Alpha-synuclein: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
m (Bot: HTTP→HTTPS (v475)) |
||
| Line 1: | Line 1: | ||
{{Infobox gene}} | |||
'''Alpha-synuclein''' is a [[protein]] that is abundant in the human brain.<ref name="ghrsnca">{{cite web | url=http://ghr.nlm.nih.gov/gene/SNCA | title = Genetics Home Reference: SNCA | publisher = U.S. National Library of Medicine | accessdate = 14 Nov 2013 | date = 12 Nov 2013}}</ref> Smaller amounts are found in the heart, muscles, and other tissues.<ref name="ghrsnca"/> In the brain, alpha-synuclein is found mainly at the tips of nerve cells (neurons) in specialized structures called [[presynaptic terminal]]s.<ref name="ghrsnca"/> Within these structures, alpha-synuclein interacts with [[phospholipid]]s<ref>{{cite journal | vauthors = Chandra S, Chen X, Rizo J, Jahn R, Südhof TC | title = A broken alpha -helix in folded alpha -Synuclein | journal = The Journal of Biological Chemistry | volume = 278 | issue = 17 | pages = 15313–8 | date = Apr 2003 | pmid = 12586824 | doi = 10.1074/jbc.M213128200 }}</ref> and proteins.<ref name="ghrsnca"/> Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function.<ref name="ghrsnca"/> | |||
Although the function of alpha-synuclein is not well understood, studies suggest that it plays a role in maintaining a supply of synaptic vesicles in presynaptic terminals by clustering synaptic vesicles.<ref name="pmid23638301">{{cite journal | vauthors = Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Südhof TC, Brunger AT | title = Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2 | journal = eLife | volume = 2 | issue = | pages = e00592 | year = 2013 | pmid = 23638301 | pmc = 3639508 | doi = 10.7554/eLife.00592 }}</ref> It may also help regulate the release of dopamine, a type of neurotransmitter that is critical for controlling the start and stop of voluntary and involuntary movements.<ref name="ghrsnca"/> | |||
{{ | |||
| | |||
| | |||
| | |||
| | |||
| | |||
}}< | |||
The human alpha-synuclein protein is made of 140 amino acids and is encoded by the ''SNCA'' [[gene]].<ref name="pmid8248242">{{cite journal | vauthors = Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T | title = Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 90 | issue = 23 | pages = 11282–6 | date = Dec 1993 | pmid = 8248242 | pmc = 47966 | doi = 10.1073/pnas.90.23.11282 | url = http://www.pnas.org/content/90/23/11282 }}</ref><ref name="pmid12214035">{{cite journal | vauthors = Xia Y, Saitoh T, Uéda K, Tanaka S, Chen X, Hashimoto M, Hsu L, Conrad C, Sundsmo M, Yoshimoto M, Thal L, Katzman R, Masliah E | title = Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms | journal = Journal of Alzheimer's Disease | volume = 3 | issue = 5 | pages = 485–494 | date = Oct 2001 | pmid = 12214035 | doi = | url = http://iospress.metapress.com/content/jpmvj4dubjpm3b73/ }}</ref><ref>{{cite journal | vauthors = Xia Y, Saitoh T, Uéda K, Tanaka S, Chen X, Hashimoto M, Hsu L, Conrad C, Sundsmo M, Yoshimoto M, Thal L, Katzman R, Masliah E | title = Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms: Erratum p489 Fig 3 | journal = J. Alzheimers Dis. | volume = 4 | issue = 4 | pages = 337 | year = 2002 | pmid = | doi = | url = http://iospress.metapress.com/content/jpmvj4dubjpm3b73/ | issn = }}</ref> An alpha-synuclein fragment, known as the non-[[Abeta]] component (NAC) of [[Alzheimer's disease]] [[amyloid]], originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP.<ref name="pmid8248242"/> It was later determined that NACP was the human homologue of ''[[Torpedo (genus)|Torpedo]]'' synuclein. Therefore, NACP is now referred to as human alpha-synuclein. | |||
== Tissue expression == | |||
Alpha-synuclein is | Alpha-synuclein is a [[synuclein]] [[protein]] of unknown function primarily found in [[neural tissue]], making up as much as 1% of all proteins in the [[cytosol]] of brain cells.<ref name="pmid7857654">{{cite journal | vauthors = Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T | title = The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system | journal = Neuron | volume = 14 | issue = 2 | pages = 467–75 | date = Feb 1995 | pmid = 7857654 | doi = 10.1016/0896-6273(95)90302-X }}</ref> It is predominantly expressed in the [[neocortex]], [[hippocampus]], [[substantia nigra]], [[thalamus]], and [[cerebellum]]. It is predominantly a neuronal protein, but can also be found in the neuroglial cells.{{Citation needed|date=March 2012}} In melanocytic cells, SNCA protein expression may be regulated by [[Microphthalmia-associated transcription factor|MITF]].<ref name="pmid19067971">{{cite journal | vauthors = Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, Valgeirsdottir S, Bergsteinsdottir K, Schepsky A, Dummer R, Steingrimsson E | title = Novel MITF targets identified using a two-step DNA microarray strategy | journal = Pigment Cell & Melanoma Research | volume = 21 | issue = 6 | pages = 665–76 | date = Dec 2008 | pmid = 19067971 | doi = 10.1111/j.1755-148X.2008.00505.x }}</ref> | ||
It has been established that alpha-synuclein is extensively localized in the nucleus of mammalian brain neurons, suggesting a role of alpha-synuclein in the nucleus.<ref name="pmid17275196">{{cite journal | vauthors = Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Uéda K, Chan P | title = Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody | journal = Neuroscience | volume = 145 | issue = 2 | pages = 539–55 | date = Mar 2007 | pmid = 17275196 | doi = 10.1016/j.neuroscience.2006.12.028 }}</ref> Synuclein is however found predominantly in the [[presynaptic]] termini, in both free or membrane-bound forms,<ref name="pmid10722726">{{cite journal | vauthors = McLean PJ, Kawamata H, Ribich S, Hyman BT | title = Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations | journal = The Journal of Biological Chemistry | volume = 275 | issue = 12 | pages = 8812–6 | date = Mar 2000 | pmid = 10722726 | doi = 10.1074/jbc.275.12.8812 }}</ref> with roughly 15% of synuclein being membrane-bound in any moment in neurons.<ref name="pmid11679584">{{cite journal | vauthors = Lee HJ, Choi C, Lee SJ | title = Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form | journal = The Journal of Biological Chemistry | volume = 277 | issue = 1 | pages = 671–8 | date = Jan 2002 | pmid = 11679584 | doi = 10.1074/jbc.M107045200 }}</ref> | |||
It has also been shown that alpha-synuclein is localized in neuronal mitochondria.<ref name="pmid 18817762">{{cite journal | vauthors = Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Uéda K, Yu S, Yang H | title = Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody | journal = Brain Research | volume = 1244 | issue = | pages = 40–52 | date = Dec 2008 | pmid = 18817762 | doi = 10.1016/j.brainres.2008.08.067 }}</ref><ref name="pmid 19429081">{{cite journal | vauthors = Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K, Chan P, Yu S | title = alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity | journal = Neuroscience Letters | volume = 454 | issue = 3 | pages = 187–92 | date = May 2009 | pmid = 19429081 | doi = 10.1016/j.neulet.2009.02.056 }}</ref> Alpha-synuclein is highly expressed in the mitochondria in olfactory bulb, hippocampus, striatum and thalamus, where the cytosolic alpha-synuclein is also rich. However, the cerebral cortex and cerebellum are | |||
two exceptions, which contain rich cytosolic alpha-synuclein but very low levels of mitochondrial alpha-synuclein. It has been shown that alpha-synuclein is localized in the inner membrane of mitochondria, and that the inhibitory effect of alpha-synuclein on complex I activity of mitochondrial respiratory chain is dose-dependent. Thus, it is suggested that alpha-synuclein in mitochondria is differentially expressed in different brain regions and the background levels of mitochondrial alpha-synuclein may be a potential factor affecting mitochondrial function and predisposing some neurons to degeneration.<ref name="pmid 19429081"/> | |||
[[ | At least three isoforms of synuclein are produced through [[alternative splicing]].<ref name="Beyer_2006">{{cite journal | vauthors = Beyer K | title = Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers | journal = Acta Neuropathologica | volume = 112 | issue = 3 | pages = 237–51 | date = Sep 2006 | pmid = 16845533 | doi = 10.1007/s00401-006-0104-6 }}</ref> The majority form of the protein, and the one most investigated, is the full-length protein of 140 amino acids. Other isoforms are alpha-synuclein-126, which lacks residues 41-54 due to loss of exon 3; and alpha-synuclein-112,<ref name="pmid 7802671">{{cite journal | vauthors = Uéda K, Saitoh T, Mori H | title = Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid | journal = Biochemical and Biophysical Research Communications | volume = 205 | issue = 2 | pages = 1366–72 | date = Dec 1994 | pmid = 7802671 | doi = 10.1006/bbrc.1994.2816 }}</ref> which lacks residue 103-130 due to loss of exon 5.<ref name="Beyer_2006"/> | ||
== | == Structure == | ||
== | Alpha-synuclein in solution is considered to be an [[intrinsically disordered protein]], i.e. it lacks a single stable 3D structure.<ref name="pmid19837084" >{{cite journal | vauthors = van Rooijen BD, van Leijenhorst-Groener KA, Claessens MM, Subramaniam V | title = Tryptophan fluorescence reveals structural features of alpha-synuclein oligomers | journal = Journal of Molecular Biology | volume = 394 | issue = 5 | pages = 826–33 | date = Dec 2009 | pmid = 19837084 | doi = 10.1016/j.jmb.2009.10.021 }}</ref> As of 2014, an increasing number of reports suggest, however, the presence of partial structures or mostly structured oligomeric states in the solution structure of alpha-synuclein even in the absence of lipids. This trend is also supported by a large number of single molecule ([[optical tweezers]]) measurements on single copies of monomeric alpha-synuclein as well as covalently enforced [[protein dimer|dimer]]s or [[tetramer]]s of alpha-synuclein.<ref name="pmid24475132">{{cite journal | vauthors = Neupane K, Solanki A, Sosova I, Belov M, Woodside MT | title = Diverse metastable structures formed by small oligomers of α-synuclein probed by force spectroscopy | journal = PLOS ONE | volume = 9 | issue = 1 | pages = e86495 | date = January 2014 | pmid = 24475132 | pmc = 3901707 | doi = 10.1371/journal.pone.0086495 }}</ref> | ||
{{ | |||
== Function == | |||
Alpha-synuclein is specifically [[Regulation of gene expression|upregulated]] in a discrete population of presynaptic terminals of the brain during a period of acquisition-related synaptic rearrangement.<ref name="pmid7646890">{{cite journal | vauthors = George JM, Jin H, Woods WS, Clayton DF | title = Characterization of a novel protein regulated during the critical period for song learning in the zebra finch | journal = Neuron | volume = 15 | issue = 2 | pages = 361–72 | date = Aug 1995 | pmid = 7646890 | doi = 10.1016/0896-6273(95)90040-3 }}</ref> | |||
It has been shown that alpha-synuclein significantly interacts with [[tubulin]],<ref name="pmid11867079">{{cite journal | vauthors = Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K | title = Tubulin seeds alpha-synuclein fibril formation | journal = The Journal of Biological Chemistry | volume = 277 | issue = 3 | pages = 2112–7 | date = Jan 2002 | pmid = 11698390 | pmc = | doi = 10.1074/jbc.M102981200 }}</ref> and that alpha-synuclein may have activity as a potential microtubule-associated protein, like [[Tau protein|tau]].<ref name="pmid15345814">{{cite journal | vauthors = Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T, Kaji H, Yoshii M, Hisanaga S, Uéda K | title = Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein | journal = Journal of Alzheimer's Disease | volume = 6 | issue = 4 | pages = 435–42; discussion 443–9 | date = Aug 2004 | pmid = 15345814 | doi = }}</ref> | |||
Recent evidence suggests that alpha-synuclein functions as a molecular [[chaperone (protein)|chaperone]] in the formation of [[SNARE (protein)|SNARE]] complexes.<ref name="pmid16269324">{{cite journal | vauthors = Bonini NM, Giasson BI | title = Snaring the function of alpha-synuclein | journal = Cell | volume = 123 | issue = 3 | pages = 359–61 | date = Nov 2005 | pmid = 16269324 | doi = 10.1016/j.cell.2005.10.017 }}</ref><ref name="pmid16269331">{{cite journal | vauthors = Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC | title = Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration | journal = Cell | volume = 123 | issue = 3 | pages = 383–96 | date = Nov 2005 | pmid = 16269331 | doi = 10.1016/j.cell.2005.09.028 }}</ref> In particular, it simultaneously binds to phospholipids of the [[plasma membrane]] via its N-terminus domain and to [[synaptobrevin]]-2 via its C-terminus domain, with increased importance during synaptic activity.<ref name="pmid20798282">{{cite journal | vauthors = Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC | title = Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro | journal = Science | volume = 329 | issue = 5999 | pages = 1663–7 | date = Sep 2010 | pmid = 20798282 | pmc = 3235365 | doi = 10.1126/science.1195227 }}</ref> Indeed, there is growing evidence that alpha-synuclein is involved in the functioning of the neuronal [[Golgi apparatus]] and [[Vesicle (biology)|vesicle]] trafficking.<ref name="aacooper">{{cite journal | vauthors = Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S | title = Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models | journal = Science | volume = 313 | issue = 5785 | pages = 324–8 | date = Jul 2006 | pmid = 16794039 | pmc = 1983366 | doi = 10.1126/science.1129462 }}</ref> | |||
Apparently, alpha-synuclein is essential for normal development of the cognitive functions. Knock-out mice with the targeted inactivation of the expression of alpha-synuclein show impaired spatial learning and working memory.<ref name="pmid22469626">{{cite journal | vauthors = Kokhan VS, Afanasyeva MA, Van'kin GI | title = α-Synuclein knockout mice have cognitive impairments | journal = Behavioural Brain Research | volume = 231 | issue = 1 | pages = 226–30 | date = May 2012 | pmid = 22469626 | pmc = | doi = 10.1016/j.bbr.2012.03.026 }}</ref> | |||
=== Interaction with lipid membranes === | |||
Experimental evidence has been collected on the interaction of alpha-synuclein with [[cell membrane|membrane]] and its involvement with membrane composition and turnover. [[Saccharomyces cerevisiae|Yeast]] genome screening has found that several genes that deal with lipid metabolism play a role in alpha-synuclein toxicity.<ref name="Willingham_2003">{{cite journal | vauthors = Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ | title = Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein | journal = Science | volume = 302 | issue = 5651 | pages = 1769–72 | date = Dec 2003 | pmid = 14657499 | doi = 10.1126/science.1090389 }}</ref> Conversely, alpha-synuclein expression levels can affect the viscosity and the relative amount of fatty acids in the lipid bilayer.<ref name="Uversky_2007">{{cite journal | vauthors = Uversky VN | title = Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation | journal = Journal of Neurochemistry | volume = 103 | issue = 1 | pages = 17–37 | date = Oct 2007 | pmid = 17623039 | doi = 10.1111/j.1471-4159.2007.04764.x }}</ref> | |||
Alpha-synuclein is known to directly bind to lipid membranes, associating with the negatively charged surfaces of [[phospholipids]].<ref name="Uversky_2007"/> Alpha-synuclein forms an extended helical structure on small unilamellar vesicles.<ref name="Jao_2008">{{cite journal | vauthors = Jao CC, Hegde BG, Chen J, Haworth IS, Langen R | title = Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 105 | issue = 50 | pages = 19666–71 | date = Dec 2008 | pmid = 19066219 | pmc = 2605001 | doi = 10.1073/pnas.0807826105 }}</ref> A preferential binding to small vesicles has been found.<ref name="Zhu_2003">{{cite journal | vauthors = Zhu M, Li J, Fink AL | title = The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation | journal = The Journal of Biological Chemistry | volume = 278 | issue = 41 | pages = 40186–97 | date = Oct 2003 | pmid = 12885775 | doi = 10.1074/jbc.M305326200 }}</ref> The binding of alpha-synuclein to lipid membranes has complex effects on the latter, altering the bilayer structure and leading to the formation of small vesicles.<ref name="Madine_2006">{{cite journal | vauthors = Madine J, Doig AJ, Middleton DA | title = A study of the regional effects of alpha-synuclein on the organization and stability of phospholipid bilayers | journal = Biochemistry | volume = 45 | issue = 18 | pages = 5783–92 | date = May 2006 | pmid = 16669622 | doi = 10.1021/bi052151q }}</ref> Alpha-synuclein has been shown to bend membranes of negatively charged phospholipid vesicles and form tubules from large lipid vesicles.<ref name="pmid20693280">{{cite journal | vauthors = Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R | title = Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins | journal = The Journal of Biological Chemistry | volume = 285 | issue = 42 | pages = 32486–93 | date = Oct 2010 | pmid = 20693280 | pmc = 2952250 | doi = 10.1074/jbc.M110.139576 }}</ref> Using [[cryo-EM]] it was shown that these are micellar tubes of ~5-6 nm diameter.<ref>Remodeling of lipid vesicles into cylindrical micelles by α-synuclein in an extended α-helical conformation Naoko Mizuno, Jobin Varkey, Natalie C. Kegulian, Balachandra G. Hegde, Naiqian Cheng, Ralf Langen and Alasdair C. Steven</ref> Alpha-synuclein has also been shown to form lipid disc-like particles similar to [[apolipoproteins]] [http://www.jbc.org/content/288/24/17620.short].<ref>α-Synuclein Oligomers with Broken Helical Conformation Form Lipoprotein Nanoparticles. Jobin Varkey, Naoko Mizuno, Balachandra G. Hegde, Naiqian Cheng, Alasdair C. Steven and Ralf Langen</ref> Studies have also suggested a possible [[antioxidant]] activity of alpha-synuclein in the membrane.<ref name="Zhu_2006">{{cite journal | vauthors = Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL | title = Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles | journal = Biochemistry | volume = 45 | issue = 26 | pages = 8135–42 | date = Jul 2006 | pmid = 16800638 | doi = 10.1021/bi052584t }}</ref> | |||
[[File:Lewy bodies (alpha synuclein inclusions).svg|right|thumb|Photomicrographs of regions of substantia nigra in a patient showing Lewy bodies and Lewy neurites in various magnifications]] It has also been reported that aggregated states of alpha-synuclein permeate the membrane of lipid vesicles.<ref>P. Flagmeier et al., Ultrasensitive Measurement of Ca2+ Influx into Lipid Vesicles Induced by Protein Aggregates. Angew. Chemie Int. Ed. (2017), {{doi|10.1002/anie.201700966}}.</ref> | |||
== Sequence == | |||
Alpha-synuclein [[primary structure]] is usually divided in three distinct domains: | |||
* Residues 1-60: An [[amphiphile|amphipathic]] N-terminal region dominated by four 11-residue repeats including the [[consensus sequence]] KTKEGV. This sequence has a structural [[alpha helix]] propensity similar to apolipoproteins-binding domains<ref>{{cite journal |vauthors=Clayton DF, George JM | title = The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease | journal= Trends in Neurosciences | volume=21 | issue=6 | pages=249–254 | year=1998 | doi = 10.1016/S0166-2236(97)01213-7}}</ref> | |||
* Residues 61-95: A central hydrophobic region which includes the ''non-amyloid-β component'' (NAC) region, involved in protein aggregation<ref name="pmid8248242"/> | |||
* Residues 96-140: a highly acidic and [[proline]]-rich region which has no distinct structural propensity | |||
== Autoproteolytic activity == | |||
The use of high-resolution [[Ion-mobility spectrometry–mass spectrometry|ion-mobility mass spectrometry]] (IMS-MS) on HPLC-purified alpha-synuclein ''in vitro'' has shown alpha-synuclein to be autoproteolytic (self-[[Proteolysis|proteolytic]]), generating a variety of small [[molecular weight]] fragments upon incubation.<ref name="pmid22162214">{{cite journal | vauthors = Vlad C, Lindner K, Karreman C, Schildknecht S, Leist M, Tomczyk N, Rontree J, Langridge J, Danzer K, Ciossek T, Petre A, Gross ML, Hengerer B, Przybylski M | title = Autoproteolytic fragments are intermediates in the oligomerization/aggregation of the Parkinson's disease protein alpha-synuclein as revealed by ion mobility mass spectrometry | journal = Chembiochem | volume = 12 | issue = 18 | pages = 2740–4 | date = Dec 2011 | pmid = 22162214 | pmc = 3461308 | doi = 10.1002/cbic.201100569 }}</ref> The 14.46 [[Kilodalton|kDa]] protein was found to generate numerous smaller fragments, including 12.16 kDa ([[amino acids]] 14-133) and 10.44 kDa (40-140) fragments formed through [[C-terminus|C-]] and [[N-terminus|N-terminal]] truncation and a 7.27 kDa C-terminal fragment (72-140). The 7.27 kDa fragment, which contains the majority of the NAC region, aggregated considerably faster than full-length alpha-synuclein. It is possible that these autoproteolytic products play a role as intermediates or cofactors in the aggregation of alpha-synuclein ''in vivo''. | |||
== Clinical significance == | |||
[[File:Lewy Body alphaSynuclein.jpg|thumb|Positive α-Synuclein staining of a [[Lewy body]] from a patient who had Parkinson's disease.]] | |||
Classically considered an [[Intrinsically unstructured proteins|unstructured]] soluble protein, unmutated α-synuclein forms a stably folded [[tetramer]] that resists [[protein aggregation|aggregation]].<ref name="pmid21841800">{{cite journal | vauthors = Bartels T, Choi JG, Selkoe DJ | title = α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation | journal = Nature | volume = 477 | issue = 7362 | pages = 107–10 | date = August 2011 | pmid = 21841800 | pmc = 3166366 | doi = 10.1038/nature10324 | laysummary = http://www.focushms.com/features/new-clue-to-parkinsons/ | laysource = Harvard Medical School New Focus }}</ref> This observation, though reproduced and extended by several labs,<ref name="DettmerNewman2013">{{cite journal | vauthors = Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D | title = In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells | journal = The Journal of Biological Chemistry | volume = 288 | issue = 9 | pages = 6371–85 | date = March 2013 | pmid = 23319586 | pmc = 3585072 | doi = 10.1074/jbc.M112.403311 }}</ref><ref name="WestphalChandra2012">{{cite journal | vauthors = Westphal CH, Chandra SS | title = Monomeric synucleins generate membrane curvature | journal = The Journal of Biological Chemistry | volume = 288 | issue = 3 | pages = 1829–40 | date = January 2013 | pmid = 23184946 | pmc = 3548493 | doi = 10.1074/jbc.M112.418871 }}</ref><ref name="TrexlerRhoades2012">{{cite journal | vauthors = Trexler AJ, Rhoades E | title = N-Terminal acetylation is critical for forming α-helical oligomer of α-synuclein | journal = Protein Science | volume = 21 | issue = 5 | pages = 601–5 | date = May 2012 | pmid = 22407793 | doi = 10.1002/pro.2056 }}</ref> is still a matter of debate in the field due to conflicting reports.<ref name="FauvetMbefo2012">{{cite journal | vauthors = Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA | title = α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer | journal = The Journal of Biological Chemistry | volume = 287 | issue = 19 | pages = 15345–64 | date = May 2012 | pmid = 22315227 | pmc = 3346117 | doi = 10.1074/jbc.M111.318949 }}</ref><ref name="BurréVivona2013">{{cite journal | vauthors = Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC | title = Properties of native brain α-synuclein | journal = Nature | volume = 498 | issue = 7453 | pages = E4-6; discussion E6-7 | date = June 2013 | pmid = 23765500 | doi = 10.1038/nature12125 }}</ref><ref name="TheilletBinolfi2016">{{cite journal | vauthors = Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P | title = Structural disorder of monomeric α-synuclein persists in mammalian cells | journal = Nature | volume = 530 | issue = 7588 | pages = 45–50 | date = February 2016 | pmid = 26808899 | doi = 10.1038/nature16531 }}</ref> Nevertheless, alpha-synuclein aggregates to form insoluble fibrils in pathological conditions characterized by [[Lewy body|Lewy bodies]], such as [[Parkinson's disease]], [[dementia with Lewy bodies]] and [[Multiple System Atrophy|multiple system atrophy]].<ref name="pmid9278044">{{cite journal | vauthors = Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M | title = Alpha-synuclein in Lewy bodies | journal = Nature | volume = 388 | issue = 6645 | pages = 839–40 | date = August 1997 | pmid = 9278044 | pmc = | doi = 10.1038/42166 }}</ref><ref name="pmid9662355">{{cite journal | vauthors = Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH, Brownstein MJ | title = Alpha synuclein in neurodegenerative disorders: murderer or accomplice? | journal = Nature Medicine | volume = 4 | issue = 7 | pages = 755–7 | date = July 1998 | pmid = 9662355 | pmc = | doi = 10.1038/nm0798-755 }}</ref> These disorders are known as [[synucleinopathies]]. Alpha-synuclein is the primary structural component of Lewy body fibrils. Occasionally, Lewy bodies contain tau protein;<ref name="pmid 10528110">{{cite journal | vauthors = Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K, Ikeda K, Kawai M | title = Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies | journal = Brain Research | volume = 843 | issue = 1-2 | pages = 53–61 | date = October 1999 | pmid = 10528110 | doi = 10.1016/S0006-8993(99)01848-X }}</ref> however, alpha-synuclein and tau constitute two distinctive subsets of filaments in the same inclusion bodies.<ref name="pmid10963357">{{cite journal | vauthors = Arima K, Mizutani T, Alim MA, Tonozuka-Uehara H, Izumiyama Y, Hirai S, Uéda K | title = NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies | journal = Acta Neuropathologica | volume = 100 | issue = 2 | pages = 115–21 | date = August 2000 | pmid = 10963357 | doi = 10.1007/s004010050002 }}</ref> Alpha-synuclein pathology is also found in both sporadic and familial cases with Alzheimer's disease.<ref name="pmid12410385">{{cite journal | vauthors = Yokota O, Terada S, Ishizu H, Ujike H, Ishihara T, Nakashima H, Yasuda M, Kitamura Y, Uéda K, Checler F, Kuroda S | title = NACP/alpha-synuclein, NAC, and beta-amyloid pathology of familial Alzheimer's disease with the E184D presenilin-1 mutation: a clinicopathological study of two autopsy cases | journal = Acta Neuropathologica | volume = 104 | issue = 6 | pages = 637–48 | date = December 2002 | pmid = 12410385 | doi = 10.1007/s00401-002-0596-7 }}</ref> | |||
The aggregation mechanism of alpha-synuclein is uncertain. There is evidence of a structured intermediate rich in [[beta sheet|beta structure]] that can be the precursor of aggregation and, ultimately, Lewy bodies.<ref name="pmid17722123">{{cite journal | vauthors = Kim HY, Heise H, Fernandez CO, Baldus M, Zweckstetter M | title = Correlation of amyloid fibril beta-structure with the unfolded state of alpha-synuclein | journal = Chembiochem | volume = 8 | issue = 14 | pages = 1671–4 | date = September 2007 | pmid = 17722123 | doi = 10.1002/cbic.200700366 }}</ref> A single molecule study in 2008 suggests alpha-synuclein exists as a mix of unstructured, [[alpha-helix]], and [[beta-sheet]]-rich conformers in equilibrium. Mutations or buffer conditions known to improve aggregation strongly increase the population of the beta conformer, thus suggesting this could be a conformation related to pathogenic aggregation.<ref name="pmid18198943">{{cite journal | vauthors = Sandal M, Valle F, Tessari I, Mammi S, Bergantino E, Musiani F, Brucale M, Bubacco L, Samorì B | title = Conformational equilibria in monomeric alpha-synuclein at the single-molecule level | journal = PLoS Biology | volume = 6 | issue = 1 | pages = e6 | date = January 2008 | pmid = 18198943 | pmc = 2174973 | doi = 10.1371/journal.pbio.0060006 }}</ref> One theory is that the majority of alpha-synuclein aggregates are located in the presynapse as smaller deposits which causes synaptic dysfunction.<ref>{{cite journal | vauthors = Schulz-Schaeffer WJ | title = The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia | language = en | journal = Acta Neuropathologica | volume = 120 | issue = 2 | pages = 131–43 | date = August 2010 | pmid = 20563819 | doi = 10.1007/s00401-010-0711-0 | url = https://link.springer.com/article/10.1007/s00401-010-0711-0 }}</ref> Among the strategies for treating synucleinopathies are compounds that inhibit aggregation of alpha-synuclein. It has been shown that the small molecule [[cuminaldehyde]] inhibits fibrillation of alpha-synuclein.<ref>{{cite journal | vauthors = Morshedi D, Aliakbari F | title = The Inhibitory Effects of Cuminaldehyde on Amyloid Fibrillation and Cytotoxicity of Alpha-synuclein | journal = modares journal of medical sciences: pathobiology | volume = 15 | issue = 1 | pages = 45–60 |date=Spring 2012 | pmid = | doi = | url = | issn = }}</ref> | |||
The [[Epstein-Barr virus]] has been implicated in these disorders.<ref>{{cite journal | vauthors = Woulfe J, Hoogendoorn H, Tarnopolsky M, Muñoz DG | title = Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain | journal = Neurology | volume = 55 | issue = 9 | pages = 1398–401 | date = November 2000 | pmid = 11087792 | doi = 10.1212/WNL.55.9.1398 }}</ref> | |||
In rare cases of familial forms of [[Parkinson's disease]], there is a mutation in the [[gene]] coding for alpha-synuclein. Five [[point mutation]]s have been identified thus far: A53T,<ref name="pmid9197268">{{cite journal | vauthors = Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL | title = Mutation in the alpha-synuclein gene identified in families with Parkinson's disease | journal = Science | volume = 276 | issue = 5321 | pages = 2045–7 | date = June 1997 | pmid = 9197268 | pmc = | doi = 10.1126/science.276.5321.2045 }}</ref> A30P,<ref name="pmid9462735">{{cite journal | vauthors = Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O | title = Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease | journal = Nature Genetics | volume = 18 | issue = 2 | pages = 106–8 | date = February 1998 | pmid = 9462735 | pmc = | doi = 10.1038/ng0298-106 }}</ref> E46K,<ref name="pmid14755719">{{cite journal | vauthors = Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG | title = The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia | journal = Annals of Neurology | volume = 55 | issue = 2 | pages = 164–73 | date = February 2004 | pmid = 14755719 | doi = 10.1002/ana.10795 }}</ref> H50Q,<ref name="pmid23457019">{{cite journal | vauthors = Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ | title = Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease | journal = Movement Disorders | volume = 28 | issue = 6 | pages = 811–3 | date = June 2013 | pmid = 23457019 | doi = 10.1002/mds.25421 }}</ref> and G51D.<ref name="pmid23526723">{{cite journal | vauthors = Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, Pieri L, Madiona K, Dürr A, Melki R, Verny C, Brice A | title = G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome | journal = Annals of Neurology | volume = 73 | issue = 4 | pages = 459–71 | date = Apr 2013 | pmid = 23526723 | doi = 10.1002/ana.23894 }}</ref> It has been reported that some mutations influence the initiation and amplification steps of the aggregation process.<ref>{{cite journal | vauthors = Flagmeier P, Meisl G, Vendruscolo M, Knowles TP, Dobson CM, Buell AK, Galvagnion C | title = Mutations associated with familial Parkinson's disease alter the initiation and amplification steps of α-synuclein aggregation | language = en | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 113 | issue = 37 | pages = 10328–33 | date = September 2016 | pmid = 27573854 | pmc = 5027465 | doi = 10.1073/pnas.1604645113 }}</ref> Genomic duplication and triplication of the gene appear to be a rare cause of Parkinson's disease in other lineages, although more common than point mutations.<ref name="pmid14593171">{{cite journal | vauthors = Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K | title = alpha-Synuclein locus triplication causes Parkinson's disease | journal = Science | volume = 302 | issue = 5646 | pages = 841 | date = Oct 2003 | pmid = 14593171 | doi = 10.1126/science.1090278 }}</ref> Hence certain mutations of alpha-synuclein may cause it to form amyloid-like fibrils that contribute to Parkinson's disease. | |||
Certain sections of the alpha-synuclein protein may play a role in the [[tauopathies]].<ref name="pmid10663973">{{cite journal | vauthors = Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Masliah E | title = C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders | journal = Acta Neuropathologica | volume = 99 | issue = 3 | pages = 296–304 | date = Mar 2000 | pmid = 10663973 | doi = 10.1007/PL00007441 }}</ref> | |||
A [[prion]] form of the protein alpha-synuclein may be a causal agent for the disease [[multiple system atrophy]].<ref>{{cite journal | vauthors = Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K | title = Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism | journal = Proceedings of the National Academy of Sciences of the United States of America | date = Aug 2015 | pmid = 26324905 | doi = 10.1073/pnas.1514475112 | volume=112 | pages=E5308-17 | pmc=4586853}}</ref><ref>{{cite web | url = http://www.ucsf.edu/news/2015/08/131416/new-type-prion-may-cause-transmit-neurodegeneration | title = New Type of Prion May Cause, Transmit Neurodegeneration | first = Nicholas | last = Weiler | name-list-format = vanc | date = 31 August 2015 }}</ref><ref>{{cite journal | url = http://www.livescience.com/52040-prions-multiple-system-atrophy.html | title = Another Fatal Brain Disease May Come from the Spread of 'Prion' Proteins | work = Wired Science | first = Rachael | last = Rettner | name-list-format = vanc | date = 31 August 2015 }}</ref> | |||
== | [[File:Events in alpha synuclein toxicity.jpg|thumb|Events in α-synuclein toxicity.<ref name="pmid19193223">{{cite journal | vauthors = Cookson MR | title = alpha-Synuclein and neuronal cell death | journal = Mol Neurodegener | volume = 4 | issue = 1| pages = 9 | year = 2009 | pmid = 19193223 | pmc = 2646729 | doi = 10.1186/1750-1326-4-9 | url = | issn = }}</ref>]] | ||
{{ | {{Refimprove section|date=November 2015}} | ||
[[Antibodies]] against alpha-synuclein have replaced antibodies against [[ubiquitin]] as the gold standard for [[immunostaining]] of Lewy bodies.<ref>{{cite journal | vauthors = Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T | title = alpha-Synuclein is phosphorylated in synucleinopathy lesions | journal = Nature Cell Biology | volume = 4 | issue = 2 | pages = 160–4 | date = February 2002 | pmid = 11813001 | doi = 10.1038/ncb748 }}</ref> The central panel in the figure to the right shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons. | |||
== Protein-protein interactions == | |||
* | Alpha-synuclein has been shown to [[Protein-protein interaction|interact]] with | ||
* [[Dopamine transporter]],<ref name="pmid12672538">{{cite journal | vauthors = Wersinger C, Sidhu A | title = Attenuation of dopamine transporter activity by alpha-synuclein | journal = Neuroscience Letters | volume = 340 | issue = 3 | pages = 189–92 | date = Apr 2003 | pmid = 12672538 | doi = 10.1016/S0304-3940(03)00097-1 }}</ref><ref name="pmid11292651">{{cite journal | vauthors = Lee FJ, Liu F, Pristupa ZB, Niznik HB | title = Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis | journal = FASEB Journal | volume = 15 | issue = 6 | pages = 916–26 | date = Apr 2001 | pmid = 11292651 | doi = 10.1096/fj.00-0334com }}</ref> | |||
* [[Parkin (ligase)]],<ref name="pmid11588587">{{cite journal | vauthors = Choi P, Golts N, Snyder H, Chong M, Petrucelli L, Hardy J, Sparkman D, Cochran E, Lee JM, Wolozin B | author10-link=Benjamin Wolozin| title = Co-association of parkin and alpha-synuclein | journal = NeuroReport | volume = 12 | issue = 13 | pages = 2839–43 | date = Sep 2001 | pmid = 11588587 | doi = 10.1097/00001756-200109170-00017 }}</ref><ref name="pmid18195004">{{cite journal | vauthors = Kawahara K, Hashimoto M, Bar-On P, Ho GJ, Crews L, Mizuno H, Rockenstein E, Imam SZ, Masliah E | title = alpha-Synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease | journal = The Journal of Biological Chemistry | volume = 283 | issue = 11 | pages = 6979–87 | date = Mar 2008 | pmid = 18195004 | doi = 10.1074/jbc.M710418200 }}</ref> | |||
* [[Phospholipase D1]],<ref name="pmid11821392">{{cite journal | vauthors = Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, Chung KC, Yoon SH, Hahn SJ, Kim MS, Jo YH, Min DS | title = alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells | journal = The Journal of Biological Chemistry | volume = 277 | issue = 14 | pages = 12334–42 | date = Apr 2002 | pmid = 11821392 | doi = 10.1074/jbc.M110414200 }}</ref> | |||
* [[SNCAIP]],<ref name="pmid12044636">{{cite journal | vauthors = Neystat M, Rzhetskaya M, Kholodilov N, Burke RE | title = Analysis of synphilin-1 and synuclein interactions by yeast two-hybrid beta-galactosidase liquid assay | journal = Neuroscience Letters | volume = 325 | issue = 2 | pages = 119–23 | date = Jun 2002 | pmid = 12044636 | doi = 10.1016/S0304-3940(02)00253-7 }}</ref><ref name="pmid1742726">{{cite journal | vauthors = Reed JC, Meister L, Tanaka S, Cuddy M, Yum S, Geyer C, Pleasure D | title = Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin | journal = Cancer Research | volume = 51 | issue = 24 | pages = 6529–38 | date = Dec 1991 | pmid = 1742726 | doi = }}</ref><ref name="pmid11331421">{{cite journal | vauthors = Kawamata H, McLean PJ, Sharma N, Hyman BT | title = Interaction of alpha-synuclein and synphilin-1: effect of Parkinson's disease-associated mutations | journal = Journal of Neurochemistry | volume = 77 | issue = 3 | pages = 929–34 | date = May 2001 | pmid = 11331421 | doi = 10.1046/j.1471-4159.2001.00301.x }}</ref><ref name="pmid10319874">{{cite journal | vauthors = Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA | title = Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions | journal = Nature Genetics | volume = 22 | issue = 1 | pages = 110–4 | date = May 1999 | pmid = 10319874 | doi = 10.1038/8820 }}</ref> | |||
* [[Tau protein]].<ref name="pmid10464279">{{cite journal | vauthors = Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R | title = alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356 | journal = The Journal of Biological Chemistry | volume = 274 | issue = 36 | pages = 25481–9 | date = Sep 1999 | pmid = 10464279 | doi = 10.1074/jbc.274.36.25481 }}</ref><ref>{{cite journal | vauthors = Giasson BI, Lee VM, Trojanowski JQ | title = Interactions of amyloidogenic proteins | journal = Neuromolecular Medicine | volume = 4 | issue = 1–2 | pages = 49–58 | year = 2003 | pmid = 14528052 | doi = 10.1385/NMM:4:1-2:49 }}</ref> | |||
* [[Beta amyloid]]<ref name=pmid22734715>{{cite journal | vauthors = Ono K, Takahashi R, Ikeda T, Yamada M | title = Cross-seeding effects of amyloid β-protein and α-synuclein | journal = Journal of Neurochemistry | volume = 122 | issue = 5 | pages = 883–90 | date = Sep 2012 | pmid = 22734715 | doi = 10.1111/j.1471-4159.2012.07847.x | url = https://www.ncbi.nlm.nih.gov/pubmed/?term=Cross-seeding+effects+of+amyloid+β-protein+and+α-synuclein }}</ref> | |||
== See also == | |||
* [[Synuclein]] | |||
* [[Contursi Terme]] - the village in Italy where a mutation in the α-synuclein gene led to a family history of Parkinson's disease | |||
* | |||
* | |||
== References == | |||
{{Reflist|33em}} | |||
==External links== | == Further reading == | ||
{{Refbegin|33em}} | |||
* {{cite web | url = https://www.nytimes.com/2002/05/21/health/in-folding-proteins-clues-to-many-diseases.html | title = In Folding Proteins, Clues to Many Diseases - | author = Blakeslee S | authorlink = | date = 2002-05-27 | work = | publisher = New York Times | pages = | archiveurl = | archivedate = | quote = | accessdate = }} | |||
* {{cite journal | vauthors = Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL | title = Mutation in the alpha-synuclein gene identified in families with Parkinson's disease | journal = Science | volume = 276 | issue = 5321 | pages = 2045–7 | date = Jun 1997 | pmid = 9197268 | doi = 10.1126/science.276.5321.2045 }} | |||
* {{cite journal | vauthors = Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Müller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C | title = Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies | journal = The Journal of Clinical Investigation | volume = 110 | issue = 10 | pages = 1429–39 | date = Nov 2002 | pmid = 12438441 | pmc = 151810 | doi = 10.1172/JCI15777 }} | |||
* {{cite journal | vauthors = George JM | title = The synucleins | journal = Genome Biology | volume = 3 | issue = 1 | pages = REVIEWS3002 | year = 2001 | pmid = 11806835 | pmc = 150459 | doi = 10.1186/gb-2001-3-1-reviews3002 }} | |||
* {{cite journal | vauthors = Lavedan C | title = The synuclein family | journal = Genome Research | volume = 8 | issue = 9 | pages = 871–80 | date = Sep 1998 | pmid = 9750188 | doi = 10.1101/gr.8.9.871 }} | |||
* {{cite journal | vauthors = Ozawa T, Wakabayashi K, Oyanagi K | title = [Recent progress in the research of multiple system atrophy with special references to alpha-synuclein and suprachiasmatic nucleus] | journal = Nō to Shinkei = Brain and Nerve | volume = 54 | issue = 2 | pages = 111–7 | date = Feb 2002 | pmid = 11889756 | doi = }} | |||
* {{cite journal | vauthors = Cole NB, Murphy DD | title = The cell biology of alpha-synuclein: a sticky problem? | journal = Neuromolecular Medicine | volume = 1 | issue = 2 | pages = 95–109 | year = 2002 | pmid = 12025860 | doi = 10.1385/NMM:1:2:95 }} | |||
* {{cite journal | vauthors = Iwatsubo T | title = [alpha-synuclein and Parkinson's disease] | journal = Seikagaku. The Journal of Japanese Biochemical Society | volume = 74 | issue = 6 | pages = 477–82 | date = Jun 2002 | pmid = 12138709 | doi = }} | |||
* {{cite journal | vauthors = Trojanowski JQ, Lee VM | title = Parkinson's disease and related synucleinopathies are a new class of nervous system amyloidoses | journal = Neurotoxicology | volume = 23 | issue = 4–5 | pages = 457–60 | date = Oct 2002 | pmid = 12428717 | doi = 10.1016/S0161-813X(02)00065-7 }} | |||
* {{cite journal | vauthors = Alves da Costa C | title = Recent advances on alpha-synuclein cell biology: functions and dysfunctions | journal = Current Molecular Medicine | volume = 3 | issue = 1 | pages = 17–24 | date = Feb 2003 | pmid = 12558071 | doi = 10.2174/1566524033361690 }} | |||
* {{cite journal | vauthors = Ma QL, Chan P, Yoshii M, Uéda K | title = Alpha-synuclein aggregation and neurodegenerative diseases | journal = Journal of Alzheimer's Disease | volume = 5 | issue = 2 | pages = 139–48 | date = Apr 2003 | pmid = 12719631 | doi = }} | |||
* {{cite journal | vauthors = Di Rosa G, Puzzo D, Sant'Angelo A, Trinchese F, Arancio O | title = Alpha-synuclein: between synaptic function and dysfunction | journal = Histology and Histopathology | volume = 18 | issue = 4 | pages = 1257–66 | date = Oct 2003 | pmid = 12973692 | doi = }} | |||
* {{cite journal | vauthors = Baptista MJ, Cookson MR, Miller DW | title = Parkin and alpha-synuclein: opponent actions in the pathogenesis of Parkinson's disease | journal = The Neuroscientist | volume = 10 | issue = 1 | pages = 63–72 | date = Feb 2004 | pmid = 14987449 | doi = 10.1177/1073858403260392 }} | |||
* {{cite journal | vauthors = Kim S, Seo JH, Suh YH | title = Alpha-synuclein, Parkinson's disease, and Alzheimer's disease | journal = Parkinsonism & Related Disorders | volume = 10 Suppl 1 | issue = | pages = S9-13 | date = May 2004 | pmid = 15109581 | doi = 10.1016/j.parkreldis.2003.11.005 }} | |||
* {{cite journal | vauthors = Sidhu A, Wersinger C, Vernier P | title = alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease | journal = FEBS Letters | volume = 565 | issue = 1–3 | pages = 1–5 | date = May 2004 | pmid = 15135042 | doi = 10.1016/j.febslet.2004.03.063 }} | |||
* {{cite journal | vauthors = Vekrellis K, Rideout HJ, Stefanis L | title = Neurobiology of alpha-synuclein | journal = Molecular Neurobiology | volume = 30 | issue = 1 | pages = 1–21 | date = Aug 2004 | pmid = 15247485 | doi = 10.1385/MN:30:1:001 }} | |||
* {{cite journal | vauthors = Chiba-Falek O, Nussbaum RL | title = Regulation of alpha-synuclein expression: implications for Parkinson's disease | journal = Cold Spring Harbor Symposia on Quantitative Biology | volume = 68 | issue = | pages = 409–15 | year = 2004 | pmid = 15338643 | doi = 10.1101/sqb.2003.68.409 }} | |||
* {{cite journal | vauthors = Pankratz N, Foroud T | title = Genetics of Parkinson disease | journal = NeuroRx | volume = 1 | issue = 2 | pages = 235–42 | date = Apr 2004 | pmid = 15717024 | pmc = 534935 | doi = 10.1602/neurorx.1.2.235 }} | |||
* {{cite journal | vauthors = Singleton AB | title = Altered alpha-synuclein homeostasis causing Parkinson's disease: the potential roles of dardarin | journal = Trends in Neurosciences | volume = 28 | issue = 8 | pages = 416–21 | date = Aug 2005 | pmid = 15955578 | doi = 10.1016/j.tins.2005.05.009 }} | |||
* {{cite journal | vauthors = Yu S, Uéda K, Chan P | title = Alpha-synuclein and dopamine metabolism | journal = Molecular Neurobiology | volume = 31 | issue = 1–3 | pages = 243–54 | year = 2005 | pmid = 15953825 | doi = 10.1385/MN:31:1-3:243 }} | |||
* {{cite journal | vauthors = Lee HG, Zhu X, Takeda A, Perry G, Smith MA | title = Emerging evidence for the neuroprotective role of alpha-synuclein | journal = Experimental Neurology | volume = 200 | issue = 1 | pages = 1–7 | date = Jul 2006 | pmid = 16780837 | doi = 10.1016/j.expneurol.2006.04.024 }} | |||
* {{cite journal | vauthors = Giorgi FS, Bandettini di Poggio A, Battaglia G, Pellegrini A, Murri L, Ruggieri S, Paparelli A, Fornai F | title = A short overview on the role of alpha-synuclein and proteasome in experimental models of Parkinson's disease | journal = Journal of Neural Transmission. Supplementum | volume = 70 | issue = 70 | pages = 105–9 | year = 2006 | pmid = 17017516 | doi = 10.1007/978-3-211-45295-0_17 }} | |||
{{Refend}} | |||
== External links == | |||
* {{commons-inline|Category:Alpha-synuclein|alpha synuclein}} | |||
* {{MeshName|alpha-Synuclein}} | * {{MeshName|alpha-Synuclein}} | ||
* {{UCSC gene info|SNCA}} | |||
{{PDB Gallery|geneid=6622}} | |||
{{Nerve tissue protein}} | |||
[[Category:Peripheral membrane proteins]] | [[Category:Peripheral membrane proteins]] | ||
Revision as of 18:07, 2 November 2017
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Alpha-synuclein is a protein that is abundant in the human brain.[1] Smaller amounts are found in the heart, muscles, and other tissues.[1] In the brain, alpha-synuclein is found mainly at the tips of nerve cells (neurons) in specialized structures called presynaptic terminals.[1] Within these structures, alpha-synuclein interacts with phospholipids[2] and proteins.[1] Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function.[1]
Although the function of alpha-synuclein is not well understood, studies suggest that it plays a role in maintaining a supply of synaptic vesicles in presynaptic terminals by clustering synaptic vesicles.[3] It may also help regulate the release of dopamine, a type of neurotransmitter that is critical for controlling the start and stop of voluntary and involuntary movements.[1]
The human alpha-synuclein protein is made of 140 amino acids and is encoded by the SNCA gene.[4][5][6] An alpha-synuclein fragment, known as the non-Abeta component (NAC) of Alzheimer's disease amyloid, originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP.[4] It was later determined that NACP was the human homologue of Torpedo synuclein. Therefore, NACP is now referred to as human alpha-synuclein.
Tissue expression
Alpha-synuclein is a synuclein protein of unknown function primarily found in neural tissue, making up as much as 1% of all proteins in the cytosol of brain cells.[7] It is predominantly expressed in the neocortex, hippocampus, substantia nigra, thalamus, and cerebellum. It is predominantly a neuronal protein, but can also be found in the neuroglial cells.[citation needed] In melanocytic cells, SNCA protein expression may be regulated by MITF.[8]
It has been established that alpha-synuclein is extensively localized in the nucleus of mammalian brain neurons, suggesting a role of alpha-synuclein in the nucleus.[9] Synuclein is however found predominantly in the presynaptic termini, in both free or membrane-bound forms,[10] with roughly 15% of synuclein being membrane-bound in any moment in neurons.[11]
It has also been shown that alpha-synuclein is localized in neuronal mitochondria.[12][13] Alpha-synuclein is highly expressed in the mitochondria in olfactory bulb, hippocampus, striatum and thalamus, where the cytosolic alpha-synuclein is also rich. However, the cerebral cortex and cerebellum are two exceptions, which contain rich cytosolic alpha-synuclein but very low levels of mitochondrial alpha-synuclein. It has been shown that alpha-synuclein is localized in the inner membrane of mitochondria, and that the inhibitory effect of alpha-synuclein on complex I activity of mitochondrial respiratory chain is dose-dependent. Thus, it is suggested that alpha-synuclein in mitochondria is differentially expressed in different brain regions and the background levels of mitochondrial alpha-synuclein may be a potential factor affecting mitochondrial function and predisposing some neurons to degeneration.[13]
At least three isoforms of synuclein are produced through alternative splicing.[14] The majority form of the protein, and the one most investigated, is the full-length protein of 140 amino acids. Other isoforms are alpha-synuclein-126, which lacks residues 41-54 due to loss of exon 3; and alpha-synuclein-112,[15] which lacks residue 103-130 due to loss of exon 5.[14]
Structure
Alpha-synuclein in solution is considered to be an intrinsically disordered protein, i.e. it lacks a single stable 3D structure.[16] As of 2014, an increasing number of reports suggest, however, the presence of partial structures or mostly structured oligomeric states in the solution structure of alpha-synuclein even in the absence of lipids. This trend is also supported by a large number of single molecule (optical tweezers) measurements on single copies of monomeric alpha-synuclein as well as covalently enforced dimers or tetramers of alpha-synuclein.[17]
Function
Alpha-synuclein is specifically upregulated in a discrete population of presynaptic terminals of the brain during a period of acquisition-related synaptic rearrangement.[18] It has been shown that alpha-synuclein significantly interacts with tubulin,[19] and that alpha-synuclein may have activity as a potential microtubule-associated protein, like tau.[20]
Recent evidence suggests that alpha-synuclein functions as a molecular chaperone in the formation of SNARE complexes.[21][22] In particular, it simultaneously binds to phospholipids of the plasma membrane via its N-terminus domain and to synaptobrevin-2 via its C-terminus domain, with increased importance during synaptic activity.[23] Indeed, there is growing evidence that alpha-synuclein is involved in the functioning of the neuronal Golgi apparatus and vesicle trafficking.[24]
Apparently, alpha-synuclein is essential for normal development of the cognitive functions. Knock-out mice with the targeted inactivation of the expression of alpha-synuclein show impaired spatial learning and working memory.[25]
Interaction with lipid membranes
Experimental evidence has been collected on the interaction of alpha-synuclein with membrane and its involvement with membrane composition and turnover. Yeast genome screening has found that several genes that deal with lipid metabolism play a role in alpha-synuclein toxicity.[26] Conversely, alpha-synuclein expression levels can affect the viscosity and the relative amount of fatty acids in the lipid bilayer.[27]
Alpha-synuclein is known to directly bind to lipid membranes, associating with the negatively charged surfaces of phospholipids.[27] Alpha-synuclein forms an extended helical structure on small unilamellar vesicles.[28] A preferential binding to small vesicles has been found.[29] The binding of alpha-synuclein to lipid membranes has complex effects on the latter, altering the bilayer structure and leading to the formation of small vesicles.[30] Alpha-synuclein has been shown to bend membranes of negatively charged phospholipid vesicles and form tubules from large lipid vesicles.[31] Using cryo-EM it was shown that these are micellar tubes of ~5-6 nm diameter.[32] Alpha-synuclein has also been shown to form lipid disc-like particles similar to apolipoproteins [2].[33] Studies have also suggested a possible antioxidant activity of alpha-synuclein in the membrane.[34]
It has also been reported that aggregated states of alpha-synuclein permeate the membrane of lipid vesicles.[35]
Sequence
Alpha-synuclein primary structure is usually divided in three distinct domains:
- Residues 1-60: An amphipathic N-terminal region dominated by four 11-residue repeats including the consensus sequence KTKEGV. This sequence has a structural alpha helix propensity similar to apolipoproteins-binding domains[36]
- Residues 61-95: A central hydrophobic region which includes the non-amyloid-β component (NAC) region, involved in protein aggregation[4]
- Residues 96-140: a highly acidic and proline-rich region which has no distinct structural propensity
Autoproteolytic activity
The use of high-resolution ion-mobility mass spectrometry (IMS-MS) on HPLC-purified alpha-synuclein in vitro has shown alpha-synuclein to be autoproteolytic (self-proteolytic), generating a variety of small molecular weight fragments upon incubation.[37] The 14.46 kDa protein was found to generate numerous smaller fragments, including 12.16 kDa (amino acids 14-133) and 10.44 kDa (40-140) fragments formed through C- and N-terminal truncation and a 7.27 kDa C-terminal fragment (72-140). The 7.27 kDa fragment, which contains the majority of the NAC region, aggregated considerably faster than full-length alpha-synuclein. It is possible that these autoproteolytic products play a role as intermediates or cofactors in the aggregation of alpha-synuclein in vivo.
Clinical significance
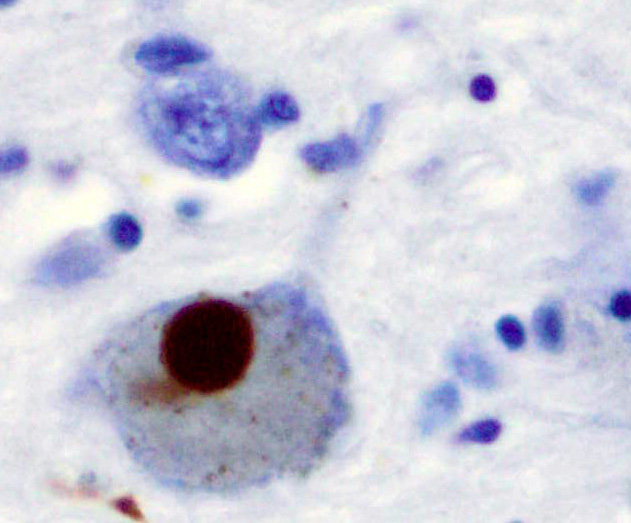
Classically considered an unstructured soluble protein, unmutated α-synuclein forms a stably folded tetramer that resists aggregation.[38] This observation, though reproduced and extended by several labs,[39][40][41] is still a matter of debate in the field due to conflicting reports.[42][43][44] Nevertheless, alpha-synuclein aggregates to form insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson's disease, dementia with Lewy bodies and multiple system atrophy.[45][46] These disorders are known as synucleinopathies. Alpha-synuclein is the primary structural component of Lewy body fibrils. Occasionally, Lewy bodies contain tau protein;[47] however, alpha-synuclein and tau constitute two distinctive subsets of filaments in the same inclusion bodies.[48] Alpha-synuclein pathology is also found in both sporadic and familial cases with Alzheimer's disease.[49]
The aggregation mechanism of alpha-synuclein is uncertain. There is evidence of a structured intermediate rich in beta structure that can be the precursor of aggregation and, ultimately, Lewy bodies.[50] A single molecule study in 2008 suggests alpha-synuclein exists as a mix of unstructured, alpha-helix, and beta-sheet-rich conformers in equilibrium. Mutations or buffer conditions known to improve aggregation strongly increase the population of the beta conformer, thus suggesting this could be a conformation related to pathogenic aggregation.[51] One theory is that the majority of alpha-synuclein aggregates are located in the presynapse as smaller deposits which causes synaptic dysfunction.[52] Among the strategies for treating synucleinopathies are compounds that inhibit aggregation of alpha-synuclein. It has been shown that the small molecule cuminaldehyde inhibits fibrillation of alpha-synuclein.[53] The Epstein-Barr virus has been implicated in these disorders.[54]
In rare cases of familial forms of Parkinson's disease, there is a mutation in the gene coding for alpha-synuclein. Five point mutations have been identified thus far: A53T,[55] A30P,[56] E46K,[57] H50Q,[58] and G51D.[59] It has been reported that some mutations influence the initiation and amplification steps of the aggregation process.[60] Genomic duplication and triplication of the gene appear to be a rare cause of Parkinson's disease in other lineages, although more common than point mutations.[61] Hence certain mutations of alpha-synuclein may cause it to form amyloid-like fibrils that contribute to Parkinson's disease.
Certain sections of the alpha-synuclein protein may play a role in the tauopathies.[62]
A prion form of the protein alpha-synuclein may be a causal agent for the disease multiple system atrophy.[63][64][65]
This section needs additional citations for verification. (November 2015) (Learn how and when to remove this template message) |
Antibodies against alpha-synuclein have replaced antibodies against ubiquitin as the gold standard for immunostaining of Lewy bodies.[67] The central panel in the figure to the right shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons.
Protein-protein interactions
Alpha-synuclein has been shown to interact with
- Dopamine transporter,[68][69]
- Parkin (ligase),[70][71]
- Phospholipase D1,[72]
- SNCAIP,[73][74][75][76]
- Tau protein.[77][78]
- Beta amyloid[79]
See also
- Synuclein
- Contursi Terme - the village in Italy where a mutation in the α-synuclein gene led to a family history of Parkinson's disease
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Genetics Home Reference: SNCA". U.S. National Library of Medicine. 12 Nov 2013. Retrieved 14 Nov 2013.
- ↑ Chandra S, Chen X, Rizo J, Jahn R, Südhof TC (Apr 2003). "A broken alpha -helix in folded alpha -Synuclein". The Journal of Biological Chemistry. 278 (17): 15313–8. doi:10.1074/jbc.M213128200. PMID 12586824.
- ↑ Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Südhof TC, Brunger AT (2013). "Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2". eLife. 2: e00592. doi:10.7554/eLife.00592. PMC 3639508. PMID 23638301.
- ↑ 4.0 4.1 4.2 Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T (Dec 1993). "Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease". Proceedings of the National Academy of Sciences of the United States of America. 90 (23): 11282–6. doi:10.1073/pnas.90.23.11282. PMC 47966. PMID 8248242.
- ↑ Xia Y, Saitoh T, Uéda K, Tanaka S, Chen X, Hashimoto M, Hsu L, Conrad C, Sundsmo M, Yoshimoto M, Thal L, Katzman R, Masliah E (Oct 2001). "Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms". Journal of Alzheimer's Disease. 3 (5): 485–494. PMID 12214035.
- ↑ Xia Y, Saitoh T, Uéda K, Tanaka S, Chen X, Hashimoto M, Hsu L, Conrad C, Sundsmo M, Yoshimoto M, Thal L, Katzman R, Masliah E (2002). "Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms: Erratum p489 Fig 3". J. Alzheimers Dis. 4 (4): 337.
- ↑ Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T (Feb 1995). "The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system". Neuron. 14 (2): 467–75. doi:10.1016/0896-6273(95)90302-X. PMID 7857654.
- ↑ Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, Valgeirsdottir S, Bergsteinsdottir K, Schepsky A, Dummer R, Steingrimsson E (Dec 2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell & Melanoma Research. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ↑ Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Uéda K, Chan P (Mar 2007). "Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody". Neuroscience. 145 (2): 539–55. doi:10.1016/j.neuroscience.2006.12.028. PMID 17275196.
- ↑ McLean PJ, Kawamata H, Ribich S, Hyman BT (Mar 2000). "Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations". The Journal of Biological Chemistry. 275 (12): 8812–6. doi:10.1074/jbc.275.12.8812. PMID 10722726.
- ↑ Lee HJ, Choi C, Lee SJ (Jan 2002). "Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form". The Journal of Biological Chemistry. 277 (1): 671–8. doi:10.1074/jbc.M107045200. PMID 11679584.
- ↑ Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Uéda K, Yu S, Yang H (Dec 2008). "Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody". Brain Research. 1244: 40–52. doi:10.1016/j.brainres.2008.08.067. PMID 18817762.
- ↑ 13.0 13.1 Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K, Chan P, Yu S (May 2009). "alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity". Neuroscience Letters. 454 (3): 187–92. doi:10.1016/j.neulet.2009.02.056. PMID 19429081.
- ↑ 14.0 14.1 Beyer K (Sep 2006). "Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers". Acta Neuropathologica. 112 (3): 237–51. doi:10.1007/s00401-006-0104-6. PMID 16845533.
- ↑ Uéda K, Saitoh T, Mori H (Dec 1994). "Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid". Biochemical and Biophysical Research Communications. 205 (2): 1366–72. doi:10.1006/bbrc.1994.2816. PMID 7802671.
- ↑ van Rooijen BD, van Leijenhorst-Groener KA, Claessens MM, Subramaniam V (Dec 2009). "Tryptophan fluorescence reveals structural features of alpha-synuclein oligomers". Journal of Molecular Biology. 394 (5): 826–33. doi:10.1016/j.jmb.2009.10.021. PMID 19837084.
- ↑ Neupane K, Solanki A, Sosova I, Belov M, Woodside MT (January 2014). "Diverse metastable structures formed by small oligomers of α-synuclein probed by force spectroscopy". PLOS ONE. 9 (1): e86495. doi:10.1371/journal.pone.0086495. PMC 3901707. PMID 24475132.
- ↑ George JM, Jin H, Woods WS, Clayton DF (Aug 1995). "Characterization of a novel protein regulated during the critical period for song learning in the zebra finch". Neuron. 15 (2): 361–72. doi:10.1016/0896-6273(95)90040-3. PMID 7646890.
- ↑ Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K (Jan 2002). "Tubulin seeds alpha-synuclein fibril formation". The Journal of Biological Chemistry. 277 (3): 2112–7. doi:10.1074/jbc.M102981200. PMID 11698390.
- ↑ Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T, Kaji H, Yoshii M, Hisanaga S, Uéda K (Aug 2004). "Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein". Journal of Alzheimer's Disease. 6 (4): 435–42, discussion 443–9. PMID 15345814.
- ↑ Bonini NM, Giasson BI (Nov 2005). "Snaring the function of alpha-synuclein". Cell. 123 (3): 359–61. doi:10.1016/j.cell.2005.10.017. PMID 16269324.
- ↑ Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC (Nov 2005). "Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration". Cell. 123 (3): 383–96. doi:10.1016/j.cell.2005.09.028. PMID 16269331.
- ↑ Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC (Sep 2010). "Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro". Science. 329 (5999): 1663–7. doi:10.1126/science.1195227. PMC 3235365. PMID 20798282.
- ↑ Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S (Jul 2006). "Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models". Science. 313 (5785): 324–8. doi:10.1126/science.1129462. PMC 1983366. PMID 16794039.
- ↑ Kokhan VS, Afanasyeva MA, Van'kin GI (May 2012). "α-Synuclein knockout mice have cognitive impairments". Behavioural Brain Research. 231 (1): 226–30. doi:10.1016/j.bbr.2012.03.026. PMID 22469626.
- ↑ Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ (Dec 2003). "Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein". Science. 302 (5651): 1769–72. doi:10.1126/science.1090389. PMID 14657499.
- ↑ 27.0 27.1 Uversky VN (Oct 2007). "Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation". Journal of Neurochemistry. 103 (1): 17–37. doi:10.1111/j.1471-4159.2007.04764.x. PMID 17623039.
- ↑ Jao CC, Hegde BG, Chen J, Haworth IS, Langen R (Dec 2008). "Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement". Proceedings of the National Academy of Sciences of the United States of America. 105 (50): 19666–71. doi:10.1073/pnas.0807826105. PMC 2605001. PMID 19066219.
- ↑ Zhu M, Li J, Fink AL (Oct 2003). "The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation". The Journal of Biological Chemistry. 278 (41): 40186–97. doi:10.1074/jbc.M305326200. PMID 12885775.
- ↑ Madine J, Doig AJ, Middleton DA (May 2006). "A study of the regional effects of alpha-synuclein on the organization and stability of phospholipid bilayers". Biochemistry. 45 (18): 5783–92. doi:10.1021/bi052151q. PMID 16669622.
- ↑ Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R (Oct 2010). "Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins". The Journal of Biological Chemistry. 285 (42): 32486–93. doi:10.1074/jbc.M110.139576. PMC 2952250. PMID 20693280.
- ↑ Remodeling of lipid vesicles into cylindrical micelles by α-synuclein in an extended α-helical conformation Naoko Mizuno, Jobin Varkey, Natalie C. Kegulian, Balachandra G. Hegde, Naiqian Cheng, Ralf Langen and Alasdair C. Steven
- ↑ α-Synuclein Oligomers with Broken Helical Conformation Form Lipoprotein Nanoparticles. Jobin Varkey, Naoko Mizuno, Balachandra G. Hegde, Naiqian Cheng, Alasdair C. Steven and Ralf Langen
- ↑ Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL (Jul 2006). "Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles". Biochemistry. 45 (26): 8135–42. doi:10.1021/bi052584t. PMID 16800638.
- ↑ P. Flagmeier et al., Ultrasensitive Measurement of Ca2+ Influx into Lipid Vesicles Induced by Protein Aggregates. Angew. Chemie Int. Ed. (2017), doi:10.1002/anie.201700966.
- ↑ Clayton DF, George JM (1998). "The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease". Trends in Neurosciences. 21 (6): 249–254. doi:10.1016/S0166-2236(97)01213-7.
- ↑ Vlad C, Lindner K, Karreman C, Schildknecht S, Leist M, Tomczyk N, Rontree J, Langridge J, Danzer K, Ciossek T, Petre A, Gross ML, Hengerer B, Przybylski M (Dec 2011). "Autoproteolytic fragments are intermediates in the oligomerization/aggregation of the Parkinson's disease protein alpha-synuclein as revealed by ion mobility mass spectrometry". Chembiochem. 12 (18): 2740–4. doi:10.1002/cbic.201100569. PMC 3461308. PMID 22162214.
- ↑ Bartels T, Choi JG, Selkoe DJ (August 2011). "α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation". Nature. 477 (7362): 107–10. doi:10.1038/nature10324. PMC 3166366. PMID 21841800. Lay summary – Harvard Medical School New Focus.
- ↑ Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D (March 2013). "In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells". The Journal of Biological Chemistry. 288 (9): 6371–85. doi:10.1074/jbc.M112.403311. PMC 3585072. PMID 23319586.
- ↑ Westphal CH, Chandra SS (January 2013). "Monomeric synucleins generate membrane curvature". The Journal of Biological Chemistry. 288 (3): 1829–40. doi:10.1074/jbc.M112.418871. PMC 3548493. PMID 23184946.
- ↑ Trexler AJ, Rhoades E (May 2012). "N-Terminal acetylation is critical for forming α-helical oligomer of α-synuclein". Protein Science. 21 (5): 601–5. doi:10.1002/pro.2056. PMID 22407793.
- ↑ Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA (May 2012). "α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer". The Journal of Biological Chemistry. 287 (19): 15345–64. doi:10.1074/jbc.M111.318949. PMC 3346117. PMID 22315227.
- ↑ Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC (June 2013). "Properties of native brain α-synuclein". Nature. 498 (7453): E4–6, discussion E6-7. doi:10.1038/nature12125. PMID 23765500.
- ↑ Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P (February 2016). "Structural disorder of monomeric α-synuclein persists in mammalian cells". Nature. 530 (7588): 45–50. doi:10.1038/nature16531. PMID 26808899.
- ↑ Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (August 1997). "Alpha-synuclein in Lewy bodies". Nature. 388 (6645): 839–40. doi:10.1038/42166. PMID 9278044.
- ↑ Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH, Brownstein MJ (July 1998). "Alpha synuclein in neurodegenerative disorders: murderer or accomplice?". Nature Medicine. 4 (7): 755–7. doi:10.1038/nm0798-755. PMID 9662355.
- ↑ Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K, Ikeda K, Kawai M (October 1999). "Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies". Brain Research. 843 (1–2): 53–61. doi:10.1016/S0006-8993(99)01848-X. PMID 10528110.
- ↑ Arima K, Mizutani T, Alim MA, Tonozuka-Uehara H, Izumiyama Y, Hirai S, Uéda K (August 2000). "NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies". Acta Neuropathologica. 100 (2): 115–21. doi:10.1007/s004010050002. PMID 10963357.
- ↑ Yokota O, Terada S, Ishizu H, Ujike H, Ishihara T, Nakashima H, Yasuda M, Kitamura Y, Uéda K, Checler F, Kuroda S (December 2002). "NACP/alpha-synuclein, NAC, and beta-amyloid pathology of familial Alzheimer's disease with the E184D presenilin-1 mutation: a clinicopathological study of two autopsy cases". Acta Neuropathologica. 104 (6): 637–48. doi:10.1007/s00401-002-0596-7. PMID 12410385.
- ↑ Kim HY, Heise H, Fernandez CO, Baldus M, Zweckstetter M (September 2007). "Correlation of amyloid fibril beta-structure with the unfolded state of alpha-synuclein". Chembiochem. 8 (14): 1671–4. doi:10.1002/cbic.200700366. PMID 17722123.
- ↑ Sandal M, Valle F, Tessari I, Mammi S, Bergantino E, Musiani F, Brucale M, Bubacco L, Samorì B (January 2008). "Conformational equilibria in monomeric alpha-synuclein at the single-molecule level". PLoS Biology. 6 (1): e6. doi:10.1371/journal.pbio.0060006. PMC 2174973. PMID 18198943.
- ↑ Schulz-Schaeffer WJ (August 2010). "The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia". Acta Neuropathologica. 120 (2): 131–43. doi:10.1007/s00401-010-0711-0. PMID 20563819.
- ↑ Morshedi D, Aliakbari F (Spring 2012). "The Inhibitory Effects of Cuminaldehyde on Amyloid Fibrillation and Cytotoxicity of Alpha-synuclein". modares journal of medical sciences: pathobiology. 15 (1): 45–60.
- ↑ Woulfe J, Hoogendoorn H, Tarnopolsky M, Muñoz DG (November 2000). "Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain". Neurology. 55 (9): 1398–401. doi:10.1212/WNL.55.9.1398. PMID 11087792.
- ↑ Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (June 1997). "Mutation in the alpha-synuclein gene identified in families with Parkinson's disease". Science. 276 (5321): 2045–7. doi:10.1126/science.276.5321.2045. PMID 9197268.
- ↑ Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O (February 1998). "Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease". Nature Genetics. 18 (2): 106–8. doi:10.1038/ng0298-106. PMID 9462735.
- ↑ Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG (February 2004). "The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia". Annals of Neurology. 55 (2): 164–73. doi:10.1002/ana.10795. PMID 14755719.
- ↑ Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ (June 2013). "Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease". Movement Disorders. 28 (6): 811–3. doi:10.1002/mds.25421. PMID 23457019.
- ↑ Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, Pieri L, Madiona K, Dürr A, Melki R, Verny C, Brice A (Apr 2013). "G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome". Annals of Neurology. 73 (4): 459–71. doi:10.1002/ana.23894. PMID 23526723.
- ↑ Flagmeier P, Meisl G, Vendruscolo M, Knowles TP, Dobson CM, Buell AK, Galvagnion C (September 2016). "Mutations associated with familial Parkinson's disease alter the initiation and amplification steps of α-synuclein aggregation". Proceedings of the National Academy of Sciences of the United States of America. 113 (37): 10328–33. doi:10.1073/pnas.1604645113. PMC 5027465. PMID 27573854.
- ↑ Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (Oct 2003). "alpha-Synuclein locus triplication causes Parkinson's disease". Science. 302 (5646): 841. doi:10.1126/science.1090278. PMID 14593171.
- ↑ Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Masliah E (Mar 2000). "C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders". Acta Neuropathologica. 99 (3): 296–304. doi:10.1007/PL00007441. PMID 10663973.
- ↑ Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K (Aug 2015). "Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism". Proceedings of the National Academy of Sciences of the United States of America. 112: E5308–17. doi:10.1073/pnas.1514475112. PMC 4586853. PMID 26324905.
- ↑ Weiler N (31 August 2015). "New Type of Prion May Cause, Transmit Neurodegeneration".
- ↑ Rettner R (31 August 2015). "Another Fatal Brain Disease May Come from the Spread of 'Prion' Proteins". Wired Science.
- ↑ Cookson MR (2009). "alpha-Synuclein and neuronal cell death". Mol Neurodegener. 4 (1): 9. doi:10.1186/1750-1326-4-9. PMC 2646729. PMID 19193223.
- ↑ Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (February 2002). "alpha-Synuclein is phosphorylated in synucleinopathy lesions". Nature Cell Biology. 4 (2): 160–4. doi:10.1038/ncb748. PMID 11813001.
- ↑ Wersinger C, Sidhu A (Apr 2003). "Attenuation of dopamine transporter activity by alpha-synuclein". Neuroscience Letters. 340 (3): 189–92. doi:10.1016/S0304-3940(03)00097-1. PMID 12672538.
- ↑ Lee FJ, Liu F, Pristupa ZB, Niznik HB (Apr 2001). "Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis". FASEB Journal. 15 (6): 916–26. doi:10.1096/fj.00-0334com. PMID 11292651.
- ↑ Choi P, Golts N, Snyder H, Chong M, Petrucelli L, Hardy J, Sparkman D, Cochran E, Lee JM, Wolozin B (Sep 2001). "Co-association of parkin and alpha-synuclein". NeuroReport. 12 (13): 2839–43. doi:10.1097/00001756-200109170-00017. PMID 11588587.
- ↑ Kawahara K, Hashimoto M, Bar-On P, Ho GJ, Crews L, Mizuno H, Rockenstein E, Imam SZ, Masliah E (Mar 2008). "alpha-Synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease". The Journal of Biological Chemistry. 283 (11): 6979–87. doi:10.1074/jbc.M710418200. PMID 18195004.
- ↑ Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, Chung KC, Yoon SH, Hahn SJ, Kim MS, Jo YH, Min DS (Apr 2002). "alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells". The Journal of Biological Chemistry. 277 (14): 12334–42. doi:10.1074/jbc.M110414200. PMID 11821392.
- ↑ Neystat M, Rzhetskaya M, Kholodilov N, Burke RE (Jun 2002). "Analysis of synphilin-1 and synuclein interactions by yeast two-hybrid beta-galactosidase liquid assay". Neuroscience Letters. 325 (2): 119–23. doi:10.1016/S0304-3940(02)00253-7. PMID 12044636.
- ↑ Reed JC, Meister L, Tanaka S, Cuddy M, Yum S, Geyer C, Pleasure D (Dec 1991). "Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin". Cancer Research. 51 (24): 6529–38. PMID 1742726.
- ↑ Kawamata H, McLean PJ, Sharma N, Hyman BT (May 2001). "Interaction of alpha-synuclein and synphilin-1: effect of Parkinson's disease-associated mutations". Journal of Neurochemistry. 77 (3): 929–34. doi:10.1046/j.1471-4159.2001.00301.x. PMID 11331421.
- ↑ Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA (May 1999). "Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions". Nature Genetics. 22 (1): 110–4. doi:10.1038/8820. PMID 10319874.
- ↑ Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R (Sep 1999). "alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356". The Journal of Biological Chemistry. 274 (36): 25481–9. doi:10.1074/jbc.274.36.25481. PMID 10464279.
- ↑ Giasson BI, Lee VM, Trojanowski JQ (2003). "Interactions of amyloidogenic proteins". Neuromolecular Medicine. 4 (1–2): 49–58. doi:10.1385/NMM:4:1-2:49. PMID 14528052.
- ↑ Ono K, Takahashi R, Ikeda T, Yamada M (Sep 2012). "Cross-seeding effects of amyloid β-protein and α-synuclein". Journal of Neurochemistry. 122 (5): 883–90. doi:10.1111/j.1471-4159.2012.07847.x. PMID 22734715.
Further reading
- Blakeslee S (2002-05-27). "In Folding Proteins, Clues to Many Diseases -". New York Times.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (Jun 1997). "Mutation in the alpha-synuclein gene identified in families with Parkinson's disease". Science. 276 (5321): 2045–7. doi:10.1126/science.276.5321.2045. PMID 9197268.
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Müller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C (Nov 2002). "Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies". The Journal of Clinical Investigation. 110 (10): 1429–39. doi:10.1172/JCI15777. PMC 151810. PMID 12438441.
- George JM (2001). "The synucleins". Genome Biology. 3 (1): REVIEWS3002. doi:10.1186/gb-2001-3-1-reviews3002. PMC 150459. PMID 11806835.
- Lavedan C (Sep 1998). "The synuclein family". Genome Research. 8 (9): 871–80. doi:10.1101/gr.8.9.871. PMID 9750188.
- Ozawa T, Wakabayashi K, Oyanagi K (Feb 2002). "[Recent progress in the research of multiple system atrophy with special references to alpha-synuclein and suprachiasmatic nucleus]". Nō to Shinkei = Brain and Nerve. 54 (2): 111–7. PMID 11889756.
- Cole NB, Murphy DD (2002). "The cell biology of alpha-synuclein: a sticky problem?". Neuromolecular Medicine. 1 (2): 95–109. doi:10.1385/NMM:1:2:95. PMID 12025860.
- Iwatsubo T (Jun 2002). "[alpha-synuclein and Parkinson's disease]". Seikagaku. The Journal of Japanese Biochemical Society. 74 (6): 477–82. PMID 12138709.
- Trojanowski JQ, Lee VM (Oct 2002). "Parkinson's disease and related synucleinopathies are a new class of nervous system amyloidoses". Neurotoxicology. 23 (4–5): 457–60. doi:10.1016/S0161-813X(02)00065-7. PMID 12428717.
- Alves da Costa C (Feb 2003). "Recent advances on alpha-synuclein cell biology: functions and dysfunctions". Current Molecular Medicine. 3 (1): 17–24. doi:10.2174/1566524033361690. PMID 12558071.
- Ma QL, Chan P, Yoshii M, Uéda K (Apr 2003). "Alpha-synuclein aggregation and neurodegenerative diseases". Journal of Alzheimer's Disease. 5 (2): 139–48. PMID 12719631.
- Di Rosa G, Puzzo D, Sant'Angelo A, Trinchese F, Arancio O (Oct 2003). "Alpha-synuclein: between synaptic function and dysfunction". Histology and Histopathology. 18 (4): 1257–66. PMID 12973692.
- Baptista MJ, Cookson MR, Miller DW (Feb 2004). "Parkin and alpha-synuclein: opponent actions in the pathogenesis of Parkinson's disease". The Neuroscientist. 10 (1): 63–72. doi:10.1177/1073858403260392. PMID 14987449.
- Kim S, Seo JH, Suh YH (May 2004). "Alpha-synuclein, Parkinson's disease, and Alzheimer's disease". Parkinsonism & Related Disorders. 10 Suppl 1: S9–13. doi:10.1016/j.parkreldis.2003.11.005. PMID 15109581.
- Sidhu A, Wersinger C, Vernier P (May 2004). "alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease". FEBS Letters. 565 (1–3): 1–5. doi:10.1016/j.febslet.2004.03.063. PMID 15135042.
- Vekrellis K, Rideout HJ, Stefanis L (Aug 2004). "Neurobiology of alpha-synuclein". Molecular Neurobiology. 30 (1): 1–21. doi:10.1385/MN:30:1:001. PMID 15247485.
- Chiba-Falek O, Nussbaum RL (2004). "Regulation of alpha-synuclein expression: implications for Parkinson's disease". Cold Spring Harbor Symposia on Quantitative Biology. 68: 409–15. doi:10.1101/sqb.2003.68.409. PMID 15338643.
- Pankratz N, Foroud T (Apr 2004). "Genetics of Parkinson disease". NeuroRx. 1 (2): 235–42. doi:10.1602/neurorx.1.2.235. PMC 534935. PMID 15717024.
- Singleton AB (Aug 2005). "Altered alpha-synuclein homeostasis causing Parkinson's disease: the potential roles of dardarin". Trends in Neurosciences. 28 (8): 416–21. doi:10.1016/j.tins.2005.05.009. PMID 15955578.
- Yu S, Uéda K, Chan P (2005). "Alpha-synuclein and dopamine metabolism". Molecular Neurobiology. 31 (1–3): 243–54. doi:10.1385/MN:31:1-3:243. PMID 15953825.
- Lee HG, Zhu X, Takeda A, Perry G, Smith MA (Jul 2006). "Emerging evidence for the neuroprotective role of alpha-synuclein". Experimental Neurology. 200 (1): 1–7. doi:10.1016/j.expneurol.2006.04.024. PMID 16780837.
- Giorgi FS, Bandettini di Poggio A, Battaglia G, Pellegrini A, Murri L, Ruggieri S, Paparelli A, Fornai F (2006). "A short overview on the role of alpha-synuclein and proteasome in experimental models of Parkinson's disease". Journal of Neural Transmission. Supplementum. 70 (70): 105–9. doi:10.1007/978-3-211-45295-0_17. PMID 17017516.
External links
Media related to alpha synuclein at Wikimedia Commons
- alpha-Synuclein at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human SNCA genome location and SNCA gene details page in the UCSC Genome Browser.
- Pages with broken file links
- Genes on human chromosome
- All articles with unsourced statements
- Articles with unsourced statements from March 2012
- Articles with invalid date parameter in template
- Articles needing additional references from November 2015
- All articles needing additional references
- Peripheral membrane proteins