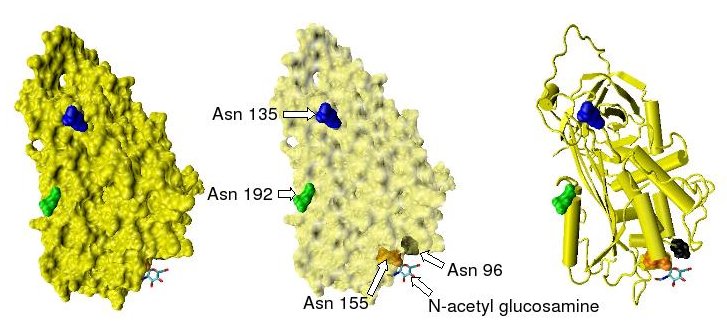
They all have four potential N-glycosylation sites. These occur at asparagine (Asn) amino acid numbers 96, 135, 155 and 192 in humans and at similar amino acid numbers in other species. All these sites are occupied by covalently attached oligosaccharide side chains in the predominant form of human antithrombin, α-antithrombin. The potential glycosylation site at asparagine 135 is not occupied in a minor form of antithrombin, β-antithrombin.[6]
Shown below are the location of the four potential glycosylation sites within the tertiary structure of an antithrombin monomer, as taken from the protein data bank file 2ANT. In this structure only Asn 155 is glycosylated by the addition of a single N-acetylglucosamine residue.
Function
Antithrombin is a serpin (serine protease inhibitor). The physiological target proteases of antithrombin are those of the contact activation pathway (formerly known as the intrinsic pathway), namely the activated forms of Factor X (Xa), Factor IX (IXa), Factor XI (XIa), Factor XII (XIIa) and Factor II (thrombin) (IIa) and also the activated form of Factor VII (VIIa) from the tissue factor pathway (formerly known as the extrinsic pathway).[7] Protease inactivation results as a consequence of the trapping the protease in an equimolar complex with antithrombin in which the active site of the protease enzyme is inaccessible to its usual substrate.[5] The formation of an antithrombin-protease complex involves an interaction between the protease and a specific reactive peptide bond within antithrombin. In human antithrombin this bond is between arginine (arg) 393 and serine (ser) 394.[5] As shown below the reactive arg 393 - ser 394 bond is located on an exposed loop at the surface of the molecule. This loop is termed the reactive site loop.
It is thought the trapping of protease enzymes in inactive antithrombin-protease complexes results as a consequence of their attack of the reactive bond. Where the attack of a similar bond within their normal substrate results in its rapid proteolytic cleavage, on initiating an attack on the antithrombin reactive bond the antithrombin inhibitor is activated to trap the enzyme at an intermediate stage during the proteolytic process. Given time thrombin is able to cleave the reactive bond within antithrombin and an inactive thrombin-antithrombin complex will dissociate, however the time it takes for this to occur may be greater than 3 days.[8]
The rate of antithrombin's inhibition of protease activity is greatly enhanced by its additional binding to heparin.
Antithrombin and heparin
AT-III binds to a specific pentasaccharide sulfation sequence contained within the heparin polymer
GlcNAc/NS(6S)-GlcA-GlcNS(3S,6S)-IdoA(2S)-GlcNS(6S)
Upon binding to this pentasaccharide sequence, inhibition of protease activity is increased by heparin as a result of two distinct mechanisms.[9] In one mechanism heparin stimulation of Factor IXa and Xa inhibition depends on a conformational change within antithrombin involving the reactive site loop and is thus allosteric. This conformational change results in an approximate 300 fold increase in Factor IXa and Xa inhibitory activity.[10] In another mechanism stimulation of thrombin inhibition depends on the formation of a ternary complex between AT-III, thrombin, and heparin. Formation of this complex results in an approximate 4 fold increase in antithrombin inhibitory activity.[10]
Allosteric activation
Increased Factor IXa and Xa inhibition requires the minimal heparin pentasaccharide sequence. The conformational changes that occur within antithrombin in response to pentasaccharide binding are well documented.[11][12][13] Shown below are two crystal structures for antithrombin. Model A is taken from the pdb file 2ANT and model B from pdb file 1AZX. Model B is complexed with a pentasaccharide while model A is uncomplexed.

The conformational change most relevant for Factor IXa and Xa inhibition occurs within the N-terminal portion of the reactive site loop and is circled in model B above. This region has been termed the hinge region and it has been shown that by preventing the normal extension of this region in response to heparin binding, increased Factor IXa and Xa inhibition does not occur.[10] It is thought that the increased flexibility given to the reactive site loop as a result of the hinge region conformational change is a key factor in influencing increased Factor IXa and Xa inhibition.
Non-allosteric activation
Increased thrombin inhibition requires the minimal heparin pentasaccharide plus at least an additional 13 monomeric units.[14] This is thought to be due to a requirement that antithrombin and thrombin must bind to the same heparin chain adjacent to each other. This can be seen in the series of models below taken from the pdb file 1TB6

Role in disease
Evidence for the important role antithrombin plays in regulating normal blood coagulation is demonstrated by the correlation between inherited or acquired antithrombin deficiencies and an increased risk of any affected individual developing thrombotic disease.[15] Antithrombin deficiency generally comes to light when a patient suffers recurrent venous thrombosis and pulmonary embolism. Variability across patients in the levels of antithrombin III account for some of the variability in patients' response to heparin.
Acquired antithrombin deficiency
Acquired antithrombin deficiency may result from a range of disorders such as liver dysfunction (coagulopathy), sepsis, or premature birth or as a result of interventions such as major surgery or cardiopulmonary bypass.[16] Smoking and oral contraceptives reduce antithrombin III levels.
Inherited antithrombin deficiency
Inherited deficiencies in blood antithrombin activity may be due to a low circulating level of structurally and functionally normal antithrombin. In this case typically antithrombin levels and therefore activity may be reduced by 50% compared to normal antithrombin levels.[17] This type of deficiency is classified as type I antithrombin deficiency.
Inherited deficiencies may also be the result of a structurally and functionally abnormal antithrombin protein circulating in the blood. In this case levels of antithrombin may be normal but the activity produced by this protein again may be reduced by 50% when compared to normal antithrombin activity levels.[17] This type of deficiency is classified as type II antithrombin deficiency.
Both type I and type II antithrombin deficiency have been shown to be the result of any one of a number of frameshift mutations, missense mutations or nonsense mutations in the gene that encodes antithrombin.[17][18][19]
Renal losses of antithrombin account for an increased risk of thrombosis in patients with nephrotic syndrome.
Complete Differential Diagnosis of Disorders of Antithrombin III
Increased Antithrombin III
- Cholestasis
- Warfarin therapy
Decreased Antithrombin III
- Coronary artery bypass grafting
- Cirrhosis
- Congenital heterozygous familial AT III deficiency
- Disseminated intravascular coagulation (DIC)
- Heparin therapy
- Major surgery
- Nephrotic syndrome
- Oral contraceptives
- Premature birth
- Protein-Losing Enteropathy or exudative enteropathy
- Sepsis
- Surgery involving large wound surfaces
- Thrombolysis
- Hepatic failure
- Trauma
References
- ↑ Bjork, I (1997). Antithrombin, A bloody important serpin (in Chemistry and Biology of Serpins). Plenum Press. pp. 17–33. ISBN 0-306-45698-2. Unknown parameter
|coauthors=ignored (help) - ↑ Collen DJ, Schetz F.; et al. (1977). "Metabolism of antithrombin III (heparin cofactor) in man: Effects of venous thrombosis of heparin administration". Eur. J. Clin. Invest. 7: 27–35. PMID 65284.
- ↑ Conrad J, Brosstad M.; et al. (1983). "Molar antithrombin concentration in normal human plasma". Haemostasis. 13: 363–368. PMID 6667903.
- ↑ Jordan RE. (1983). "Antithrombin in vertebrate species: Conservation of the heparin-dependent anticoagulant mechanism". Arch. Biochem. Biophys. 227: 587–595. PMID 6607710.
- ↑ 5.0 5.1 5.2 Olson ST, Bjork I. (1994). "Regulation of thrombin activity by antithrombin and heparin". Sem. Thromb. Hemost. 20 (4): 373–409. PMID 7899869.
- ↑ Brennan SO, George PM, Jordan, RE. (1987). "Physiological variant of antithrombin-III lacks carbohydrate side chain at Asn 135". FEBS Lett. 219: 431–436. PMID 3609301.
- ↑ Persson E, Bak H and Olsen OH. (2001). "Substitution of valine for leucine 305 in factor VIIa increases the intrinsic enzymatic activity". J. Biol. Chem. 276 (31): 29195–29199. PMID 11389142.
- ↑ Danielsson A and Bjork, I (1980). "Slow, spontaneous dissociation of the antithrombin-thrombin complex produces a proteolytically modified form of the inhibitor". FEBS Lett. 119: 241–244. PMID 7428936.
- ↑ Johnson DJ, Langdown J; et al. (2006). "Crystal structure of monomeric native antithrombin reveals a novel reactive center loop conformation". J. Biol. Chem. 281 (46): 35478–35486. PMID 16973611.
- ↑ 10.0 10.1 10.2 Langdown J, Johnson DJ.; et al. (2004). "Allosteric activation of antithrombin critically depends upon hinge region extension". J. Biol. Chem. 279 (45): 47288–47297. PMID 15326167.
- ↑ Schreuder HA, de Boer B.; et al. (1994). "The intact and cleaved human antithrombin III complex as a model for serpin-proteinase interactions". Nat. Struct. Biol. 1 (1): 48–54. PMID 7656006.
- ↑ Carrell RW, Stein PE.; et al. (1994). "Biological implications of a 3 A structure of dimeric antithrombin". Structure. 2 (4): 257–270. PMID 8087553.
- ↑ Whisstock JC, Pike RN.; et al. (2000). "Conformational changes in serpins: II. The mechanism of activation of antithrombin by heparindagger". J. Mol. Biol. 301 (5): 1287–1305. PMID 10966821.
- ↑ Petitou M, Herault JP, Bernat A, Driguez PA; et al. (1999). "Synthesis of Thrombin inhibiting Heparin mimetics without side effects". Nature. 398: 417–422. PMID 10201371.
- ↑ van Boven HH and Lane DA. (1997). "Antithrombin and its inherited deficiency states". Semin. Hematol. 34 (3): 188–204. PMID 9241705.
- ↑ Maclean PS and Tait RC. (2007). "Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options". Drugs. 67 (10): 1429–1440. PMID 17600391.
- ↑ 17.0 17.1 17.2 Lane DA, Olds RJ and Thein SL. (1994). "Antithrombin III: summary of first database update". Nucleic Acids Res. 22 (17): 3556–3559. PMID 7937056.
- ↑ Lane DA, Ireland H.; et al. (1991). "Antithrombin III: a database of mutations". Thromb. Haemost. 66 (6): 657–661. PMID 1796410.
- ↑ Picard V, Nowak-Göttl U.; et al. (2006). "Molecular bases of antithrombin deficiency: twenty-two novel mutations in the antithrombin gene". Hum. Mutat. 27 (6): 600. PMID 16705712.
External links
- Antithrombin+III at the US National Library of Medicine Medical Subject Headings (MeSH)
Acknowledgements
The content on this page was first contributed by Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
