Amyloidosis pathophysiology: Difference between revisions
Megan Merlo (talk | contribs) No edit summary |
|||
| (42 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Amyloidosis}} | {{Amyloidosis}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}}{{SHH}}{{Sab}} | ||
==Overview== | == Overview == | ||
[[Amyloid]] is | [[Amyloid]] is an abnormal insoluble [[extracellular]] [[protein]] that deposits in the different tissues and causes organic dysfunction and a wide variety of clinical syndromes. In systemic amyloidosis, [[amyloid]] gradually accumulate and [[amyloid]] deposition is widespread in the viscera, [[blood vessel]] walls, and in the different [[Connective tissue|connective tissues]]. [[AL amyloidosis|Primary (AL) amyloidosis)]] is the most common type of amyloidosis. It results from aggregation and deposition of monoclonal [[Immunoglobulin|immunoglobulin (Ig)]] [[Light chain|light chains]] that usually produced by [[plasma cell]] clones. [[AA amyloidosis|Secondary amyloidosis]] is associated with chronic [[inflammation]] (such as [[tuberculosis]] or [[rheumatoid arthritis]]). Hereditary (or familial) amyloidosis are [[Autosome|autosomal]] [[Dominance relationship|dominant]] diseases that [[inherited]] variant [[Protein|proteins]] cause the production and deposition of [[amyloid]] fibrils. Some [[Neurodegenerative disease|neurodegenerative disorders]] such as [[Parkinson's disease]], [[Alzheimer's disease|Alzheimer]], and [[Huntington's disease]] may occur in localised amyloidosis. On microscopic pathology, typical green [[birefringence]] under [[Polarization|polarized]] light after [[Congo red]] staining (appears in red under normal light). | ||
==Pathophysiology== | == Pathophysiology == | ||
*[[Amyloid]] is an abnormal insoluble [[extracellular]] [[protein]] that deposits in the different tissues and causes organic dysfunction and a wide variety of clinical syndromes.<ref name="pmid26719234">{{cite journal |vauthors=Wechalekar AD, Gillmore JD, Hawkins PN |title=Systemic amyloidosis |journal=Lancet |volume=387 |issue=10038 |pages=2641–2654 |date=June 2016 |pmid=26719234 |doi=10.1016/S0140-6736(15)01274-X |url=}}</ref> | |||
*These abnormal [[Amyloid|amyloids]] are derived from misfolding and aggregation of normally soluble [[Protein|proteins]]. | |||
*[[Amyloid]] deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.<ref name="pmid267192342">{{cite journal |vauthors=Wechalekar AD, Gillmore JD, Hawkins PN |title=Systemic amyloidosis |journal=Lancet |volume=387 |issue=10038 |pages=2641–2654 |date=June 2016 |pmid=26719234 |doi=10.1016/S0140-6736(15)01274-X |url=}}</ref> | |||
<ref> | |||
===Systemic Amyloidosis=== | ===Systemic Amyloidosis=== | ||
*In systemic amyloidosis, [[amyloid]] gradually accumulates and [[amyloid]] deposition is widespread in the viscera, [[blood vessel]] walls, and different [[Connective tissue|connective tissues]].<ref name="pmid23227278">{{cite journal |vauthors=Baker KR, Rice L |title=The amyloidoses: clinical features, diagnosis and treatment |journal=Methodist Debakey Cardiovasc J |volume=8 |issue=3 |pages=3–7 |date=2012 |pmid=23227278 |pmc=3487569 |doi= |url=}}</ref><ref name="pmid16409147">{{cite journal |vauthors=Pepys MB |title=Amyloidosis |journal=Annu. Rev. Med. |volume=57 |issue= |pages=223–41 |date=2006 |pmid=16409147 |doi=10.1146/annurev.med.57.121304.131243 |url=}}</ref> | |||
* | |||
=== | ====Primary Amyloidosis (AL)==== | ||
*[[AL amyloidosis|Primary (AL) amyloidosis)]] is the most common type of amyloidosis.<ref name="pmid267192343">{{cite journal |vauthors=Wechalekar AD, Gillmore JD, Hawkins PN |title=Systemic amyloidosis |journal=Lancet |volume=387 |issue=10038 |pages=2641–2654 |date=June 2016 |pmid=26719234 |doi=10.1016/S0140-6736(15)01274-X |url=}}</ref> | |||
* [[ | *It results from aggregation and deposition of monoclonal [[Immunoglobulin|immunoglobulin (Ig)]] [[Light chain|light chains]] that are usually produced by [[plasma cell]] clones. | ||
* [[ | *Change in the [[secondary structure]] or [[tertiary structure]] of a monoclonal [[light chain]] results in abnormal folding of the [[light chain]] that abnormally form [[amyloid]] [[Fibril|fibrils]]. | ||
*This type of amyloidosis most frequently involve the [[kidney]] ([[nephrotic syndrome]]) and the [[heart]]. | |||
* [[ | *In [[AL amyloidosis|primary (AL) amyloidosis]] survival rate depends on: | ||
**Type of organ involvement ([[amyloid]] heart disease is the main prognostic factor) | |||
**The severity of different organ involvement | |||
* | **[[Hematology|Hematological]] response to treatment | ||
*The median [[Survival analysis|survival]] of patients with [[AL amyloidosis]] is approximately 3.8 years. | |||
* [[ | |||
* | |||
** | |||
* [[ | |||
* [[ | |||
=== | ====Secondary Amyloidosis (AA)==== | ||
{| | *[[AA amyloidosis|Secondary amyloidosis]] occurs as a reaction to an existing illness. | ||
| | *[[AA amyloidosis|Secondary amyloidosis]] is associated with chronic [[inflammation]] (such as [[tuberculosis]] or [[rheumatoid arthritis]]).<ref name="pmid116772762">{{cite journal |vauthors=Khan MF, Falk RH |title=Amyloidosis |journal=Postgrad Med J |volume=77 |issue=913 |pages=686–93 |date=November 2001 |pmid=11677276 |pmc=1742163 |doi= |url=}}</ref> | ||
| | *[[AA amyloidosis|Secondary or reactive amyloidosis (AA)]] comprises approximately 45% of the systemic amyloidoses. | ||
| | *[[Pathogenesis]] of [[AA amyloidosis|secondary amyloidosis]] is multifactorial, including: | ||
|[[ | **[[Primary structure]] of the [[precursor]] protein | ||
|[[ | **Acute phase response | ||
|[[ | **Nonfibril [[Protein|proteins]] ([[amyloid]] P component, [[Apolipoprotein E|apo E]], [[Glycosaminoglycan|GAGs]], [[Proteoglycan|proteoglycans]] and [[basement membrane]] [[Protein|proteins]]) | ||
| | **[[Receptor (biochemistry)|Receptors]] | ||
**[[Lipid metabolism]] | |||
**[[Protease|Proteases]] | |||
====Hereditary Amyloidosis==== | |||
*Hereditary amyloidosis is an [[Autosome|autosomal]] [[Dominance relationship|dominant]] disorder.<ref name="pmid11261421">{{cite journal |vauthors=Hund E, Linke RP, Willig F, Grau A |title=Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment |journal=Neurology |volume=56 |issue=4 |pages=431–5 |date=February 2001 |pmid=11261421 |doi=10.1212/wnl.56.4.431 |url=}}</ref> | |||
|- | *It can have a heterogeneous nature of presentation and can be complicated by significant disability and mortality.<ref name="pmid28978215">{{cite journal |vauthors=Gertz MA |title=Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges |journal=Am J Manag Care |volume=23 |issue=7 Suppl |pages=S107–S112 |date=June 2017 |pmid=28978215 |doi= |url=}}</ref> | ||
| | *[[Inherited]] variant [[Protein|proteins]] cause the production and deposition of [[amyloid]] fibrils.<ref name="pmid116772762" /> | ||
*Hereditary amyloidosis is due to amyloidogenic [[Mutation|mutations]] and the subsequent deposition of [[Amyloid|amyloids]] which include: | |||
**[[Transthyretin|Transthyretin (TTR)]] (most common [[inherited]] [[mutation]]) | |||
**[[Fibrinogen]] | |||
**[[Apolipoprotein A1]] | |||
**[[Apolipoprotein A2]] | |||
| | **[[Lysozyme]] | ||
**[[Gelsolin]] [[Gene|genes]] | |||
=== | ===Organ-specific Amyloidosis=== | ||
*In this type of amyloidosis, [[amyloid]] deposition occurs only in the organ of origin or tissue of [[precursor]] [[protein]]. | |||
*[[Neurodegenerative disease|Neurodegenerative disorders]], such as [[Parkinson's disease]], [[Alzheimer's disease]], and [[Huntington's disease]], may occur in localized amyloidosis. | |||
*Localized amyloidoses can develop due to the deposition of [[intracellular]] and/or [[extracellular]] [[amyloid]]. | |||
**[[Huntington's disease]]: [[intracellular]] [[protein]] deposition | |||
**[[Parkinson's disease]]: [[intracellular]] [[protein]] deposition | |||
**[[Alzheimer's disease]]: [[intracellular]] ([[Tau protein]] [[Fibril|fibrils]]) and [[extracellular]] ([[amyloid]] β fibrils) deposition | |||
== | ==Genetics== | ||
==== Hereditary Amyloidosis ==== | |||
* Hereditary amyloidosis involves [[Mutation|mutations]] in the following genes:<ref name="pmid8464497">{{cite journal |vauthors=Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ |title=Human lysozyme gene mutations cause hereditary systemic amyloidosis |journal=Nature |volume=362 |issue=6420 |pages=553–7 |date=April 1993 |pmid=8464497 |doi=10.1038/362553a0 |url=}}</ref> | |||
** LYZ gene | |||
** Fibrinogen A alpha polypeptide gene | |||
** FGA gene | |||
** APOA1 gene | |||
** Lysozyme gene | |||
** B2M gene | |||
==== Primary Localized Cutaneous Amyloidosis ==== | |||
* Primary localized cutaneous amyloidosis is inherited in autosomal dominant manner.<ref name="pmid18179886">{{cite journal |vauthors=Arita K, South AP, Hans-Filho G, Sakuma TH, Lai-Cheong J, Clements S, Odashiro M, Odashiro DN, Hans-Neto G, Hans NR, Holder MV, Bhogal BS, Hartshorne ST, Akiyama M, Shimizu H, McGrath JA |title=Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis |journal=Am. J. Hum. Genet. |volume=82 |issue=1 |pages=73–80 |date=January 2008 |pmid=18179886 |pmc=2253984 |doi=10.1016/j.ajhg.2007.09.002 |url=}}</ref> | |||
* Primary localized cutaneous amyloidosis involves mutations in the following genes:<ref name="pmid181798862">{{cite journal |vauthors=Arita K, South AP, Hans-Filho G, Sakuma TH, Lai-Cheong J, Clements S, Odashiro M, Odashiro DN, Hans-Neto G, Hans NR, Holder MV, Bhogal BS, Hartshorne ST, Akiyama M, Shimizu H, McGrath JA |title=Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis |journal=Am. J. Hum. Genet. |volume=82 |issue=1 |pages=73–80 |date=January 2008 |pmid=18179886 |pmc=2253984 |doi=10.1016/j.ajhg.2007.09.002 |url=}}</ref> | |||
** OSMR gene | |||
== Associated Conditions == | |||
Conditions associated with amyloidosis include:<ref name="pmid8757765">{{cite journal |vauthors=Hofstra RM, Sijmons RH, Stelwagen T, Stulp RP, Kousseff BG, Lips CJ, Steijlen PM, Van Voorst Vader PC, Buys CH |title=RET mutation screening in familial cutaneous lichen amyloidosis and in skin amyloidosis associated with multiple endocrine neoplasia |journal=J. Invest. Dermatol. |volume=107 |issue=2 |pages=215–8 |date=August 1996 |pmid=8757765 |doi=10.1111/1523-1747.ep12329651 |url=}}</ref> | |||
* MEN2A | |||
== Gross Pathology == | |||
On gross pathology, the organs affected by amyloidosis can be characterized by the following features: | |||
*Porcelain like or waxy appearance | |||
*Enlargement | |||
===Images=== | |||
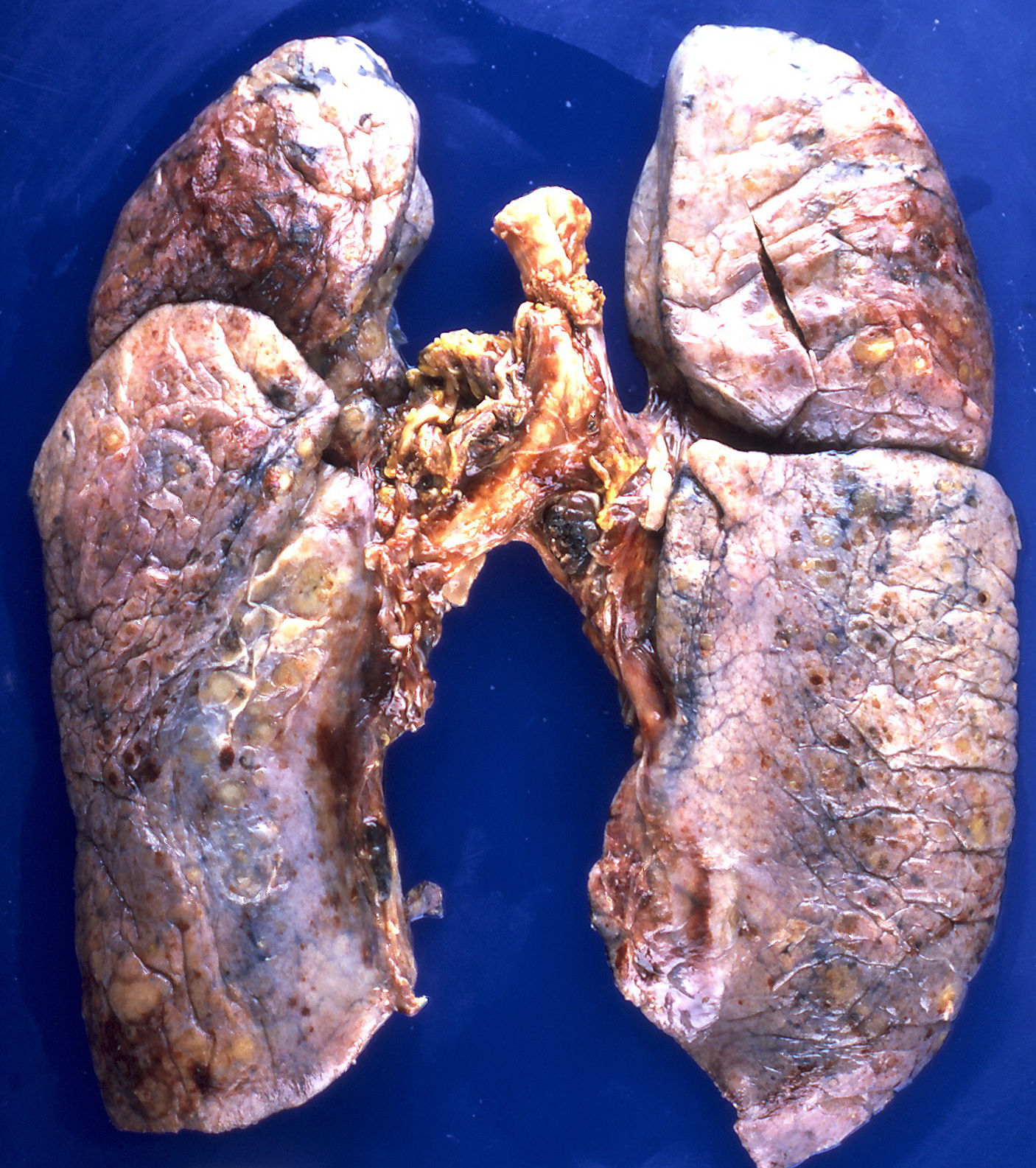
[[File:Amyloidosis (4867136708).jpg|300px|left|thumb|Nodular deposits of amyloid on the pleural surfaces.<ref>By Yale Rosen from USA - Amyloidosis, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=31127928</ref>]] | |||
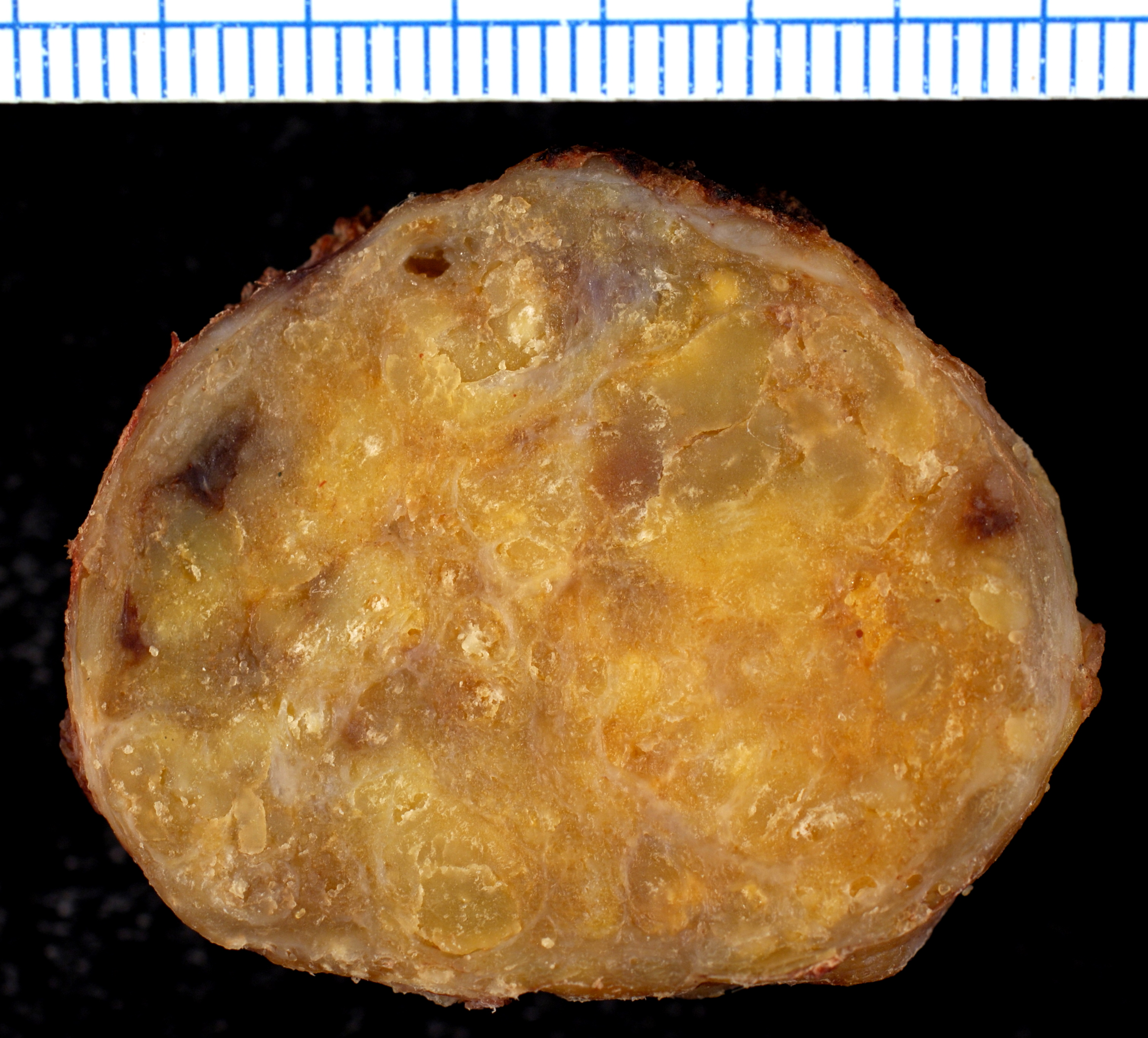
[[File:Amyloidosis, Node, Gross.jpg|400px|center|thumb|Cut section of an inguinal lymph node showing firm and waxy consistency.<ref>By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377537238/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629764</ref>]] | |||
[[File:Amyloidosis, Node, Lugol's Reaction.jpg|300px|left|thumb|A slice of the affected node (left) has turned black after treatment with Lugol's solution. A piece of normal myometrium (right) treated similarly with no reaction is also shown.<ref>By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377538012/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629740</ref>]] | |||
<br style="clear:left"> | |||
==Microscopic Pathology== | |||
On microscopic histopathological analysis, amyloidosis is characterized by:<ref name="pmid116772762" /><ref name="pmid119640392">{{cite journal |vauthors=Röcken C, Shakespeare A |title=Pathology, diagnosis and pathogenesis of AA amyloidosis |journal=Virchows Arch. |volume=440 |issue=2 |pages=111–122 |date=February 2002 |pmid=11964039 |doi=10.1007/s00428-001-0582-9 |url=}}</ref> | |||
*Green [[birefringence]] under [[Polarization|polarized]] light after [[Congo red]] staining (appears red under normal light) | |||
*Linear non-branching [[Fibril|fibrils]] (indefinite length with an approximately same diameter) | |||
*Distinct [[X-rays|X-ray]] diffraction pattern consistent with Pauling's model of a cross-beta fibril | |||
===Images=== | |||
[[File:Small bowel duodenum with amyloid deposition congo red 10X.jpg|300px|left|thumb|Small bowel duodenum with amyloid deposition Congo red.<ref>By Michael Feldman, MD, PhDUniversity of Pennsylvania School of Medicine - http://www.healcentral.org/healapp/showMetadata?metadataId=38717, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=870218</ref>]] | |||
[[File:Amyloidosis, Node, Congo Red.jpg|300px|center|thumb|Amyloidosis (black arrows) in a lymph node after staining with Congo Red.<ref>By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559787/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629716</ref>]] | |||
[[File:Amyloidosis, lymph node, polarizer.jpg|300px|left|thumb|Green [[birefringence]] under [[Polarization|polarized]] light.<ref>By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559955/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629705</ref>]] | |||
<br style="clear:left"> | |||
== References == | == References == | ||
{{reflist|2}} | {{reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 19:03, 29 October 2019
|
Amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Amyloidosis pathophysiology |
|
Risk calculators and risk factors for Amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shaghayegh Habibi, M.D.[2]Sabawoon Mirwais, M.B.B.S, M.D.[3]
Overview
Amyloid is an abnormal insoluble extracellular protein that deposits in the different tissues and causes organic dysfunction and a wide variety of clinical syndromes. In systemic amyloidosis, amyloid gradually accumulate and amyloid deposition is widespread in the viscera, blood vessel walls, and in the different connective tissues. Primary (AL) amyloidosis) is the most common type of amyloidosis. It results from aggregation and deposition of monoclonal immunoglobulin (Ig) light chains that usually produced by plasma cell clones. Secondary amyloidosis is associated with chronic inflammation (such as tuberculosis or rheumatoid arthritis). Hereditary (or familial) amyloidosis are autosomal dominant diseases that inherited variant proteins cause the production and deposition of amyloid fibrils. Some neurodegenerative disorders such as Parkinson's disease, Alzheimer, and Huntington's disease may occur in localised amyloidosis. On microscopic pathology, typical green birefringence under polarized light after Congo red staining (appears in red under normal light).
Pathophysiology
- Amyloid is an abnormal insoluble extracellular protein that deposits in the different tissues and causes organic dysfunction and a wide variety of clinical syndromes.[1]
- These abnormal amyloids are derived from misfolding and aggregation of normally soluble proteins.
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[2]
Systemic Amyloidosis
- In systemic amyloidosis, amyloid gradually accumulates and amyloid deposition is widespread in the viscera, blood vessel walls, and different connective tissues.[3][4]
Primary Amyloidosis (AL)
- Primary (AL) amyloidosis) is the most common type of amyloidosis.[5]
- It results from aggregation and deposition of monoclonal immunoglobulin (Ig) light chains that are usually produced by plasma cell clones.
- Change in the secondary structure or tertiary structure of a monoclonal light chain results in abnormal folding of the light chain that abnormally form amyloid fibrils.
- This type of amyloidosis most frequently involve the kidney (nephrotic syndrome) and the heart.
- In primary (AL) amyloidosis survival rate depends on:
- Type of organ involvement (amyloid heart disease is the main prognostic factor)
- The severity of different organ involvement
- Hematological response to treatment
- The median survival of patients with AL amyloidosis is approximately 3.8 years.
Secondary Amyloidosis (AA)
- Secondary amyloidosis occurs as a reaction to an existing illness.
- Secondary amyloidosis is associated with chronic inflammation (such as tuberculosis or rheumatoid arthritis).[6]
- Secondary or reactive amyloidosis (AA) comprises approximately 45% of the systemic amyloidoses.
- Pathogenesis of secondary amyloidosis is multifactorial, including:
- Primary structure of the precursor protein
- Acute phase response
- Nonfibril proteins (amyloid P component, apo E, GAGs, proteoglycans and basement membrane proteins)
- Receptors
- Lipid metabolism
- Proteases
Hereditary Amyloidosis
- Hereditary amyloidosis is an autosomal dominant disorder.[7]
- It can have a heterogeneous nature of presentation and can be complicated by significant disability and mortality.[8]
- Inherited variant proteins cause the production and deposition of amyloid fibrils.[6]
- Hereditary amyloidosis is due to amyloidogenic mutations and the subsequent deposition of amyloids which include:
Organ-specific Amyloidosis
- In this type of amyloidosis, amyloid deposition occurs only in the organ of origin or tissue of precursor protein.
- Neurodegenerative disorders, such as Parkinson's disease, Alzheimer's disease, and Huntington's disease, may occur in localized amyloidosis.
- Localized amyloidoses can develop due to the deposition of intracellular and/or extracellular amyloid.
- Huntington's disease: intracellular protein deposition
- Parkinson's disease: intracellular protein deposition
- Alzheimer's disease: intracellular (Tau protein fibrils) and extracellular (amyloid β fibrils) deposition
Genetics
Hereditary Amyloidosis
- Hereditary amyloidosis involves mutations in the following genes:[9]
- LYZ gene
- Fibrinogen A alpha polypeptide gene
- FGA gene
- APOA1 gene
- Lysozyme gene
- B2M gene
Primary Localized Cutaneous Amyloidosis
- Primary localized cutaneous amyloidosis is inherited in autosomal dominant manner.[10]
- Primary localized cutaneous amyloidosis involves mutations in the following genes:[11]
- OSMR gene
Associated Conditions
Conditions associated with amyloidosis include:[12]
- MEN2A
Gross Pathology
On gross pathology, the organs affected by amyloidosis can be characterized by the following features:
- Porcelain like or waxy appearance
- Enlargement
Images

Microscopic Pathology
On microscopic histopathological analysis, amyloidosis is characterized by:[6][16]
- Green birefringence under polarized light after Congo red staining (appears red under normal light)
- Linear non-branching fibrils (indefinite length with an approximately same diameter)
- Distinct X-ray diffraction pattern consistent with Pauling's model of a cross-beta fibril
Images



References
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Baker KR, Rice L (2012). "The amyloidoses: clinical features, diagnosis and treatment". Methodist Debakey Cardiovasc J. 8 (3): 3–7. PMC 3487569. PMID 23227278.
- ↑ Pepys MB (2006). "Amyloidosis". Annu. Rev. Med. 57: 223–41. doi:10.1146/annurev.med.57.121304.131243. PMID 16409147.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ 6.0 6.1 6.2 Khan MF, Falk RH (November 2001). "Amyloidosis". Postgrad Med J. 77 (913): 686–93. PMC 1742163. PMID 11677276.
- ↑ Hund E, Linke RP, Willig F, Grau A (February 2001). "Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment". Neurology. 56 (4): 431–5. doi:10.1212/wnl.56.4.431. PMID 11261421.
- ↑ Gertz MA (June 2017). "Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges". Am J Manag Care. 23 (7 Suppl): S107–S112. PMID 28978215.
- ↑ Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ (April 1993). "Human lysozyme gene mutations cause hereditary systemic amyloidosis". Nature. 362 (6420): 553–7. doi:10.1038/362553a0. PMID 8464497.
- ↑ Arita K, South AP, Hans-Filho G, Sakuma TH, Lai-Cheong J, Clements S, Odashiro M, Odashiro DN, Hans-Neto G, Hans NR, Holder MV, Bhogal BS, Hartshorne ST, Akiyama M, Shimizu H, McGrath JA (January 2008). "Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis". Am. J. Hum. Genet. 82 (1): 73–80. doi:10.1016/j.ajhg.2007.09.002. PMC 2253984. PMID 18179886.
- ↑ Arita K, South AP, Hans-Filho G, Sakuma TH, Lai-Cheong J, Clements S, Odashiro M, Odashiro DN, Hans-Neto G, Hans NR, Holder MV, Bhogal BS, Hartshorne ST, Akiyama M, Shimizu H, McGrath JA (January 2008). "Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis". Am. J. Hum. Genet. 82 (1): 73–80. doi:10.1016/j.ajhg.2007.09.002. PMC 2253984. PMID 18179886.
- ↑ Hofstra RM, Sijmons RH, Stelwagen T, Stulp RP, Kousseff BG, Lips CJ, Steijlen PM, Van Voorst Vader PC, Buys CH (August 1996). "RET mutation screening in familial cutaneous lichen amyloidosis and in skin amyloidosis associated with multiple endocrine neoplasia". J. Invest. Dermatol. 107 (2): 215–8. doi:10.1111/1523-1747.ep12329651. PMID 8757765.
- ↑ By Yale Rosen from USA - Amyloidosis, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=31127928
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377537238/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629764
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377538012/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629740
- ↑ Röcken C, Shakespeare A (February 2002). "Pathology, diagnosis and pathogenesis of AA amyloidosis". Virchows Arch. 440 (2): 111–122. doi:10.1007/s00428-001-0582-9. PMID 11964039.
- ↑ By Michael Feldman, MD, PhDUniversity of Pennsylvania School of Medicine - http://www.healcentral.org/healapp/showMetadata?metadataId=38717, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=870218
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559787/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629716
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559955/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629705